Abstract
Objective: To compare treatment patterns and economic outcomes of dasatinib and nilotinib as 1st-line therapies for chronic myeloid leukemia (CML).
Methods: Adult CML patients initiated on first-line dasatinib or nilotinib in 2010–2014 were identified from two large US administrative claims databases. Treatment patterns, tyrosine kinase inhibitor (TKI) adherence and healthcare resource utilization (HRU) and costs were measured from the 1st-line TKI initiation (index date) to the end of follow-up.
Results: A total of 604 and 418 patients were included in the dasatinib and nilotinib cohorts (mean ages = 50.9 and 52.5 years, 46.4% and 45.7% female), respectively. Among the dasatinib patients, 91% started with 100 mg/day, 3% with <100 mg/day, and 6% with >100 mg/day. Among the nilotinib patients, 76% started with 600 mg/day, 16% with >600 mg/day, and 8% <600 mg/day. The dasatinib cohort had a higher hazard of dose decrease (hazard ratio [HR] = 1.66; p = .002) and of switching to another TKI (HR =1.62; p = .019) compared to the nilotinib cohort. The hazard of dose increase (HR =0.76; p = .423) and treatment discontinuation (HR =1.10; p = .372) were not significantly different between cohorts. There was also no significant difference in TKI adherence levels (mean proportion of days covered [PDC] difference over first 6 months = −0.0003, p = .981; mean PDC difference over first 12 months = −0.0022, p = .880) and HRU (inpatient day incidence rate ratio [IRR] = 1.03, p = .930; emergency room IRR =1.26, p = .197; and days with outpatient services IRR = 1.01, p = .842). The dasatinib cohort incurred higher healthcare costs by $749 per patient per month (p = .044) compared to the nilotinib cohort.
Limitation: Information on CML phase and Sokal score was not available.
Conclusions: Dasatinib was associated with an increased hazard of dose decrease and switching to another TKI and higher healthcare costs, vs nilotinib.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative neoplasm of the hematopoietic stem cells and a form of leukemia, representing ∼15% of all adult leukemia cases diagnosed in the USCitation1. In 2013, there were ∼44,885 people in the US living with CML, and 8,220 new cases are estimated to occur in 2016Citation1. Approximately 95% of patients with CML display a reciprocal translocation between chromosomes 9–22, with the derivative of 22 known as the Philadelphia chromosome (Ph+)Citation2,Citation3. This translocation involves the ABL1 (v-abl Abelson murine leukemia viral oncogene homolog 1) and the BCR (breakpoint cluster region) genes in the formation of a chimeric transcript coding the constitutively active, oncogenic BCR-ABL1 tyrosine kinaseCitation4. Tyrosine kinase inhibitors (TKIs) have been the standard-of care for the past decade. In 2010, the US Food and Drug Administration (FDA) approved the second-generation TKIs nilotinib (Tasigna)1 and dasatinib (Sprycel)2 as alternative first-line therapies to imatinib (Gleevec)3, the first targeted therapy approved for CML, for newly-diagnosed patients with Ph + CML in chronic phase (CP)Citation5,Citation6.
NilotinibCitation7,Citation8 and dasatinibCitation9,Citation10 have both been demonstrated to be well tolerated and have superior efficacy compared to imatinib in Phase III clinical trialsCitation11,Citation12. However, to date, no head-to-head randomized clinical trials or real-world evidence studies have directly compared nilotinib or dasatinib as first-line therapy for the treatment of CML patients. A network meta-analysis, indirectly comparing rates of major molecular response (MMR) between nilotinib and dasatinib in newly diagnosed CML-CP patients from available clinical trials, concluded that nilotinib had a higher rate of MMR during the first year of treatment compared to dasatinibCitation13. In addition, a mixed treatment comparison of those same trials found that nilotinib had a higher rate of deeper molecular response at 60 months compared to dasatinib, and was found to have a better survival profileCitation12. According to the wholesale acquisition cost (WAC), the daily cost at the recommended dose of the two treatments as first-line for CML-CPCitation14,Citation15 slightly differs: nilotinib costs $376.58 at 600 mg/day, and dasatinib costs $362.15 at 100 mg/dayCitation16. However, given that the cost of treatment depends on adherence to TKI therapy, the prescribed dose, and dose changes, it is unclear how the cost of therapy of nilotinib and dasatinib differs in real-world practice. Furthermore, to compare economic outcomes between nilotinib and dasatinib patients, medical service costs and other pharmacy costs should also be considered. To the best of our knowledge, no studies have compared the economic outcomes between nilotinib and dasatinib patients in first-line therapy for CML. Thus, this study aimed to address this knowledge gap by comparing treatment patterns, adherence to TKI therapy, healthcare resource utilization (HRU), and healthcare costs (from the payers’ perspective) of newly diagnosed CML patients using the second generation TKIs dasatinib or nilotinib as first-line therapy in a US real-world setting.
Methods
Data source
Data spanning from 2002 to the end of 2014 from two large US administrative claims databases were used in this study. Data related to prior diagnoses or treatments pertinent to the sample selection criteria were extracted from 2002–2014, while the data used to measure patient characteristics and outcomes included years 2010–2014. These two databases include detailed information on pharmacy and medical claims from more than 200 health plans and represent, in most recent years, over 115 million annually covered lives from all US census regions, with higher prevalence in the South and North Central (Midwest) regions. Data elements in these databases include detailed information on health plan enrollment, demographics, diagnoses, and procedures for medical care received in inpatient (IP) or outpatient (OP) settings, and pharmacy services for covered employees and their dependents. Data were de-identified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act.
Study design and sample selection
This study used a retrospective cross-sectional cohort design (). The index date was defined as the first TKI prescription fill date (dasatinib or nilotinib), and the first TKI was defined as the index TKI. The baseline period was defined as the 6 months before the index date, and the study period extended from the index date to the end of continuous health plan coverage or data availability, whichever occurred first.
Figure 1. Study design. CML, chronic myeloid leukemia; TKI, tyrosine-kinase inhibitor; HSCT, hematopoietic stem cell transplant; ESRD, end stage renal disease; Tx, treatment; Q1, first quarter; Q4, fourth quarter.
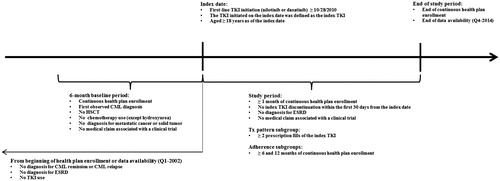
Adult patients (≥18 years old at the index date) newly-diagnosed with CML (i.e. diagnosed with International Statistical Classification of Diseases, Ninth Revision [ICD-9] diagnosis codes 205.1x within the 6 months before the index date) who were initiated on dasatinib or nilotinib as first-line therapy on or after October 28, 2010 (i.e. the date after which both treatment options were approved by the FDA for the treatment of newly-diagnosed adult patients with Ph + CML-CP) were included. Patients were also required to have continuous health plan coverage for ≥6 months prior to and ≥1 month after the index date.
Patients were excluded if they had a diagnosis for end stage renal disease (ESRD), CML remission, or CML relapse from the beginning of health plan enrollment or data availability to the index date; had a procedure for hematopoietic stem cell transplant (HSCT), chemotherapy treatment (except for hydroxyurea), or a diagnosis for metastatic cancer or solid tumor during the 6-month baseline period or at the index date; had a medical claim associated with a clinical trial during the baseline or study periods; or did not remain on the first-line therapy for at least ≥1 month following treatment initiation.
Patients meeting all sample selection criteria were classified into two mutually exclusive cohorts based on their index TKI: dasatinib cohort or nilotinib cohort. From these two cohorts, a sub-group of patients with ≥2 prescription fills of the index TKI was selected for the treatment patterns analyses, and two additional sub-groups, those with ≥6 months and ≥12 months of continuous health plan coverage after the index date, were selected for the adherence to TKI analyses.
Outcomes
A dose decrease or increase of the index TKI was defined as a change of the daily dose, compared to the initial daily dose, by ≥20 mg for dasatinib and ≥100 mg for nilotinib. In addition, dose decreases preceded by diagnoses that could indicate an adverse event (AE) were identified. AEs were defined as medical claims with a diagnosis for one of the AEs reported on the FDA product label of dasatinib and/or nilotinib (i.e. fluid retention, cytopenia, and non-hematologic AEs such as arthralgia, dyspnea, fatigue, and QT cycle [QTc] prolongation)Citation14,Citation15 within the 30 days before the dose decrease. Time from the index date to the first dose decrease and to the first dose increase was analyzed in two distinct models. Patients’ observation periods were censored at treatment discontinuation (defined below) or at the end of the study period, whichever occurred first, if patients did not have a dose decrease/increase by that date.
A treatment discontinuation was defined as a gap in treatment of ≥60 consecutive days between the end of supply of the index TKI and the next prescription of the index TKI or the end of the study period. Time-to-discontinuation was measured from the index date to the first treatment discontinuation, and observation periods were censored if patients did not discontinue the index TKI by the end of the study period.
A treatment switch was defined as the initiation of a TKI (i.e. nilotinib, dasatinib, imatinib, bosutinib, or ponatinib) other than the patient’s index TKI. Time-to-switch was measured from the index date to the date of the first switch, and observation periods were censored if patients did not switch by the end of the study period.
For the analysis of adherence to TKI therapy, the proportion of days covered (PDC) was measured during the 6- and 12-month periods following the index date among sub-groups of patients with continuous health plan coverage during these periods, respectively. PDC was measured as the ratio of days in possession of the index TKI during the 6- or 12-month period divided by the number of calendar days included in these periods (180 or 365 days, respectively)Citation17.
All-cause HRU and healthcare costs were measured during the study period. The following HRU categories were reported: IP days, emergency room (ER) visits, days with OP services, and days with other medical services (e.g. independent laboratory, durable medical equipment, or other ancillary services, including home health services and skilled nursing facility services). Healthcare costs were measured from the payers’ perspective (commercial plan and co-ordination of benefits [COB]) and adjusted for inflation to 2014 US dollars using the Consumer Price Index (CPI) Medical Care componentCitation18. The following healthcare cost components were reported: medical services costs (IP, ER, OP, and other medical services), pharmacy costs (TKI-related and other pharmacy costs), and total costs (medical service + pharmacy costs). Due to the varying length of the study period per patient, HRU and healthcare costs were reported on a monthly basis (per patient per month [PPPM], i.e. a patient’s total cost was divided by the number of days during the study period and multiplied by 30 days).
Statistical analyses
Patient characteristics measured at the index date or during the baseline period were compared between the dasatinib and nilotinib cohorts using Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables. The proportion of patients with a dose decrease preceded by an AE was compared between cohorts using Fisher’s exact tests.
Kaplan–Meier (KM) analyses were used to describe rates of dose decrease, dose increase, treatment switch, and treatment discontinuation over time in each cohort. Multivariate Cox proportional-hazards regression models (Cox) were used to compare the hazard of these events between cohorts; adjusted hazard ratios (HRs) with p-values and 95% confidence intervals (CIs) were reported. The proportionality of hazard was tested using the Bagdonavicius and NikulinCitation19 proportionality of hazard test, and the goodness of fit was assessed using the Gronnesby and BorganCitation20 test.
Levels of adherence to TKI were compared between cohorts using multivariate ordinary least-squares regression models (OLS); results were reported as adjusted mean differences with p-values and 95% CIs. OLS assumptions for unbiased and robust estimates were metCitation21.
HRU was compared between cohorts using multivariate Poisson regression models; results were reported as adjusted incidence rate ratios (IRRs). Because of over-dispersion identified using the Pearson testCitation22, p-values with 95% CIs were estimated using a non-parametric bootstrap re-sampling technique with 499 iterations.
Healthcare costs were compared between cohorts using multivariate two-part regression models where the first part was a logistic regression and the second part was a generalized linear model (GLM) with a gamma distribution and a log link; results were reported as adjusted cost differences. The goodness of fit of the model for the total healthcare costs was assessed using the modified Hosmer and LemeshowCitation23 test. p-values with 95% CIs were estimated using a non-parametric bootstrap resampling technique with 499 iterations.
All multivariate regression models (Cox, OLS, and GLM) were adjusted for the following potential pre-selected confounding factors: age as of the index date, sex, initial daily dose of the index TKI, type of health plan, and the Darkow disease complexity indexCitation24, and Charlson-Quan comorbidity indexCitation25 measured during the baseline period.
Data analyses were performed using SAS® version 9.3 (SAS Institute, Inc., Cary, NC).
Results
A total of 1,022 newly-diagnosed adult patients with CML met the sample selection criteria (Supplemental Figure A). Of these, 604 patients received dasatinib and 418 received nilotinib as first-line TKI.
Patient characteristics are described in . The mean age was 50.9 (standard deviation [SD] = 13.5) years for dasatinib patients and 52.5 (SD = 12.9) years for nilotinib patients (p = .114), and the proportion that was female was 46.4% for dasatinib patients and 45.7% for nilotinib patients (p = .834). The proportion of patients who were initiated on the recommended dose for CML-CPCitation14,Citation15 was 91.2% of the dasatinib patients (100 mg/day) and 76.1% of the nilotinib patients (600 mg/day; p < .001). The proportion of patients who were initiated on a higher than recommended dose for CML-CP was 6.0% for the dasatinib patients (i.e. >100 mg/day) and 15.8% for the nilotinib patients (i.e. >600 mg/day; p < .001). During the study period, the average dose was 102 mg/day of dasatinib and 599 mg/day of nilotinib. The mean CCI scores and Darkow disease complexity index were not significantly different between the two cohorts (all p > .05), although a higher proportion of nilotinib patients had a CCI score >3 (dasatinib = 5.5%, nilotinib = 8.9%; p = .035). The most prevalent comorbidities (>10%) measured during the baseline period among both cohorts were hypertension, anemia, hyperlipidemia, diabetes, cardiovascular diseases, and fluid electrolyte disorders (all p > .05). The average study period length was 17.2 (SD = 11.8) months for dasatinib patients and 17.9 (SD =12.2) months for nilotinib patients (p = .380).
Table 1. Patient characteristics of the dasatinib and nilotinib cohorts.
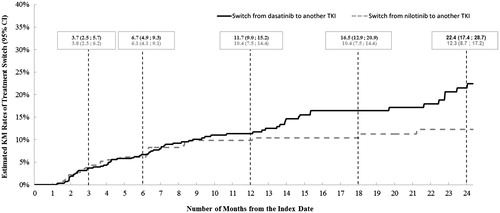
A total of 598 (99.0%) dasatinib patients and 412 (98.6%) nilotinib patients with ≥2 prescription fills of the index TKI were selected for the treatment pattern analyses. The Kaplan Meier rates of dose decrease by 12 months following the index date were 18.4% (95% CI = 15.2–22.2) for dasatinib patients and 16.0% (12.4–20.4) for nilotinib patients (). After adjusting for potential confounding factors, the hazard of dose decrease in dasatinib patients was 66% higher than that of nilotinib patients (HR [95% CI] = 1.66 [1.20–2.29], p = .002) (). Most (88.4%) of the dose decreases observed in dasatinib patients were from the recommended dose for CML-CP (100 mg/day)Citation15 to a dose ≤80 mg/day. Most (55.0%) of the dose decreases observed in nilotinib patients were from the recommended dose for CML-CP (600 mg/day) to a dose ≤450 mg/day, and 35.0% of the observed dose decreases in nilotinib patients were from a dose higher than the recommended dose (800 mg/day)Citation14 to the recommended dose of 600 mg/day. Among patients with a dose decrease, 60.7% (68 of 112 patients) of dasatinib patients and 45.0% (27 of 60) of nilotinib patients had a diagnosis potentially indicating an AE within the 30 days preceding the dose decrease. Furthermore, a higher proportion of the dasatinib patients had a dose decrease that was preceded by a diagnosis potentially indicating an AE compared to the nilotinib patients (11.4% [68 of 598] vs 6.8% [28 of 412], respectively; p = .016) (Supplemental Table 1). There were no statistically significant differences between the dasatinib and nilotinib cohorts in terms of dose increase (HR [95% CI] = 0.76 [0.39–1.48]; p = .423) (). The Kaplan-Meier rates of treatment switching by 12 months following the index date were 11.7% (95% CI =9.0–15.2) for the dasatinib patients and 10.4% (7.5–14.4) for the nilotinib patients (). After adjusting for potential confounding factors, dasatinib patients were 62% more likely to switch to another TKI compared to nilotinib patients (adjusted HR [95% CI] = 1.62 [1.08–2.42], p = .019) ().
Figure 2. Estimated rates of dose decrease for the dasatinib and nilotinib cohorts. KM, Kaplan–Meier; CI, confidence interval.
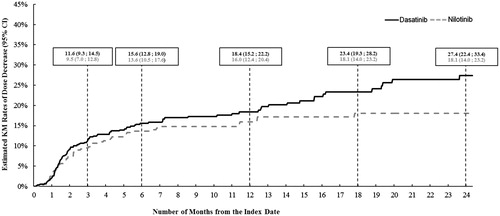
Figure 3. Adjusted comparison of treatment patterns and HRU between the dasatinib and nilotinib cohorts HR, hazard ratio; CI, confidence interval; HRU, health resource utilization; IRR, incidence rate ratio; IP, inpatient; ER, emergency room; OP, outpatient. * Significant at the 5% level.
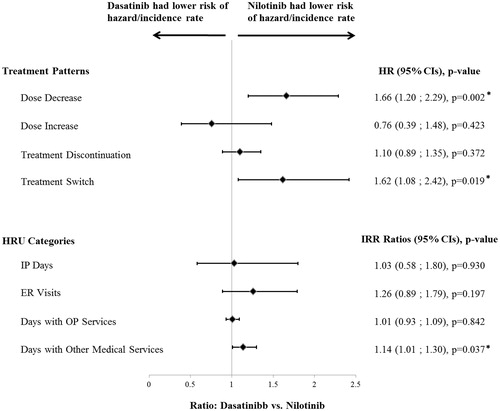
Figure 4. Rates of treatment switch for the dasatinib and nilotinib cohorts. KM, Kaplan-Meier; CI, confidence interval.
A total of 481 (79.6%) dasatinib patients and 333 (79.7%) nilotinib patients with ≥6 months of continuous health plan coverage, and 343 (56.8%) dasatinib patients and 249 (59.6%) nilotinib patients with ≥12 months of continuous health plan coverage were selected for the adherence to TKI analyses. Adherence levels were not statistically significantly different between the dasatinib and nilotinib cohorts during either the 6- or 12-month periods (results not shown). The mean PDC during the 6-month period was 0.8658 and 0.8613 among the dasatinib and nilotinib patients, respectively (adjusted difference [95% CI] = −0.0003 [−0.0233–0.0228]; p = .981), and the average PDC during the 12-month period was 0.7841 and 0.7826, respectively (adjusted difference [95% CI] = −0.0022 [−0.0302, 0.0259]; p = .880).
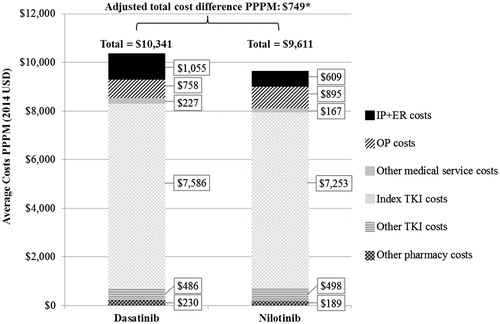
HRU was similar between dasatinib and nilotinib cohorts except for other medical services (), where dasatinib patients had 14% more days with other medical services compared to nilotinib patients (adjusted IRR [95% CI] = 1.14 [1.01–1.30], p = .037). After adjusting for potential confounding factors, dasatinib patients had higher total healthcare costs by $749 (95% CI = 11–1,497; p = .044) PPPM compared to nilotinib patients (). When healthcare costs were analyzed by cost component, each component, except for days with OP services ($758 for dasatinib and $895 for nilotinib, PPPM) and other TKI costs ($486 and $498 PPPM, respectively), was numerically higher among the dasatinib patients, resulting in significantly higher total healthcare costs among dasatinib patients compared to nilotinib patients. The main drivers of this total healthcare cost difference were the IP combined with ER costs ($1,055 for dasatinib and $609 for nilotinib, PPPM) and index TKI costs ($7,586 and $7,253 PPPM, respectively). However, the cost difference by cost component did not statistically significantly differ between the two cohorts.
Discussion
This study compared the treatment patterns, index TKI adherence, HRU, and healthcare costs among CML patients receiving first-line nilotinib or dasatinib in a real-world setting. The current study showed that patients receiving dasatinib were more likely to have a dose decrease and to switch TKI therapy, and these patients experienced higher overall healthcare costs in comparison with patients receiving nilotinib, mainly driven by higher IP with ER and index TKI costs. No significant differences were observed in terms of rates of dose increase, treatment discontinuation, adherence to TKI therapy, or other categories of HRU (except for other medical services). The direct comparison of these two first-line treatments for CML provide valuable real-world evidence on treatment patterns and health economic outcomes that may be of great interest to patients, physicians, and payers, and allow for a better understanding of the impact of the treatment choice of first-line therapy for CML.
To the best of our knowledge, this is the first study to directly compare outcomes of patients with CML receiving the second-generation TKIs nilotinib and dasatinib as first-line therapy. A few studies have used indirect comparison methods to compare efficacy and costs-per-responder of these treatments for Ph + CML-CP using clinical trial data. A first study by Signorovitch et al.Citation13 in 2014 indirectly compared rates of MMR (BCR-ABL1 fusion protein ≤0.1% on the International Scale) during the first year of treatment with nilotinib or dasatinib using data from their respective clinical trials for newly-diagnosed CML-CP patients. Following the network meta-analysis of MMR achievement by and at 12 months, the authors found that nilotinib had the highest rates of MMR, with a 97% chance of being the most effective treatment during the first year of treatment compared to dasatinib, with absolute rates of response of 55.2% for nilotinib and 44.8% for dasatinib by month 12. Another study by Signorovitch et al.Citation26 in 2011 used a matching-adjusted approach to indirectly compare efficacy between nilotinib and dasatinib using data from the ENESTndCitation7,Citation8 and DASISIONCitation9,Citation10 clinical trials for Ph + CML-CP patients. They found that, after matching, nilotinib patients experienced significantly higher rates of MMR and overall survival, and numerically higher rates of progression-free survival, compared to dasatinib patients. Additionally, a study by Guerin et al.Citation27 in 2014 estimated the 12-month costs per responder for Ph + CML-CP patients treated with first-line nilotinib vs dasatinib and found significantly higher costs for dasatinib patients by $46,079 (USD 2014), and this cost difference increased with the achievement of deeper molecular response. The Guerin et al. study noted that a substantial portion of these costs were attributable to AEs, including neutropenia, thrombocytopenia, and anemia, which were 53% lower among nilotinib patients compared to dasatinib patients. However, none of these studies were based on real-world data, and none of them analyzed treatment patterns, HRU, or healthcare costs.
In the present study, a greater proportion of dasatinib patients started on the daily recommend dose (100 mg/day)Citation15 compared to nilotinib (600 mg/day)Citation14 patients. Specifically, 2.8% of dasatinib patients and 8.2% of nilotinib patients started on a lower dose, and 6.0% and 15.8%, respectively, started on a higher dose. Despite these deviations from the recommended starting dose, patients receiving nilotinib were significantly less likely to experience a decrease in dose or to switch treatments, and had lower total healthcare costs. Additionally, in comparison with nilotinib, a much higher proportion of patients using dasatinib had a diagnosis potentially indicating an AE within the month preceding a dose decrease (11.4% for dasatinib vs 6.6% for nilotinib; p = .011) or had a treatment switch (adjusted HR [95% CI] = 1.62 [1.08–2.42], p = .019), suggesting that, in clinical settings, nilotinib may be better tolerated compared to dasatinib. However, it is unclear why physicians prescribed the TKI therapy at an initial dose that was different from the recommended dose. One hypothesis is that physicians may prescribe a higher dose for patients who have a high-risk profile (e.g. based on Sokal scoreCitation28). Further studies are warranted to explore reasons why physicians prescribe a dose that is different from the recommended one in the product label.
The findings presented in this study should be interpreted in the context of the source of the data and its corresponding measurement limitations, and the sample selection criteria. Thus, the generalizability of the findings to the overall population of CML patients may be limited to a commercially insured population newly diagnosed with CML. This study is subject to the common limitations of retrospective claims database analyses. Claims databases do not contain clinical measures of disease severity, such as Sokal et al.Citation28 or Hasford et al.Citation29 scores, phase of CML (CP, accelerated phase [AP], or blast crisis), reasons for medical decisions or treatment dosage or changes, or laboratory test results. Instead, the Darkow et al.Citation24 complexity index was used as an indicator of disease severity. Therefore, it was not possible to confirm that all selected patients were initiated on first-line therapy while in CP; the impact of the potential inclusion of newly-diagnosed CML patients in AP or blast crisis is unknown. Additionally, claims databases may contain inaccuracies or omissions in coded procedures, diagnoses, or pharmacy claims. However, such inaccuracies are expected to affect both cohorts equally. Finally, this study reports associations between dasatinib vs nilotinib as first-line therapy and HRU and healthcare costs among CML patients, but does not imply causation.
Conclusion
This study showed that in a large sample of CML patients commercially insured in the US and receiving a second-generation TKI as first-line therapy, compared to nilotinib, dasatinib was associated with an increased risk of dose decrease, despite the majority of dasatinib patients starting on the recommended dose, as well as an increased risk of switching to another TKI. In addition, dasatinib patients had significantly higher total healthcare costs compared to nilotinib patients. No differences in treatment adherence, discontinuation, or HRU were found.
Transparency
Declaration of funding
Funding for this research was provided by Novartis.
Declaration of financial/other relationships
LC and GJ are employees of Novartis and own stock/stock options. AG, DLV, and PG are employees of Analysis Group, Inc., which has received consultancy fees from Novartis. RN was an employee of Analysis Group, Inc., which has received consultancy fees from Novartis. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
A synopsis of the current research was presented in poster format at the Academy of Managed Care Pharmacy (AMCP) Managed Care and Specialty Pharmacy annual meeting in San Francisco, CA, during April 19–22, 2016, and at the 57th ASH Annual Meeting and Exposition, in Orlando, FL, during December 5–8, 2015.
Acknowledgments
Medical writing assistance was provided by Shelley Batts, PhD, an employee of Analysis Group, Inc., which has received funding from Novartis. Benjamin G. Cohen, MPH, who was an employee of Novartis, assisted authors in reviewing the manuscript for the accuracy and completeness of reported data.
Notes
Notes
1. Tasigna is a registered trademark of Novartis Pharmaceuticals East Hanover, NJ.
2. Sprycel is a registered trademark of Bristol Myers Squibb New York, NY.
3. Gleevec is a registered trademark of Novartis Pharmaceuticals.
References
- NIH National Cancer Institute. Surveillance, Epidemiology, and End Results Program (SEER) stat fact sheets: Chronic Myeloid Leukemia (CML) [Internet]. 2016. http://seer.cancer.gov/statfacts/html/cmyl.html. Accessed June 6, 2016
- Kurzrock R, Kantarjian HM, Druker BJ, et al. Philadelphia chromosome-positive leukemias: from basic mechanisms to molecular therapeutics. Ann Intern Med 2003;138:819-30
- Goldman JM, Melo JV. Chronic myeloid leukemia—advances in biology and new approaches to treatment. N Engl J Med 2003;349:1451-64
- Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 2009;113:1619-30
- Mace ML, Dahl J, Jabbour EJ. Which tyrosine-kinase inhibitor to use first in chronic phase chronic myelogenous leukemia? Expert Opin Pharmacother 2015;16:999-1007
- Jabbour E, Kantarjian H, Cortes J. Use of second- and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leuk 2015;15:323-34
- Giles FJ, Rosti G, Beris P, et al. Nilotinib is superior to imatinib as first-line therapy of chronic myeloid leukemia: the ENESTnd study. Expert Rev Hematol 2010;3:665-73
- Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010;362:2251-2259
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010;362:2260-70
- Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol 2010;28:398-404
- Wei G, Rafiyath S, Liu D. First-line treatment for chronic myeloid leukemia: dasatinib, nilotinib, or imatinib. J Hematol Oncol 2010;3:47
- Firwana B, Sonbol MB, Diab M, et al. Tyrosine kinase inhibitors as a first-line treatment in patients with newly diagnosed chronic myeloid leukemia in chronic phase: A mixed-treatment comparison. Int J Cancer 2016;138:1545-53
- Signorovitch J, Ayyagari R, Reichmann WM, et al. Major molecular response during the first year of dasatinib, imatinib or nilotinib treatment for newly diagnosed chronic myeloid leukemia: a network meta-analysis. Cancer Treat Rev 2014;40:285-92
- Nilotinib Product Label. United States Food and Drug Administration (FDA). 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022068s004s005lbl.pdf. Accessed November 2, 2015
- Dasatinib Product Label. United States Food and Drug Administration (FDA). 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021986s7s8lbl.pdf. Accessed November 2, 2015
- Red Book - Wholesale Aquisition Costs (WAC) [Internet]. Truven Health Analytics Micromedex Solutions. 2016. http://micromedex.com/products/product-suites/clinical-knowledge/redbook. Accessed June 7, 2016
- Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007;10:3-12
- Consumer Price Index (CPI). United States Department of Labor, Bureau of Labor Statistics. 2016. http://www.bls.gov/cpi/. Accessed June 15, 2016
- Bagdonavicius V, Nikulin M. On goodness‐of‐fit for homogeneity and proportional hazard. Appl Stochastic Models Busi Ind 2006;22:607-19
- Gronnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal 1996;2:315-28
- Stock JH, Watson MW. Introduction to econometrics. 2nd ed. Boston: Pearson/Addison Wesley; 2007. xlii, 796 p.
- Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. In: York SN, editor. Breakthroughs in Statistics; New York, NY: Springer, 1992. p 11-28
- Hosmer DW, Lemesbow S. Goodness of fit tests for the multiple logistic regression model. Commun Stat Theory Meth 1980;9:1043-69
- Darkow T, Kadlubek PJ, Shah H, et al. A retrospective analysis of disability and its related costs among employees with chronic obstructive pulmonary disease. J Occup Environ Med 2007;49:22-30
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83
- Signorovitch JE, Wu EQ, Betts KA, et al. Comparative efficacy of nilotinib and dasatinib in newly diagnosed chronic myeloid leukemia: a matching-adjusted indirect comparison of randomized trials. Curr Med Res Opin 2011;27:1263-71
- Guerin A, Revol C, Ramanakumar AV, et al. Twelve-month cost per responder (CPR) analysis of nilotinib and dasatinib as front-line therapy in patients with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP). 56th ASH Annual Meeting and Exposition. December 6, 2014. San Francisco, CA. https://ash.confex.com/ash/2014/webprogram/Paper69591.html. Accessed April 1, 2016
- Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in ‘good-risk’ chronic granulocytic leukemia. Blood 1984;63:789-99
- Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst 1998;90:850-8