Abstract
Objective: The recent development of the EDWARDS INTUITY Elite™ (EIE) valve system enables the rapid deployment of a prosthetic surgical heart valve in an aortic valve replacement (AVR) procedure via both the minimally invasive (MISAVR) and conventional (CAVR) approaches. In order to understand its economic value, this study performed a cost evaluation of the EIE valve system used in a MIS rapid-deployment approach (MIS-RDAVR) vs MISAVR and CAVR, respectively, compared to standard prosthetic aortic valves.
Methods: A simulation model was developed using TreeAge (and validated with MS Excel) to compare the inpatient utilization and complication costs for each treatment arm. Thirty-day clinical end-points for the MIS-RDAVR (mortality and complications) were taken from the TRANSFORM trial; and a best evidence review of the published literature was used for the MISAVR and CAVR approaches. Studies were pooled and parameter estimates were weighted by sample size in order to compare the TRANSFORM patients. Cost data (2016 USD) were taken from the Premier database. Incremental cost and cost-effectiveness was assessed and one-way/probabilistic sensitivity analyses performed to gauge the robustness of the results.
Results: MIS-RDAVR costs $2,621 less than CAVR and had lower mortality rates, making it a superior (dominant) technology relative to CAVR. MIS-RDAVR costs $4,560 more than MISAVR, but was associated with an additional 0.20 life years-per-patient. This implies a cost-effectiveness ratio of $22,903 per-life-year-gained. Thus, MIS-RDAVR is cost-effective compared to MISAVR.
Conclusions: The EIE valve system deployed in a MIS approach appears to be a cost-effective technology compared to MISAVR and CAVR. When compared to CAVR it may achieve cost savings as well. These results suggest that MIS-RDAVR confers superior economic value compared to both standard MISAVR and CAVR via lowered key complication rates (re-operation, renal complications, wound infection, TIA, endocarditis) and utilization (cross-clamp time, hospital ward days).
Introduction
An estimated 4.4–5.2 million adults (1.8%) in the US have been diagnosed with aortic valve diseaseCitation1. In 2013, ∼33,900 deaths were found to be related to aortic valve disordersCitation2. Aortic stenosis (AS) is the most common valvular heart diseaseCitation3. If left untreated, outcomes in AS patients are poor, and an aortic valve replacement (AVR) procedure remains the only effective treatment for this patient populationCitation4–7. Without AVR, annual mortality in severe symptomatic AS patients is 25%, with an average survival time of only 2–3 years from diagnosisCitation8. Additional clinical advantages of AVR have been well-established with documented improvements in symptoms, preservation of left ventricular function, and overall quality-of-lifeCitation4,Citation5,Citation7,Citation9–14. Surgical AVR is recommended for symptomatic AS patients who are not considered high-risk for peri-operative morbidity or mortality, or are high-risk but have severe multi-vessel coronary disease that precludes other treatment optionsCitation15. Roughly 67,500 surgical aortic valve replacement (SAVR) procedures are performed every year in the USCitation16, via both minimally invasive (MISAVR) and conventional open (CAVR) approaches.
Although CAVR remains the gold standard for AS patients, the benefits associated with MISAVR has led to the gradual adoption of this approachCitation17,Citation18. More recently, the EDWARDS INTUITY Elite™ (EIE) Valve System (Edwards Lifesciences LLC, Irvine, CA) received FDA approval. Built on the long-term durability, safety, and efficacy of the Carpentier-Edwards Perimount (Edwards Lifesciences LLC) family of valves, this valve system is designed for ‘rapid deployment’ during an AVR (RD-AVR), and was found to be safe and efficacious in an initial clinical trialCitation19. The EIE design enables rapid deployment of the prosthetic valve by using a balloon expanded frame and three guiding sutures. This design provides a greater ease of implantation and better visualization as opposed to a traditional surgical valve which may require up to 18 individual sutures. In clinical practice, the EIE valve system has been shown to facilitate reductions in cross-clamp, bypass, and operative times and appears to facilitate minimally invasive (MIS) surgeryCitation20. This, in turn, is associated with potential benefits of shorter hospital stays, faster recovery, and improvements in morbidity and mortality outcomesCitation18.
In the current environment of rapidly escalating healthcare expenditures in the US, and policymakers’ and providers’ goals to contain costs while enhancing and improving patient care, it is vital to assess the economic impact and value of new technologies such as the EIE valve system. Hence, in order to understand the cost and comparative value of this new technology compared to standard prosthetic aortic valves, this study performs economic evaluations of the EIE valve systems in MIS-RDAVR vs MISAVR and CAVR approaches.
Data and methods
This study developed an economic simulation model using TreeAge (TreeAge Pro 2015 analysis software and validated using an MS Excel build) comparing the inpatient utilization and complication costs for three treatment arms: MIS-RDAVR, MISAVR, and CAVR. and show the clinical and economic outcomes examined for each of the three procedure arms, respectively.
Figure 1. Model scaffold—clinical outcomes. *The MISAVR and CAVR cohorts have the same clinical outcomes as the MIS-RDAVR arm. MIS-RDAVR: minimally invasive rapid deployment aortic valve replacement; MISAVR: minimally invasive aortic valve replacement; CAVR: conventional aortic valve replacement, TIA: transient ischemic attack.
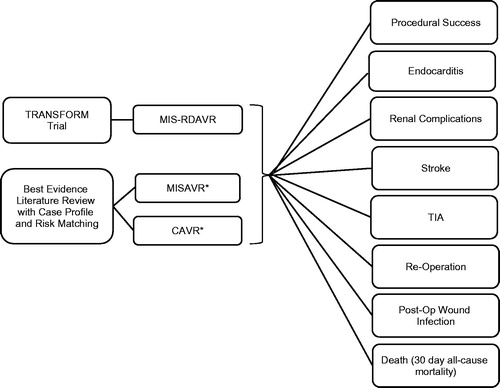
Figure 2. Model scaffold—expected cost calculations. *Costs to the hospital per episode were calculated from the Premier database. †Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38: 65–71. ‡Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day; 2014. Available at: http://kff.org/other/state-indicator/expenses-per-inpatient-day/.
Clinical end-points
Clinical end-points estimates for the MIS-RDAVR arm were derived from a sub-set (n = 276) of Multi-CenTer Experience With the Rapid Deployment EDWARDS INTUITY Valve System FOR Aortic Valve ReplaceMent (TRANSFORM) trial patientsCitation21. The TRANSFORM trial was a prospective, non-randomized, multi-center trial which enrolled up to 839 subjects at 25 centers in the US. These end-points were based on 30-day sample-size weighted outcomes for patients undergoing an isolated AVR (mini upper sternotomy [n = 214] or right thoracotomy [n = 62]) with the EIE valve system (Edwards Lifesciences LLC). Clinical end-points estimates for the MISAVR and CAVR arms were obtained from a best evidence review of the published literature. Thirty-one studies were found that satisfied the following inclusion criteria: (1) English language publications from 2003–2015; (2) adult subjects (≥18 years of age) who received isolated AVR—mini upper sternotomy or right thoracotomy—through MISAVR and/or CAVR approaches; (3) reported one or more of the following outcomes: procedure times, complications, ICU, and/or hospital LOS. Studies were excluded if they involved: (1) endovascular AVR surgeries; (2) AVR done in conjunction with aortic root replacement; (3) only pediatrics; (4) in vitro, animal, or cadaver research; (5) transcatheter (transapical) AVR, percutaneous AVR, valve-in-valve operations; or (6) case reports, commentaries, or editorials.
Parameter estimates from the 31 included studies were pooled and weighted by their respective sample sizes (see and ). These weighed average estimates were then used in the simulation model as parameters for MISAVR and CAVR arms. To check for clinical comparability of the MISAVR and CAVR arms to TRANSFORM, we examined the risk profiles of MIS-RDAVR patients in TRANSFORM vs the cohorts obtained from the literature review. While the TRANSFORM and the literature-derived cohort patients had similar pre-operative profiles, the TRANSFORM patients were slightly older and had a higher rate of co-morbidities. Average age for the MIS-RDAVR TRANSFORM patients was 72 compared to 66–67 years for the MISAVR and CAVR arms, respectively. Additionally, TRANSFORM patients had higher STS predicted mortality scores of 2.0 vs 1.4 for MISAVR and 1.5 for CAVR, respectively. TRANSFORM cohort also had a modestly higher percentage of patients with diabetes, hypertension, peripheral arterial disease, and/or renal failure than either comparator cohort. With the TRANSFORM cohort overall being older and sicker with a higher proportion of co-morbidities, any potential bias would lead to conservative model estimates.
Table 1. Best evidence literature review summary for isolated AVR.
Table 2. Adverse events, mortality, and utilization, by procedure type.
Cost data
Cost data were primarily taken from the Premier hospital database to represent a hospital perspective, and converted to 2016 dollars using the 2016 Medical Care Component of the Consumer Price Index. The Premier database contains data from ∼500 hospitals across the US. This database contains more than 310 million patients treated each year through healthcare alliance members and is representative of one in every five discharges in the nation. It is the nation’s largest clinical and financial comparative database. depicts how episode costs from the Premier database were combined with utilization measures and complication rates to obtain expected costs. Information on expected costs was used to generate estimates of incremental cost. The EIE valve system was estimated to cost an additional $3,000–6,000 over the standard prosthetic valves (Edwards Lifesciences LLC). The highest price point ($6,000) was used as the baseline cost in this investigation.
Incremental cost-effectiveness ratios
Incremental cost-effectiveness ratios (ICERs) were calculated as the difference in cost between MIS-RDAVR and a comparator treatment divided by the change in life years gained. The change in life years was estimated as the difference in mortality between MIS-RDAVR and a comparator treatment multiplied by the life expectancy of the individual. The analysis assumed an average life expectancy of 11 years for surgical AVR individuals in this elderly population, based on a study by Likosky et al.Citation22. The Likosky et al. study found that the long-term survival in AVR patients was similar to the general population of similar age. Moreover, survival varied little, whether or not AVR patients received concomitant bypass surgery. Given this evidence, we assumed that long-term survival was identical across all the treatment groups.
Sensitivity analysis
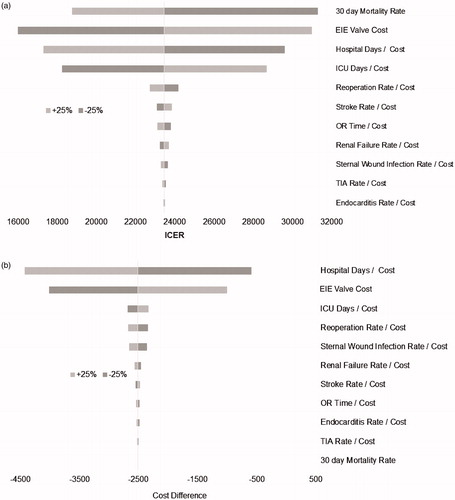
One-way sensitivity analyses were performed to gauge the robustness of the results. Model parameters were varied ±25% from their baseline values one at a time, and the resultant economic valuations were recalculated for each treatment arm. Using these cost values, a tornado diagram was then generated to visually demonstrate the magnitude of change for each of the individual model parameter values ().
Figure 3. (a) MIS-RDAVR vs MISAVR 1-way and (b) MIS-RDAVR vs CAVR 1-way sensitivity analysis tornado diagram. MIS-RDAVR, Edwards INTUITY Elite™ minimally invasive rapid deployment aortic valve replacement; MISAVR, minimally invasive aortic valve replacement; CAVR, conventional aortic valve replacement; ICU, intensive care unit; OR, operating room; TIA, transient ischemic attack; ICER, incremental cost-effectiveness ratios.
In addition, probabilistic sensitivity analyses were performed by running Monte-Carlo simulations with 10,000 trials for each comparison. For the control arms, a normal and beta distribution were assumed for the utilization and complication estimates, respectivelyCitation23 with the sample-size weighted means and standard deviations used as the distribution parameters. Given the single source for the clinical outcomes data in TRANSFORM, we lacked dispersion data to help generate more detailed distributions such as the normal distribution. Hence, we assumed that the MIS-RDAVR parameters followed the same distribution as their corresponding comparative parameters; the TRANSFORM trial point estimates were used to set up the mean (normal) and alpha components (beta) of the distributions, while the standard deviation was derived from the corresponding parameter on the comparator side. The probabilistic sensitivity analysis yielded the proportion of trials for which MIS-RDAVR demonstrates economic value vs MISAVR and CAVR, respectively.
Results
Adverse events and mortality rates
Adverse event and mortality rates for each of the three procedures are provided in . Compared to MISAVR, MIS-RDAVR had lower rates of endocarditis (0 vs 0.3%), TIAs (0.4 vs. 1.3%), re-operation (0 vs. 3.5%), post-operative wound/thoracic infection (0.8 vs. 1.2%), and mortality (0.4 vs 2.2%). However, the stroke (2.6%) and renal complication (3.4%) rate for MIS-RDAVR was marginally higher than for MISAVR (1.5% and 2.6%). Relative to CAVR, MIS-RDAVR had lower rates of endocarditis (0 vs 1.2%), renal complications (3.4 vs 4.3%), TIAs (0.4 vs 0.8%), re-operations (0 vs 4.0%), post-operative wound/thoracic infection (0.8 vs 2.7%), and mortality (0.4 vs 2.8%), with the exception of stroke (2.6% vs 2.1%).
Utilization
Utilization measures by procedure type are also provided in . Time spent in the operating room and cross clamp time were lower for MIS-RDAVR (191.3 and 63.2 min) in comparison to MISAVR (203.7 and 73.4 min) or CAVR (198.4 and 68.4 min). ICU days were higher for the MIS-RDAVR (2.6 days) vs MISAVR (1.8 days) or CAVR (2.5 days), but hospital ward days were lower (6.6 days) relative to MISAVR (8.7 days) or CAVR (9.9 days).
Expected costs and effectiveness
Expected costs and effectiveness (life years gained) for each procedure are shown in . MIS-RDAVR costs $2,621 less than CAVR, while generating more life years gained. Hence, it is a superior (dominant) technology relative to CAVR. MIS-RDAVR costs $4,560 dollars more than MISAVR; however, it adds 0.20 life years per patient. This implies an ICER of $22,903 per life year gained. Thus, MIS-RDAVR is cost-effective compared to MISAVR.
Table 3. Expected costs and effectiveness, by procedure type.
Sensitivity analysis
One-way sensitivity analyses demonstrated that the MIS-RDAVR remained cost-effective in comparison to MISAVR. Varying each model input parameter by ±25% led to ICERs that remained far below accepted cost-effectiveness thresholds of $50,000–$100,000 per life year gain, as is evident from . The results were most sensitive to 30-day mortality rate, cost of the EIE valve, and ICU and hospital ward stay and/or costs. Similarly, one-way sensitivity analysis also revealed that the comparisons of MIS-RDAVR to CAVR were robust. The results were most sensitive to hospital ward days and/or costs and the cost of the EIE valve (see ). In the probabilistic sensitivity analysis, the ICER for MIS-RDAVR remained below the $100,000 per life year gained threshold in 96.8% of the Monte Carlo simulations and below the $50,000 per life year gained threshold in 78.1% of these simulations.
Discussion
This study is, to our knowledge, the first that compares the economic value of the MIS-RDAVR to both MISAVR and CAVR procedures. The results suggest that the MIS-RDAVR is cost-effective. Moreover, it advances the care of AVR patients by facilitating the MIS approachCitation20. These findings suggest that the use of the EDWARDS INUITY Elite Valve System™ confers superior economic value compared to both MISAVR and CAVR.
The prevalence of AS in the general population increases with age, from 2.5% at 75 years to 8.1% at 85 yearsCitation3. With an aging population, the prevalence of aortic valve disease and valve replacement surgery are expected to continue this growth trend. Indeed, the AVR patient profile has become older and sicker, and this trend is only expected to growCitation17. Furthermore, for patients at high risk of surgical mortality, options become increasingly limited, and the potential benefit of AVR must outweigh both the surgical risk as well as risk associated with no treatment at all. For this patient population, a MISAVR treatment may be the only viable treatment optionCitation16. In light of these circumstances, and the clinical and cost benefits observed above, MIS-RDAVR offers a potentially attractive treatment option for these at-risk patient groups.
Limitations
This study has several important limitations that must be acknowledged. Because the TRANSFORM trial did not include control arms for MISAVR or CAVR, outcomes for these procedures were not available from the trial itself. Thus, comparative parameters had to be generated from the published literature and were representative of controlled trial and real-world experience. Therefore, it was difficult to account for patient selection bias and other differences in these studies. For instance, the use of mechanical valves in the comparator arm studies would have also necessitated the use of peri-operative anti-coagulation therapy which may subsequently have affected TIA and stroke incidence rates. While the downstream impact of this argument may have influenced the cost comparison either way, discerning the actual impact of these kinds of differences on the post-operative outcomes was outside the scope of the current study protocol. Nevertheless, the patient characteristics were similar, albeit slightly healthier in the comparator cohorts. This led to conservative economic evaluations of MIS-RDAVR. Being a relatively new approach, only one study was available for the treatment arm, while many were available for the comparator arms. Thus, the comparator arms reflect outcomes from a broader range of settings and with more experience on average.
While the most common adverse events associated with AVRs were considered in this analysis, there were some potentially relevant adverse events (e.g. pneumonia) that could not be included due to data limitations. Moreover, with the analysis limited to 30-day post-surgery (data being unavailable for a longer timeframe), the economic value of the EIE valve over a longer follow-up could not be ascertained. As such, the current study results should be viewed as a preliminary evaluation only. Future studies should be aimed at incorporating the durability and long-term impact of the EIE valve on both clinical and economic outcomes. Finally, the study did not assess the societal impact of MIS-RDAVR vs the currently available surgery techniques, owing to the paucity of data.
Despite these limitations, the results were quite robust in sensitivity analysis, and suggest that a MISAVR approach using an EIE valve system warrants further study as a potential improvement for surgical AVR. Additional studies and ‘real-world’ analyses will be useful in further understanding the behaviour and potential value of the EIE valve system in this approach.
Conclusion
The EDWARDS INTUITY Elite valve system™ deployed in a MIS approach appears to be a cost-effective technology compared to MISAVR and CAVR that potentially advances the care of AVR patients. Compared to CAVR, it may achieve cost savings as well. These results suggest that MIS-RDAVR confers superior economic value compared to both standard MISAVR and CAVR via lowered key complication rates (e.g. re-operation, renal complications, wound infection, TIA, endocarditis) and utilization (e.g. cross-clamp time, hospital stay).
Transparency
Declaration of funding
This study was funded by Edwards Lifesciences, Inc. The publication of study results was not contingent on the sponsor’s approval or censorship of the manuscript.
Declaration of financial/other relationships
MM is an employee of Edwards Lifesciences. WRC is a consultant to Edwards Lifesciences. GRB is a consultant for AtriCure, Inc, Edwards Lifesciences, and On-X Life Technologies. EAG is a consultant to and holds intellectual property with Edwards Lifesciences and Medtronic. CG and SRP are employees and JR is a consultant to CTI Clinical Trial and Consulting Services, Inc. which is a paid consultant to Edwards Lifesciences.
References
- Bach DS, Radeva JI, Birnbaum HG, et al. Prevalence, referral patterns, testing, and surgery in aortic valve disease: leaving women and elderly patients behind? J Heart Valve Dis 2007;16:362-9
- Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:447-54
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43
- Bouma BJ, van Den Brink RB, van Der Meulen JH, et al. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart 1999;82:143-8
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010;121:1502-9
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982;66:1105-10
- Varadarajan P, Kapoor N, Bansal RC, et al. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: results from a cohort of 277 patients aged > or =80 years. Eur J Cardiothorac Surg 2006;30:722-7
- Bach DS, Siao D, Girard SE, et al. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2009;2:533-9
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997;95:2395-400
- Filsoufi F, Rahmanian PB, Castillo JG, et al. Excellent early and late outcomes of aortic valve replacement in people aged 80 and older. J Am Geriatr Soc 2008;56:255-61
- Kvidal P, Bergstrom R, Horte LG, et al. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000;35:747-56
- Murphy ES, Lawson RM, Starr A, et al. Severe aortic stenosis in patients 60 years of age or older: left ventricular function and 10-year survival after valve replacement. Circulation 1981;64:II184-8
- Sundt TM, Bailey MS, Moon MR, et al. Quality of life after aortic valve replacement at the age of >80 years. Circulation 2000;102:III70-4
- Thourani VH, Myung R, Kilgo P, et al. Long-term outcomes after isolated aortic valve replacement in octogenarians: a modern perspective. Ann Thorac Surg 2008;86:1458-64; discussion 1464–55
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132
- Clark MA, Duhay FG, Thompson AK, et al. Clinical and economic outcomes after surgical aortic valve replacement in Medicare patients. Risk Manag Healthc Policy 2012;5:117-26
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-9
- Phan K, Xie A, Di Eusanio M, et al. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg 2014;98:1499-1511
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the surgical treatment of aortic stenosis with a next generation surgical aortic valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-15; discussion 115-16
- Borger MA, Dohmen PM, Knosalla C, et al. Haemodynamic benefits of rapid deployment aortic valve replacement via a minimally invasive approach: 1-year results of a prospective multicentre randomized controlled trialdagger. Eur J Cardiothorac Surg. 2016 Mar 2. pii: ezw042. [Epub ahead of print]
- Chitwood WR Jr, Barnhart GRR, Accola KD, et al. TRANSFORM US clinical trial: safety and performance of a rapid deployment aortic valve. Oral Presentation. American Association for Thoracic Surgery (AATS) Annual Meeting. Baltimore, MD. May 16, 2016
- Likosky DS, Sorensen MJ, Dacey LJ, et al. Long-term survival of the very elderly undergoing aortic valve surgery. Circulation 2009;120:S127-S33
- Diehr P, Yanez D, Ash A, et al. Methods for analysing health care utilization and costs. Annu Rev Public Health 1999;20:125-44
- Bakir I, Casselman FP, Wellens F, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg 2006;81:1599-604
- Tabata M, Aranki SF, Fox JA, et al. Minimally invasive aortic valve replacement in left ventricular dysfunction. Asian Cardiovasc Thorac Ann 2007;15:225-8
- Corbi P, Rahmati M, Donal E, et al. Prospective comparison of minimally invasive and standard techniques for aortic valve replacement: initial experience in the first hundred patients. J Card Surg 2003;18:133-9
- Furukawa N, Kuss O, Aboud A, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: matched propensity score analysis of 808 patients. Eur J Cardiothorac Surg 2014;46:221-6; discussion 226–7
- Klokocovnik T, Kersnik Levart T, Bunc M. Double venous drainage through the superior vena cava in minimally invasive aortic valve replacement: a retrospective study. Croat Med J 2012;53:11-16
- Lamelas J, Sarria A, Santana O, et al. Outcomes of minimally invasive valve surgery versus median sternotomy in patients age 75 years or greater. Ann Thorac Surg 2011;91:79-84
- Murzi M, Cerillo AG, Bevilacqua S, et al. Traversing the learning curve in minimally invasive heart valve surgery: a cumulative analysis of an individual surgeon's experience with a right minithoracotomy approach for aortic valve replacement. Eur J Cardiothorac Surg 2012;41:1242-6
- Neely RC, Boskovski MT, Gosev I, et al. Minimally invasive aortic valve replacement versus aortic valve replacement through full sternotomy: the Brigham and Women’s Hospital experience. Ann Cardiothorac Surg 2015;4:38-48
- Ruttmann E, Gilhofer TS, Ulmer H, et al. Propensity score-matched analysis of aortic valve replacement by mini-thoracotomy. J Heart Valve Dis 2010;19:606-14
- Sharony R, Grossi EA, Saunders PC, et al. Propensity score analysis of a six-year experience with minimally invasive isolated aortic valve replacement. J Heart Valve Dis 2004;13:887-93
- Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17-25
- Bowdish ME, Hui DS, Cleveland JD, et al. A comparison of aortic valve replacement via an anterior right minithoracotomy with standard sternotomy: a propensity score analysis of 492 patients. Eur J Cardiothorac Surg 2016;49:456-63
- Ghanta RK, Lapar DJ, Kern JA, et al. Minimally invasive aortic valve replacement provides equivalent outcomes at reduced cost compared with conventional aortic valve replacement: a real-world multi-institutional analysis. J Thorac Cardiovasc Surg 2015;149:1060-5
- Gilmanov D, Bevilacqua S, Murzi M, et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Ann Thorac Surg 2013;96:837-43
- Glauber M, Miceli A, Gilmanov D, et al. Right anterior minithoracotomy versus conventional aortic valve replacement: a propensity score matched study. J Thorac Cardiovasc Surg 2013;145:1222-6
- Glower DD, Desai BS, Hughes GC, et al. Aortic valve replacement via right minithoracotomy versus median sternotomy: a propensity score analysis. Innovations (Phila) 2014;9:75-81; discussion 81
- Mahesh B, Navaratnarajah M, Mensah K, et al. Mini-sternotomy aortic valve replacement: is it safe and effective? Comparison with standard techniques. J Heart Valve Dis 2011;20:650-6
- Sansone F, Punta G, Parisi F, et al. Right minithoracotomy versus full sternotomy for the aortic valve replacement: preliminary results. Heart Lung Circ 2012;21:169-73
- Stamou SC, Kapetanakis EI, Lowery R, et al. Allogeneic blood transfusion requirements after minimally invasive versus conventional aortic valve replacement: a risk-adjusted analysis. Ann Thorac Surg 2003;76:1101-6
- Vanoverbeke H, Van Belleghem Y, Francois K, et al. Operative outcome of minimal access aortic valve replacement versus standard procedure. Acta Chir Belg 2004;104:440-4
- Korach A, Shemin RJ, Hunter CT, et al. Minimally invasive versus conventional aortic valve replacement: a 10-year experience. J Cardiovasc Surg (Torino) 2010;51:417-21
- Calderon J, Richebe P, Guibaud JP, et al. Prospective randomized study of early pulmonary evaluation of patients scheduled for aortic valve surgery performed by ministernotomy or total median sternotomy. J Cardiothorac Vasc Anesth 2009;23:795-801
- De Smet JM, Rondelet B, Jansens JL, et al. Assessment based on EuroSCORE of ministernotomy for aortic valve replacement. Asian Cardiovasc Thorac Ann 2004;12:53-7
- Farhat F, Lu Z, Lefevre M, et al. Prospective comparison between total sternotomy and ministernotomy for aortic valve replacement. J Card Surg 2003;18:396-401; discussion 402–3
- Moustafa MA, Abdelsamad AA, Zakaria G, et al. Minimal vs median sternotomy for aortic valve replacement. Asian Cardiovasc Thorac Ann 2007;15:472-5
- Ahangar AG, Charag AH, Wani ML, et al. comparing aortic valve replacement through right anterolateral thoracotomy with median sternotomy. Int Cardiovasc Res J 2013;7:90-4
- Suenaga E, Suda H, Katayama Y, et al. Comparison of limited and full sternotomy in aortic valve replacement. Jpn J Thorac Cardiovasc Surg 2004;52:286-91
- Dogan S, Dzemali O, Wimmer-Greinecker G, et al. Minimally invasive versus conventional aortic valve replacement: a prospective randomized trial. J Heart Valve Dis 2003;12:76-80
- Foghsgaard S, Schmidt TA, Kjaergard HK. Minimally invasive aortic valve replacement: late conversion to full sternotomy doubles operative time. Tex Heart Inst J. 2009;36:293-297
- Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg. 2012;93:1483-1488
- Candaele S, Herijgers P, Demeyere R, et al. Chest pain after partial upper versus complete sternotomy for aortic valve surgery. Acta Cardiol. 2003;58:17-21
