Abstract
Aims: To determine if EuroQoL 5-Dimension Health Questionnaire (EQ-5D) health utility scores were able to discriminate among different levels of improvement in psoriasis severity following therapy.
Materials and methods: Data were from three placebo-controlled phase 3 ixekizumab studies (UNCOVER-1, UNCOVER-2, and UNCOVER-3) with patients who had baseline Dermatology Life Quality Index scores >10 (DLQI >10). Psoriasis severity (Psoriasis Area and Severity Index [PASI]), general health utility (EQ-5D), and psoriasis-specific utility (EQ-PSO, UNCOVER-3 only) were assessed. EQ-5D-5L utility scores were generated using the England EQ-5D-5L value set, a crosswalk applied to the EQ-5D-3L United States (US) and United Kingdom (UK) value sets, and a regression-based exploratory scoring function for the EQ-PSO (UK). Analysis of variance was used to estimate change in EQ-5D-5L from baseline to Week 12 per PASI improvement level: PASI <50, PASI 50 to <75, PASI 75 to <90, PASI 90 to <100, and PASI 100. Missing data were imputed using the last observation carried forward method. Value sets for the UK, England, and the US were applied.
Results: In total, 2085 patients across UNCOVER-1, UNCOVER-2, and UNCOVER-3 had baseline DLQI >10 and available utility scores. At Week 12, mean EQ-5D utility scores increased with increasing PASI improvement levels (p < 0.001, all analyses). Mean health utilities for PASI 90 to <100 and PASI 100 were similar when based on the generic classifier, whereas a clear differentiation between PASI 90 to <100 and PASI 100 was observed for EQ-PSO mean scores (UNCOVER-3 only, n = 645; PASI 90 to <100: 0.141, PASI 100: 0.200; adjusted p = 0.043).
Limitations: EQ-5D-5L index-based scores have limited ability to differentiate among psoriasis patients at the highest PASI improvement levels.
ConclusionsL Adding psoriasis-specific EQ-PSO dimensions to the EQ-5D may enhance responsiveness to improvement in skin clarity at the highest PASI levels, and, therefore, generate utility scores that better reflect treatment benefit in cost-utility models.
Introduction
Psoriasis is a chronic skin disease that has a negative impact on health-related quality-of-life (HRQoL)Citation1,Citation2. Although psoriasis patients may experience improvements in their condition with treatment, those who do not achieve complete skin clearance may continue to report sub-optimal HRQoLCitation3,Citation4. Recently, Strober et al.Citation5 demonstrated that the majority of patients who achieved complete resolution of their skin symptoms experienced improved HRQoL compared with patients who did not.
The EuroQoL 5-Dimension Health Questionnaire (EQ-5D) is a generic measure of health that can be used to capture treatment effects on HRQoL as they relate to a common set of core dimensionsCitation6. Utility scores can be derived from EQ-5D responses by applying value sets or utility functions that have been published for many countries, and they serve to facilitate the calculation of Quality Adjusted Life Years (QALYs) that are commonly used in economic evaluations of healthcare interventions.
With newer biologic therapies like ixekizumab and secukinumab, more patients are achieving higher levels of skin improvementCitation7–10. Randomized, controlled, and open-label studies have demonstrated improvements in both EQ-5D values and psoriasis severity (measured by the percentage of improvement from baseline on the Psoriasis Area and Severity Index [PASI]) following biologic treatmentCitation11,Citation12. Additionally, a longitudinal observational study based on the Swedish National Registry for Systemic Treatment of Psoriasis reported greater improvement in EQ-5D values among patients with more severe psoriasisCitation13. However, to date, health utility scores of patients with near complete resolution (i.e. PASI 90 to <100) have not been differentiated from those with complete resolution (i.e. PASI 100) of psoriasis. Given the effectiveness of the newer biologics, there is a need for valid, responsive health outcome measures in psoriasis that can identify differences at the highest levels of improvement in skin clearance.
In cost-utility analyses, utility scores derived from health state classifiers like the EQ-5D are used to express the clinical benefit generated by the drugs under assessment. Thus, it is important to ensure that the health utilities adequately reflect the benefits that the drug can bring to patients. In the area of psoriasis, where higher PASI thresholds, and, therefore, higher levels of skin improvements are achieveable with newer biologics, it is important to explore whether clinical benefit at the highest levels of improvement (i.e. PASI 90 to <100 and 100) is adequately translated into health utilities, and, ultimately, integrated into economic evaluations.
The aim of this research was to determine if EQ-5D health utility scores were able to discriminate among different levels of clinical improvement in psoriasis severity following therapy with ixekizumab, a high-affinity monoclonal antibody that selectively targets interleukin (IL)-17ACitation14, with specific interest in patients with higher levels of treatment response (near complete [i.e. PASI 90 to <100] and complete resolution [i.e. PASI 100]). A secondary objective was to examine if this differentiation could be better achieved with a modified version of the EQ-5D, with additional dimensions developed specifically for psoriasis.
Methods
Patients
Patients enrolled in three randomized, double-blind, placebo-controlled phase 3 studies of ixekizumab (UNCOVER-1, UNCOVER-2, and UNCOVER-3) were included. The eligibility criteria were similar across the three studies, and are described in greater detail elsewhereCitation7–9. Briefly, patients were ≥18 years of age with moderate-to-severe plaque psoriasis who had been diagnosed ≥6 months before randomization and were candidates for phototherapy and/or systemic therapy with ≥10% body surface area involvement, a static Physician’s Global Assessment score ≥3 (on a scale from 0–5, with higher scores indicating more severe disease), and a PASI score ≥12 (on a scale from 0–72, with higher scores indicating more severe disease), at both screening and baseline visits. Patients with forms of psoriasis other than chronic plaque psoriasis or with drug-induced psoriasis were excluded. The use of medications that might confound efficacy was not allowed.
Study design
In the first 12 weeks of the UNCOVER studies, patients were randomized to receive subcutaneous placebo, etanercept 50-mg twice weekly (in UNCOVER-2 and UNCOVER-3 only), or 80-mg ixekizumab every 2 weeks (IXE Q2W) or every 4 weeks (IXE Q4W) following a 160-mg loading dose. Additional study details have been previously reportedCitation7–9. All patients gave informed written consent for all studies. Individual protocols received ethical review board approval. For the present research, analysis was restricted to the 12-week induction period due to re-randomization and further differences in subsequent study periods.
Outcome measures
The clinical trial end-points selected for analysis in the present study included the PASI, the Dermatology Life Quality Index (DLQI), and the EQ-5D, which focus on different aspects of psoriatic disease and associated treatment benefits. Norlin et al.Citation15 reported the merits of each of these measures in assessing treatments for psoriasis.
Improvement in psoriasis severity was evaluated by percentage PASI improvement from baseline to Week 12. PASI improvement is an end-point commonly used in clinical trials of psoriasisCitation16. For example, a patient with a baseline absolute PASI score of 20 (on a scale of 0–72, with higher score indicating more severe disease) and a Week 12 absolute PASI score of 10 would demonstrate a 50% PASI improvement (i.e. PASI 50). For the purpose of generating health utilities, percentage PASI improvement at Week 12 was used to assign patients to one of five improvement levels: (1) no response, less than 50% improvement (PASI <50), (2) 50% to less than 75% improvement (PASI 50 to <75), (3) 75% to less than 90% improvement (PASI 75 to <90), (4) near complete resolution, 90% to less than 100% improvement (PASI 90 to <100), and (5) complete resolution, 100% improvement (PASI 100). These PASI response categories were based on previously established categories used in health utility analyses of other biologicsCitation17–20.
The DLQI, a 30-point scale with higher scores indicating greater impairment, was administered to assess dermatology-specific HRQoL impact at baseline and various time points over the 12-week treatment periodCitation21. For the purposes of this analysis, the DLQI was utilized to identify patients for whom HRQoL was impacted by psoriasis most severely (i.e. DLQI >10 at baseline), which is consistent with previous approaches to health utilities for psoriasis treatmentsCitation17–20. The stratification by baseline DLQI is consistent with previous health technology assessments where a DLQI >10 or ≥10 formed the basis for the base case.
The EQ-5D-5L, which was used in the UNCOVER trials, is a generic, patient-reported outcome measure consisting of a descriptive system and a Visual Analog Scale (EQ-5D VAS)Citation22,Citation23. The descriptive system has five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each with five levels or responses (no problems, slight problems, moderate problems, severe problems, and extreme problems). The EQ-5D-5L is a relatively new derivative of the standard EQ-5D-3L, which had three levels per dimension. The EQ-5D-5L was designed to capture smaller changes between the different health statesCitation24. In addition to the descriptive system, the EQ-5D includes a VAS component where the respondent is asked to self-rate their health today on a 20-cm vertical VAS numbered from 0–100, where 0 represents worst health imaginable and 100 indicates best health imaginable.
Notably, the EQ-5D-5L is a generic instrument and may not be sufficiently sensitive to capture important aspects of a disease like psoriasis. To capture disease-specific elements, research was conducted to add two additional dimensions (skin irritation and self-confidence) to the EQ-5D-5L as a psoriasis-specific “bolt on” version, hereafter referred to as the EQ-PSOCitation25. The format of the EQ-PSO questionnaire, developed after soliciting extensive patient insights to identify important elements of their health status, is similar to the EQ-5D-5L, with five levels (no problems, slight problems, moderate problems, severe problems, and extreme problems) across seven dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression, skin irritation, and self-confidence). The EQ-PSO has been shown by regression analysis to be better at predicting psoriasis outcomes (DLQI and self-assessed PASI) than the original, unmodified version of the EQ-5D-5LCitation25.
For all three UNCOVER studies, the EQ-5D-5L questionnaire and the EQ-5D VAS were administered at baseline and Week 12. For the UNCOVER-3 study only, the EQ-PSO questionnaire was administered additionally at the same time points.
Value sets
To estimate health state utilities, country-specific weights were applied to EQ-5D responses. EQ-5D-3L value sets are available for many countries, including the UKCitation26 and the USCitation27, and value sets are beginning to emerge for the more recently developed EQ-5D-5L, including a value set for EnglandCitation28. In the absence of a value set for the EQ-5D-5L, a validated crosswalk is available that maps responses to the 5L onto the 3L descriptive system which then allows for the application of existing 3L value setsCitation29. Here, the crosswalk was employed to generate scores using value sets for the UK and the US. EQ-5D-5L scores were generated using the EQ-5D-5L value set for EnglandCitation28, and EQ-PSO scores were generated using the UK value setCitation26.
Statistical analysis
The analyses of EQ-5D data were conducted on the intent-to-treat population of patients with a baseline DLQI >10. This sub-group was identified as being the most relevant and is consistent with previous economic evaluationsCitation17–20.
Patients who discontinued before the end of Week 12 were encouraged to complete the EQ-5D at that drop-out visit. If available, this EQ-5D value was used as an estimate for the Week 12 value (last-observation carried forward [LOCF] approach). For the PASI response, the same LOCF approach was applied to ensure that all available EQ-5D-5L and EQ-5D VAS values were utilized and the vast majority was aligned with the PASI response from the same time point.
Descriptive summary statistics were produced for baseline demographics and clinical characteristics as well as for the EQ-5D dimensions at baseline and Week 12. Analysis of variance (ANOVA [LOCF]), including multiple adjusted pairwise comparisons, was used as the primary analysis to estimate the change in EQ-5D-5L from baseline to Week 12 per PASI improvement level (i.e. PASI <50, PASI 50 to <75, PASI 75 to <90, PASI 90 to <100, and PASI 100) at 12 weeks. These analyses were conducted for the EQ-5D-5L (England), the EQ-5D-3L Crosswalk (UK and US), and the EQ-PSO (UK) data.
Results
For the intent-to-treat population with a baseline DLQI >10 (n = 2165), utility scores were available for 2085 patients (96.3%) across all three UNCOVER studies combined and were used for the EQ-5D-5L (England) and the EQ-5D-3L Crosswalk (UK and US) analyses. Additionally, for the EQ-PSO questionnaire (UK), utility scores were available for 645 patients (92.1% of 700 with baseline DLQI >10) in UNCOVER-3 only. Patients were on average 45 years of age and were predominantly white (all UNCOVER studies: 91.3%; UNCOVER-3: 90.9%) men (all UNCOVER studies: 62.6%; UNCOVER-3: 62.3%) who, on average, had a diagnosis of psoriasis for 17–18 years, a baseline absolute PASI score of 21–22, and a baseline DLQI of 17.5 ().
Table 1. Baseline demographics and clinical characteristics in patients from all UNCOVER studies and from UNCOVER-3 only—intent-to-treat patients with baseline DLQI >10.
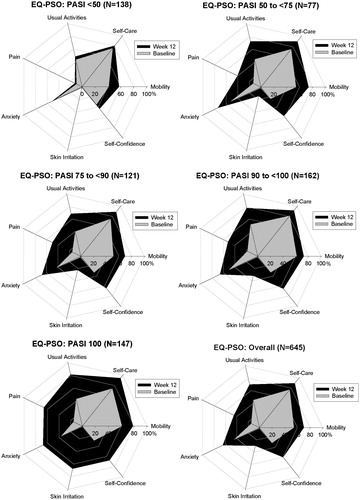
For the EQ-5D-5L descriptive system, for each dimension the observed proportions of patients reporting no problems increased among all patients experiencing improvements (i.e. ≥ PASI 75) from baseline to Week 12 (). A similar trend was observed on the EQ-PSO dimensions (for UNCOVER-3 only) where proportionally more patients reported fewer problems as the PASI improvement level increased.
Table 2. EQ-5D-5L and EQ-PSO responses at baseline and Week 12 by PASI improvement level—intent-to-treat patients with baseline DLQI >10.
and show the changes over time in the percentages of patients reporting no problems on the different dimensions of the EQ-5D-5L and the EQ-PSO, respectively, across the five different PASI improvement levels. Consistent with the descriptive data in , there was a trend toward higher proportions of patients reporting no problems on each dimension of the EQ-5D-5L and EQ-PSO as PASI improvement level increased. On the EQ-5D-5L, the Pain dimension was the most impacted at Week 12 and was also the dimension with the greatest potential for improvement from baseline. Similarly, on the EQ-PSO, the Pain dimension and the Skin Irritation dimension had the greatest potential for improvement and showed the greatest impact at Week 12.
Figure 1. No problems on the EQ-5D-5L Dimensions—Overall Population From UNCOVER-1, UNCOVER-2, and UNCOVER-3. Change in the percentage of patients reporting no problems on each of the five dimensions of the EQ-5D-5L questionnaire over time by PASI improvement level. Percentage of patients reporting no problems at baseline are shown in grey and the percentage of patients reporting no problems at Week 12 are shown in black. Includes patients from UNCOVER -1, UNCOVER-2, and UNCOVER-3 with baseline DLQI >10 and non-missing EQ-5D-5L values (n = 2085). DLQI, Dermatology Life Quality Index; EQ-5D-5L, EuroQoL 5-Dimension Health Questionnaire–5 levels; PASI, psoriasis area and severity index.
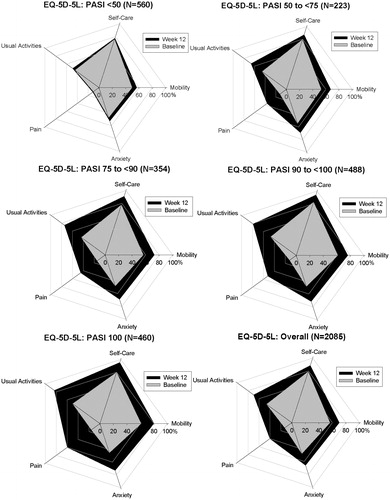
Figure 2. No problems on the EQ-PSO Dimensions—Population From UNCOVER-3 Only. Change in the percentage of patients reporting no problems on each of the five dimensions of the EQ-PSO questionnaire over time by PASI improvement level. Percentage of patients reporting no problems at baseline are shown in grey and the percentage of patients reporting no problems at Week 12 are shown in black. Includes patients from UNCOVER-3 only with baseline DLQI >10 and non-missing EQ-PSO values (n = 645). DLQI, Dermatology Life Quality Index; EQ-PSO, EuroQoL 5-Dimension Health Questionnaire—psoriasis-specific dimensions; PASI, psoriasis area and severity index.
Across PASI improvement levels, mean baseline PASI scores ranged from 20–23 () and mean baseline EQ-5D VAS scores ranged from 64–67 (), indicating that baseline score differences were small. Importantly, mean EQ-5D VAS scores improved monotonically, with greater improvement in skin clarity and a larger mean change in EQ-5D VAS associated with the PASI 100 improvement level.
Table 3. EQ-5D VAS descriptive analysis at baseline and week 12 by PASI improvement level in patients from all UNCOVER studies—intent-to-treat patients with baseline DLQI >10.
Health utilities generally increased with higher PASI improvement levels (p < 0.001 for all three index-based approaches to generating generic utilities) (). The estimated least square (LS) mean health utilities for the PASI 90 to <100 improvement level and the PASI 100 improvement level were similar for the EQ-5D-5L (PASI 90 to <100: 0.139, PASI 100: 0.141; adjusted p > 0.99) and EQ-5D-3L Crosswalk (PASI 90 to <100: 0.184, PASI 100: 0.189; adjusted p > 0.99), whereas the EQ-PSO mean scores were significantly different between PASI 90 to <100 and PASI 100 (PASI 90 to <100: 0.141, PASI 100: 0.200; adjusted p = 0.043).
Table 4. LS mean health utility improvement from baseline by PASI improvement level.
Estimations of health utilities using the US value set also indicated an increase in health utilities with higher PASI improvement levels (p < 0.001, ). Again, no substantial difference between the PASI 90 to <100 and the PASI 100 improvement levels were observed (LS means, PASI 90 to <100: 0.130, PASI 100: 0.135; adjusted p > 0.99). To contrast to our findings, health utility data used in economic evaluations of other psoriasis therapies are also provided in .
Discussion
One of the challenges for users of generic utility measures is the possibility that important and meaningful changes in the condition of patients with a specific disease may not be captured. We sought to examine this issue in psoriasis by comparing mean EQ-5D utility scores across five different levels of PASI improvement, with specific interest in whether a differentiation could be detected between PASI 90 to <100 and PASI 100 in terms of greater health utility. Notably, in dermatologic studies the benchmark for efficacy of a drug in the treatment of psoriasis is PASI 75 [16]. Newer biologics surpass that benchmark, frequently achieving PASI 90 to <100 and PASI 100 in patients. Thus, an examination of health utilities at the highest levels of PASI improvement will support more precise cost-utility assessments. Results showed that mean EQ-5D-5L index-based utility scores and VAS-based scores became larger as PASI improvements were greater, but the general utility scores were unable to differentiate among higher levels of PASI improvement categories. However, the EQ-PSO scores, which included two additional dimensions relevant to psoriasis, were able to significantly differentiate among the highest PASI improvement categories.
Overall baseline utility values for the EQ-5D questionnaire variations ranged from 0.66–0.76. These values are largely in line with the range of baseline index values reported for patients with psoriasis in other studiesCitation11,Citation12,Citation30–32. The relationships observed between the measures used in the current study supported their convergent validity. The responses to each dimension on the EQ-5D showed a smaller proportion of problems were reported among patients with progressively better PASI-based improvement levels. Similarly, the magnitude of mean change scores on the EQ-VAS became larger as PASI-based improvements increased.
The results of the current study complement the evidence on the responsiveness of utility measures in skin conditions, which was summarized in a review published in 2010Citation33. The review by Bronsard et al.Citation33 reported the measurement properties of instruments used from 1988–2009 in psoriasis and did not identify evidence available for any preference-based measures. The more recent systematic review by Yang et al.Citation34 identified 16 papers reporting EQ-5D utilities, 12 of which were in psoriasis. They reported that eight of the studies showed that the EQ-5D was able to detect change in HRQL over time and concluded that the EQ-5D had good validity and responsiveness, and found no other studies of alternative preference-based measures (i.e. the SF-6D and HUI). Our present study found that the responsiveness of the upper limits of the EQ-5D index-based scale can potentially be further improved with additional dimensions.
Some limitations of the analyses presented here should be taken into consideration. The EQ-5D-5L was utilized for data collection and there is presently no EQ-5D-5L value set for the US population. For this reason, the crosswalk methodology was used to transfer the index values to the EQ-5D-3L. It is possible that patients may have experienced a ceiling effect with less noticeable differences between PASI 90 to <100 and PASI 100 than between PASI <50 and PASI 50 to <75, making further differentiation at the highest levels difficult when applied to health utilities. This might be reinforced by the generic nature of the EQ-5D, which makes it not ideal to capture benefit in a disease such as psoriasis. This aspect is supported by the results when the psoriasis-specific EQ-PSO questionnaire was used, where a difference between the PASI 90 to <100 and PASI 100 levels was evident. Because the EQ-PSO was only utilized in one of the three trials, these findings may be limited in their generalizability, and more research on the EQ-PSO and its scoring function is needed.
Conclusions
Newer biologic therapies for the treatment of moderate-to-severe psoriasis have demonstrated higher levels of skin clearance than existing therapies, but this clinical benefit may not be adequately captured by generic preference-based measures of health such as the EQ-5D. Results showed that adding or “bolting on” disease-specific questions to the EQ-5D instrument may improve the responsiveness of the EQ-5D at the highest PASI improvement levels, and thus health utility values, and, therefore, better capture the benefits of treatment when developing cost-utility models.
Transparency
Declaration of funding
This research was funded by Eli Lilly and Company and/or one of its subsidiaries. (UNCOVER-1, UNCOVER-2, and UNCOVER-3 ClinicalTrials.gov numbers NCT01474512, NCT01597245, and NCT01646177, respectively).
Declaration of financial/other relationships
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose. ASP is partner at Second City Outcomes Research LLC, which provides health outcomes related consulting services, and is a member of the EuroQol Group, developers of the EQ-5D. MG has been an investigator, a speaker, on an advisory board for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, and Pfizer, and investigator for Dermira, UCB, and Coherus. SH and CN are employees and stockholders of Eli Lilly and Company Ltd.
Acknowledgments
The authors would like to thank Kelly Guerrettaz, inVentiv Health Clinical (Princeton, NJ) for her assistance with the manuscript writing, and Russel Burge, Eli Lilly and Company (Indianapolis, IN) for his assistance with the development of the manuscript.
References
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol 2016;41:514-21
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PLoS One 2012;7:e52935
- Takeshita J, Callis Duffin K, Shin DB, et al. Patient-reported outcomes for psoriasis patients with clear versus almost clear skin in the clinical setting. J Am Acad Dermatol 2014;71:633-41
- Viswanathan HN, Chau D, Milmont CE, et al. Total skin clearance results in improvements in health-related quality of life and reduced symptom severity among patients with moderate to severe psoriasis. J Dermatolog Treat 2015;26:235-9
- Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis J Am Acad Dermatol 2016;75:77-82.e7
- Rabin R, deCharro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016; 375:345-56
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386:541-51
- Gordon K, Blauvelt A, Langley R, et al. Ixekizumab for treatment of moderate-to-severe plaque psoriasis: 60-week results from a double-blind phase 3 induction and randomized withdrawal study (UNCOVER-1) [abstract]. Late-Breaking Research Abstract Forums: American Academy of Dermatology Annual Meeting; 2015, San Francisco, CA, March 20–24; Abstract Book, p 19
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014;371:326-38
- Kalb RE, Blauvelt A, Sofen HL, et al. Effect of infliximab on health-related quality of life and disease activity by body region in patients with moderate-to-severe psoriasis and inadequate response to etanercept: results from the PSUNRISE trial. J Drugs Dermatol 2013;12:874-80.
- Revicki D, Willian MK, Saurat JH, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol 2008;158:549-57
- Norlin JM, Steen Carlsson K, Persson U, et al. Switch to biological agent in psoriasis significantly improved clinical and patient-reported outcomes in real-world practice. Dermatology 2012;225:326-32
- Liu L, Lu J, Allan BW, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res 2016;9:39-50
- Norlin JM, Steen Carlsson K, Persson U, et al. Analysis of three outcome measures in moderate to severe psoriasis: a registry-based study of 2450 patients. Br J Dermatol 2012;166:797-802
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005;64(2 Suppl):ii65-8
- Wade R, Hinde S, Yang H, et al. Apremilast for treating moderate to severe plaque psoriasis: a single technology appraisal. Heslington, York (England): CRD and CHE Technology Group, 2015. http://www.nets.nihr.ac.uk/__data/assets/pdf_file/0011/159977/ERGReport-13-168-01.pdf. Accessed July 21, 2016
- National Institute for Health and Clinical Excellence. Ustekinumab (Stelara™) for the treatment of moderate to severe plaque psoriasis in England & Wales. London (England): Janssen-Cilag Ltd, 2009. https://www.nice.org.uk/guidance/ta180/documents/psoriasis-ustekinumab-manufacturer-submission-janssencilag2. Accessed July 21 2016
- Cummins E, Scott N, Cruickshank M, et al. Secukinumab for treating moderate to severe plaque psoriasis: a single technology appraisal. Aberdeen, UK: Aberdeen HTA Group, 2015. http://www.nets.nihr.ac.uk/__data/assets/pdf_file/0005/146426/ERGReport-13-129-01.pdf. Accessed July 21 2016
- National Institute for Health and Care Excellence. Adalimumab for the treatment of adults with psoriasis. NICE technology appraisal. London (England): Abbott Laboratories, Ltd, 2008. https://www.nice.org.uk/guidance/TA146/documents/psoriasis-adalimumab-appraisal-consultation-manufacturer-submission-abbott-laboratories-ltd2. Accessed July 21, 2016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-6
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36
- Van Reenen M, Janssen B. EQ-5D-5L User guide: basic information on how to use the EQ-5D-5L instrument. Rotterdam (Netherlands): EuroQol Group, 2015
- EuroQol [Internet]. How to obtain EQ-5D. Rotterdam, Netherlands: EUROQOL, c2016. http://www.euroqol.org/eq-5d-products/how-to-obtain-eq-5d.html. Accessed May 19, 2016
- Swinburn P, Lloyd A, Boye KS, et al. Development of a disease-specific version of the EQ-5D-5L for use in patients suffering from psoriasis: lessons learned from a feasibility study in the UK. Value Health 2013;16:1156-62
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203-20
- Devlin N, Shah K, Feng Y, et al. Valuing health-related quality of life: an EQ-5D-5L value set for England. London, UK: University of Sheffield, 2015, https://www.shef.ac.uk/polopoly_fs/1.546914!/file/An_EQ-5D-5L_Value_Set_for_England_DP_Final.pdf. Accessed August 4, 2016
- van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15
- Guh D, Papp K, Lynde C, et al. Impact of adalimumab on quality of life and depression in psoriasis patients: Results from PRIDE [abstract]. Value Health 2010;13:A148
- Reich K, Segaert S, van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mg improves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology 2009;219:239-49
- Shikiar R, Heffernan M, Langley RG, et al. Adalimumab treatment is associated with improvement in health-related quality of life in psoriasis: patient-reported outcomes from a phase II randomized controlled trial. J Dermatolog Treat 2007;18:25-31
- Bronsard V, Paul C, Prey S, et al. What are the best outcome measures for assessing quality of life in plaque type psoriasis? A systematic review of the literature. J Eur Acad Dermatol Venereol 2010;24(2 Suppl):17-22
- Yang Y, Brazier J, Longworth L. EQ-5D in skin conditions: an assessment of validity and responsiveness. Eur J Health Econ 2015;16:927-39
