Abstract
Background: Mitral regurgitation (MR) is a common valvular heart disorder requiring intervention once it becomes severe. Transcatheter mitral repair with the MitraClip device is a safe and effective therapy for selected patients denied surgery. The authors sought to evaluate the clinical outcomes and economic impact of this therapy compared to medical management in heart-failure patients with symptomatic mitral regurgitation.
Methods and results: The study was comprised of two phases; an observational study of patients with heart failure and mitral regurgitation treated with either medical therapy or the MitraClip, and an economic model. Results of the observational study were used to estimate parameters for the decision model, which estimated costs, and benefits in a hypothetical cohort of patients with heart failure and moderate-to-severe mitral regurgitation treated with either standard medical therapy or MitraClip. The cohort of patients treated with the MitraClip was propensity matched to a population of heart failure patients, and their outcomes compared. At a mean follow-up of 22 months, all-cause mortality was 21% in the MitraClip cohort and 42% in the medical management cohort (p = .007). The decision model demonstrated that MitraClip increased life expectancy from 1.87–3.60 years and quality-adjusted life years (QALY) from 1.13–2.76 years. The incremental cost was $52,500 Canadian dollars, corresponding to an incremental cost-effectiveness ratio (ICER) of $32,300.00 per QALY gained. Results were sensitive to the survival benefit.
Conclusion: In heart failure patients with symptomatic moderate–severe mitral regurgitation, therapy with the MitraClip is associated with superior survival and is cost-effective compared to medical therapy.
Introduction
Mitral regurgitation (MR) is one of the most common valvular heart disorders, with an estimated prevalence in the US of ∼1.7%, increasing with age to ∼9.3% in those >75 yearsCitation1. In heart failure (HF) patients, the presence of significant MR has been shown to independently predict mortality and hospitalizationsCitation2. HF is costly for the healthcare system, exceeding $40 billion dollars in 2012 in the USCitation3, therefore effective therapies may provide significant clinical and economic benefits.
Transcatheter mitral valve repair using the MitraClip (Abbott Vascular, Menlo Park, CA) has been commercially available in Europe since 2008 and in Canada since 2010. Such therapy involves the transcatheter placement of a metal clip on the leaflets of the mitral valve at the site of valvular regurgitation, thereby reducing MR and resulting in a double-orifice mitral valveCitation4. Evaluation of this technology in surgical candidates has established superior safety, albeit with less efficacy when compared with surgical repair or replacementCitation4.
Large-scale randomized controlled trials are currently underway in heart failure patients to evaluate the efficacy of MitraClip compared to medical therapy. We sought to compare a cohort of patients with heart failure and functional mitral regurgitation treated with a MitraClip to a cohort of medically managed patients at our institution. Given the economic burden of heart failure on the healthcare system, the lack of effective interventional options for many patients, and the substantial up-front costs of such technology, we sought to evaluate the cost-effectiveness of the MitraClip based on data from patients treated with this device at our institution.
Methods
The study was comprised of two phases: a comparison of propensity matched populations from an observational study of patients with heart failure and MR that were treated with either medical management or the MitraClip; and an economic model. Results of the observational study were used to estimate parameters for the economic model. The local Institutional Review Board and Ethics Committee approved the study.
Observational study
MitraClip cohort
This prospective cohort was comprised of patients treated with the MitraClip (Abbott Vascular, Menlo Park, CA) from 2010–2013. Indications for the procedure were determined by local institutional practice and following consultation with the treating physician, cardiologist, and cardiac surgeon. Eligible patients had symptomatic or asymptomatic moderate-to-severe (3+) or severe (4+) MR and were considered high risk for surgical intervention following multidisciplinary team discussion. Patients underwent trans-thoracic and trans-esophageal echocardiography to evaluate anatomical suitability. Exclusion criteria for the procedure included the following: mitral valve area <4 cm2 by planimetry, significant valvular or annular calcification, or visible thrombus in the left atrium. Procedures were performed as previously describedCitation4,Citation5, and all patients signed informed consent and were approved under the Health Canada Special Access program to undergo the intervention.
Medical management cohort
This retrospective comparator group consisted of medically managed patients with moderate-to-severe and severe (3–4+) MR followed at our Heart Failure Clinic from 2008–2010. Patients’ entry into the cohort was considered to be the date that significant MR was diagnosed by echocardiography.
Medical management patients were matched to those treated with the MitraClip using a propensity score. Briefly, a multivariate logistic regression model with presence of MitraClip as the dependent variable included the following independent variables: age, gender, left ventricular ejection fraction, history of ischemic heart disease, atrial fibrillation, hypertension, previous coronary artery bypass graft surgery, previous percutaneous coronary intervention, implantable cardioverter-defibrillator, cardiac resynchronization therapy, diabetes, and therapy with beta-blockers, ACE inhibitors, diuretics, angiotensin receptor blockers, and spironolactone. Propensity scores (predicted probability of having MitraClip) were obtained for each subject with MitraClip (MC) and medical therapy (MM). Absolute differences between propensity scores were computed for each pair of MC and MM subjects. Each MC subject was matched with the MM subject that yielded the smallest absolute difference in a 1:5 greedy matching scheme, matching was performed with replacement. Patients were not matched according to the baseline NYHA functional class.
The medical cohort was less symptomatic, with the majority of patients reporting NYHA class II symptoms, compared to the predominantly class IV symptoms in the MitraClip group. Given the significant differences in baseline functional class between the MitraClip and medical management cohorts, it was not possible to include NYHA class in the propensity matching.
Economic model
An economic model was developed in Excel (Microsoft Corporation, Redmond, WA) to estimate the costs, life-years, and quality-adjusted life years (QALY) for the studied patients. This data was then used to calculate the incremental cost per QALY gained and per life-year gained.
The model followed a hypothetical cohort of heart failure patients with significant MR in 1-month time increments from age 75 years until death or age 85. Patients were treated with either standard medical therapy (including cardiac resynchronization therapy as indicated) or the MitraClip device. We programmed the model inputs such that the estimates of survival, emergency room (ER) visits, hospitalizations, and rates of mitral valve surgery were the same as the results obtained in the observational study. Outcomes of interest were life expectancy (measured in years), QALYs, and costs (reported in 2013 Canadian dollars) and the incremental cost-effectiveness ratio. The model was analyzed from the perspective of the Canadian publicly funded healthcare system. All health outcomes and costs were discounted at 5% per year as per the recommendations of the Canadian Agency for Drugs and Technologies in HealthCitation6. For external model validation, we compared outcomes of the modeled cohort over time with outcomes in independent registriesCitation2,Citation7.
Model overview: data and assumptions
We constructed a decision model of symptomatic severe MR to simulate disease progression and added MitraClip as a treatment option (see ). In the first month of the model, at time zero, patients in the MitraClip group underwent the procedure and may have survived or died. Additionally in that first month they may have also experienced a complication related to the procedure, mitral valve surgery, re-intervention with the MitraClip, hospitalization for heart failure (CHF), and/or visits to the ER. In every subsequent monthly cycle, MitraClip patients may have died, undergone mitral valve surgery, had re-intervention, or have had ER visits or hospitalizations for heart failure. For the medical therapy group, in any 1-month cycle, patients may have undergone mitral valve surgery, been hospitalized for heart failure, visited the ER, or died.
We estimated mortality and peri-procedural complication rates after MitraClip using data from the observational study. The probability of death, hospitalization for heart failure, ER visits, and mitral valve surgery in the medical cohort were likewise obtained from the observational study. Mortality was extrapolated using parametric survival models for a time horizon of 10 years. An exponential, log-normal and Weibull extrapolation were performed, the Weibull was chosen as it was a better fit for the data according to the Akaike information criterion (AIC).
During each cycle of the model, cohort specific probabilities calculated from actual event rates, for heart failure hospitalizations and ER visits, were applied such that all patients alive remained at risk for these outcomes. The cohort-specific probability for mitral valve surgery was applied to both groups during the first 12 months only. Model parameters are detailed in Supplementary Appendix A. The time horizon for the model was 10 years. Model assumptions for the base case analysis included; baseline NYHA for the medical therapy cohort was equivalent to that of MitraClip patients prior to intervention, NYHA class was increased in both cohorts by one class every 2 years, and the probability of mitral valve surgery was applied only in the first year for both cohorts.
Costs
Detailed resource utilization and costs were collected for the MitraClip and medical therapy cohorts, as outlined in Supplementary Appendix B. Costs were calculated using the most important cost drivers from clinical data, including diagnostic evaluation costs directly incurred as a result of the MitraClip procedure, procedural costs, and inpatient treatment costs at a large tertiary care hospital in Montreal (Montreal Heart Institute). Follow-up costs included protocol driven visits and tests. Costs of hospitalizations and emergency room visits were obtained from the Montreal Heart Institute. Data on costs for the medical therapy cohort were obtained by reviewing outpatient hospital clinic visits, emergency room visits, and hospitalizations recorded in the Heart Failure Clinic Database. Costs are summarized in Supplementary Appendix B. Hospitalization costs at other centers were assumed to be equal to those incurred at our tertiary care center in 2013.
Utilities
Quality adjusted life years (life expectancy adjusted for quality-of-life of the health state experienced) were calculated for each patient in the alive state using published health utilities, which measure quality-of-life from a 0 (dead) to 1 (perfect health) scale, for heart failure according to NYHA ClassCitation8. We assumed that patients in the medical therapy cohort remained in NYHA Class III–IV for the duration of the model. The NYHA Class assigned to patients treated with MitraClip during the first year of follow-up was based on actual data. For projected time intervals beyond the clinical study data, an assumption was made that patients would deteriorate by one NYHA Class every 2 years.
Short-term utility decrements (i.e. disutility) for the MitraClip procedure were approximated using published decrements for percutaneous coronary interventionCitation9 and were applied in the first cycle of the model. A utility decrement for mitral valve surgery obtained from the literatureCitation10 was applied to both groups for the first year of the model only. Utility decrements were also applied for heart failure hospitalizationsCitation11 and emergency room visitsCitation12 according to the proportion of patients alive and at risk.
Analysis
We performed extensive deterministic sensitivity analyses to explore the impact of uncertainty in key parameters on the analysis results. A probabilistic sensitivity analysis to further characterize uncertainty in model parameters was performed using 10,000 simulations. A beta distribution was applied to all probabilities and utilities, gamma distributions to all costs, and a log normal distribution for all hazard ratios (see Supplementary Appendix B). Results are represented in the form of a scatter plot and cost-effectiveness acceptability curve (CEAC) representing the probability of the MitraClip being cost-effective over a range of different willingness-to-pay thresholds.
Results
Observational study of MitraClip
A total of 50 patients underwent the MitraClip procedure from December 2010 until March 2013 and their baseline characteristics are described in . The average age was 75.4 ± 9.1 years and 74% were male. The majority of patients (78%) had a previous history of ischemic heart disease, with 52% (n = 26) having had previous CABG and 40% (n = 20) previous coronary intervention (PCI). Atrial fibrillation was present in over half the cohort (n = 29) and device therapy was used in 54% (n = 27) of patients. Patients had symptomatic heart failure, 98% were NYHA class III or IV. Mitral regurgitation severity was assessed as 3+ or 4+ in all patients and the underlying etiology was functional in 90% of cases. A small sub-set of patients had high-risk degenerative mitral regurgitation (n = 5). The mean ejection fraction was 38.3 ± 15.8%.
Table 1. Baseline characteristics of MitraClip and medical management cohorts.
MitraClip procedure
The MitraClip procedure was performed under general anesthetic using trans-esophageal guidance as previously describedCitation4. MitraClip device placement was successful in 96% of patients (n = 48). Failure to place a device occurred in two patients in whom there was severe restriction of a shortened posterior leaflet that precluded grasping. Mitral regurgitation severity was reduced to ≤2+ in 94% (n = 47) of the initial cohort. Two clips were used in 71% (n = 34) of patients, with one clip in the remaining 29% (n = 14).
30-day major adverse events
Four patients (8%) died within 30 days of the MitraClip procedure. All were considered procedure-related, but none occurred intra-procedurally. The two patients with unsuccessful MitraClip procedures (i.e. no clip implanted) died due to progressive heart failure and low cardiac output. A third patient had single-leaflet device attachment 48 h post-procedure requiring surgical mitral valve replacement and subsequently died due to post-operative complications. The fourth patient died 48 h post-intervention due to an acute intra-cerebral hemorrhage on warfarin for chronic atrial fibrillation. An additional patient required surgical mitral valve replacement 2 days post-procedure due to persistent severe mitral regurgitation in the setting of a mitral valve cleft, with an uneventful recovery.
Medical management cohort
The medical management cohort was comprised of 42 patients that were matched to the MitraClip group on the basis of comorbidities and medical therapy. The baseline characteristics of the medical management cohort are described in . In comparison to the MitraClip cohort, the patients were younger, with a mean age of 68.2 ± 15.5 years. Comorbidities were similar; however, there were lower rates of ischemic heart disease (71%), CABG (48%), and previous PCI (33%). Patients were less symptomatic, with the majority of patients being NYHA Class II or III, but this parameter was not used in the matching process. Echocardiographic assessment demonstrated mitral regurgitation severity of 3+ or 4+ in all patients; however, the mean ejection fraction of 31.8 ± 13.6% was lower than that measured in the MitraClip cohort. Accordingly, there were higher rates of device therapy in this cohort, 59% with a pacemaker or implantable defibrillator.
Clinical follow-up
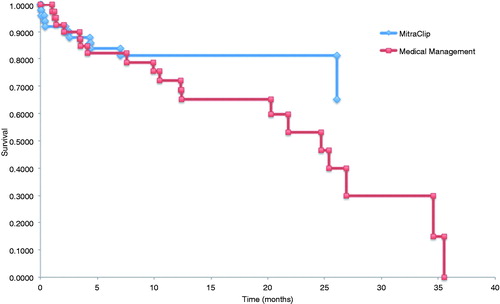
Clinical follow-up of patients in both cohorts at 12 months are shown in . All-cause mortality was 18% and 24% in the MitraClip and medical management cohorts, respectively. The number of hospitalizations for heart failure was 0.16 and 0.57 per patient in each group. Emergency room visits at 12 months were 0.08 and 0.60 per patient. Longer-term follow-up was available in the MitraClip and medical management cohorts at a mean of 22 ± 15 and 33 ± 21 months, respectively. At these time points, all-cause mortality was 21% in the MitraClip cohort and 42% in the medical management cohort (p = .007). Kaplan-Meier survival curves are plotted in , with Weibull extrapolations for a time horizon of 10 years overlaid in .
Figure 2. (A) Kaplan-Meier survival curves for MitraClip and medical management cohorts. (B) Kaplan-Meier survival curves with Weibull extrapolations for MitraClip and medical therapy cohorts.
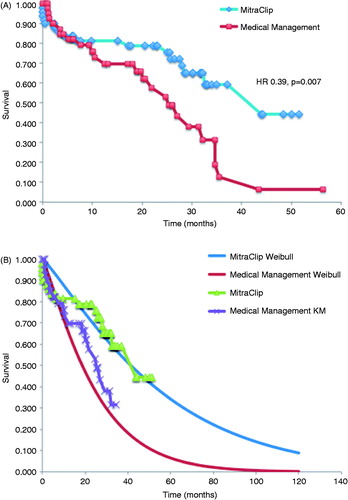
Table 2. Comparison of outcomes in MitraClip and medical therapy cohorts at 30 days and 1 year.
Cost-effectiveness of MitraClip vs medical therapy
Costs of the MitraClip device and procedure (∼$80,000 CDN) were partially offset ($30,000) by lower hospitalization, ER visits, and mitral valve surgical costs when compared to medical therapy.
summarizes the discounted results of the cost-effectiveness analysis. Under the above assumptions, the discounted cost of a MitraClip per patient was $88,200.00 compared to $35,600.00 for medical therapy over a 10-year time horizon. The discounted life years gained was 3.60 in the MitraClip cohort and 1.87 in the medical therapy cohort. Given an incremental difference in QALYs of 1.63 this results in an ICER of $32,300.00 per QALY gained.
Table 3. Economic outcomes for MitraClip and medical therapy.
Sensitivity analyses
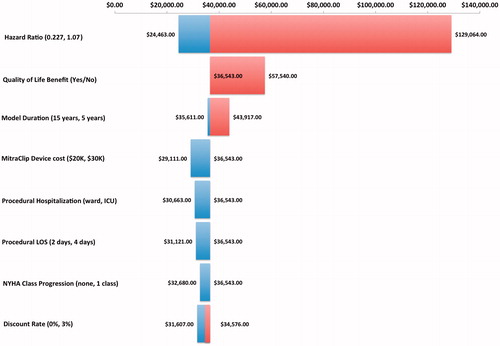
The model was robust for the majority of variables on one-way sensitivity analyses, as shown in the tornado diagram (). The model was sensitive to changes in the hazard ratio for survival, and time horizon with the MitraClip remaining cost-effective (assuming a threshold of $100,000 per QALY gainedCitation13) at the upper 95% CI of the hazard ratio, 0.795; however, with an increase in the ICER to $66,300.00. At the time horizon of 2 years the ICER increased to ∼$89,000.00, suggesting that overall life expectancy has an impact on the cost-effectiveness. In the absence of an improvement of quality-of-life in those treated with the MitraClip, the ICER also increased but remained below the threshold of $100,000.00 as displayed in the tornado diagram. The major incremental cost drivers in the model were implant costs (+$50,000.00 CDN) and disease management costs ($11,500.00 CDN) over the time horizon of the model. One-way sensitivity analyses showed that shorter length of hospital stay (2 vs 4 days) for the procedure and place of hospitalization (critical care unit vs regular ward) had a minor impact on the incremental costs.
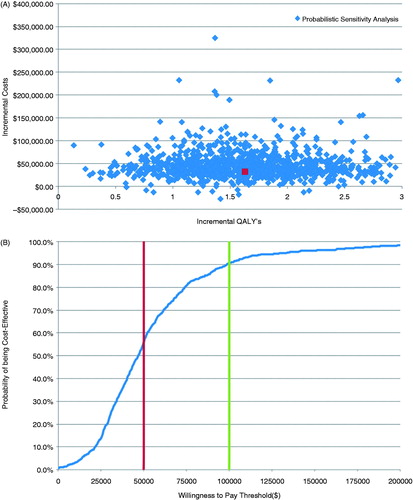
Probabilistic sensitivity analyses demonstrated that treatment with the MitraClip compared with medical therapy was cost-effective in 67% of simulations using a willingness-to-pay threshold of $50,000 and in 95% of simulations using a willingness-to-pay threshold of $100,000 ().
Figure 4. (A) Incremental cost-effectiveness of MitraClip compared with medical therapy. Scatterplot of incremental cost (in Canadian dollars; $CDN) vs incremental quality-adjusted life year (QALY) for the 10,000 simulations comparing MitraClip with medical therapy (a willingness-to-pay threshold of $100,000 CAD is indicated). (B) Cost-effectiveness acceptability curve created from a probabilistic sensitivity analysis performed with 10,000 Monte Carlo simulations.
Discussion
Heart failure is increasing in prevalence and incidence and the outcome associated with it is poor. As this global problem increases, so does the economic burden of the diseaseCitation14. Mitral regurgitation is common in the heart failure population, with 25% of patients in one series having severe MRCitation2. In patients with left ventricular dysfunction, the presence of severe MR has been associated with an all-cause mortality of 45% at 4 years, despite optimal medical therapyCitation15. Whether or not treating mitral regurgitation will impact mortality in such patients is unclear and currently the subject of study in randomized controlled trials. Observational studies, to date, have demonstrated that transcatheter therapy using the MitraClip is safe and effective at reducing mitral regurgitation. The impact of MitraClip on mortality is currently the subject of study in randomized controlled trials.
Published European experience in patients with predominantly functional MR and lower ejection fractions has been equally encouraging. Recently, data from the Pilot European Sentinel Registry showed similar success and mortality rates to ACCESS-EU with higher incidence of re-hospitalization for heart failure and NYHA class III–IV in patients with functional MRCitation16. In comparison to the two groups described above, the patient cohort at the MHI was higher risk, with more advanced left ventricular dysfunction (mean EF 38%) and mostly functional mitral regurgitation. Despite these differences, treatment with MitraClip in our cohort demonstrated comparable success rates and 12-month survival as registry data, as shown in .
Table 4. Comparison of outcomes in MitraClip cohort with published registry data.
The EVEREST High risk Registry demonstrated clinical effectiveness in a high-risk surgical population with a mean ejection fraction of 54% and 3–4+ MR with a procedural success of 96%, 30-day mortality of 7.7% and a 12-month survival of 76%Citation17. In comparison to the concurrent comparator group treated medically, an improvement in survival became evident at 6 months and continued out to 12 months. Our matched analysis demonstrates similar survival at 10 months, at which point the curves began separating with a mortality benefit that was statistically significant for those treated with MitraClip.
The cost-effectiveness analysis was performed to examine the cost utility of the MitraClip in high-risk patients with predominantly functional mitral regurgitation. This analysis demonstrates that as much as 38% of MitraClip procedure and device costs may be offset by reductions in hospitalizations, ER visits, and mitral valve surgeries compared to medical therapy. Given the Canadian societal willingness-to-pay threshold of $20,000–$100,000Citation18, MitraClip is cost-effective with a deterministic ICER of $32,300 per QALY gained and median probabilistic ICER of just under $50,000 CDN per QALY gained. Our analysis is unique in that it utilizes a propensity-matched cohort of patients with heart failure and functional mitral regurgitation in order to minimize differences in patient populations undergoing each treatment. The model was most influenced by the hazard ratio for survival, demonstrating that the therapy is not cost-effective in the absence of a survival benefit.
Our results are similar to an analysis of the MitraClip published from the perspective of the National Health Service in the UK. This evaluation utilized data from the high-risk registry of EVEREST to create a decision model. Our results are consistent with this analysis, which demonstrated that treatment with the MitraClip was cost-effective in high-risk patients. The UK model was found to be most sensitive to the time horizon chosen rather than device or procedure costCitation19.
Study limitations
The key limitations of this study are the small sample size; differences in functional class at baseline in both groups, and the limited follow-up data available requiring certain assumptions for the purposes of the economic model. Although the sample size was limited by the recent availability and restricted access to the MitraClip in Canada, we feel that the patients included are representative of the larger target population for this therapy. Patients referred for MitraClip were, in the majority of cases, those that were very symptomatic despite medical therapy and, therefore, referred for this new therapy. It must be noted, however, that the patients treated with MitraClip represent the early experience of the operators and include the procedural learning curve of this technology.
The medical management cohort was comprised of patients followed in the Heart Failure Clinic in the 2 years prior to availability of the MitraClip. Patients in this cohort received guideline-directed medical therapy for heart failure. The patients in this group did have a lower ejection fraction than the MitraClip cohort, despite the matching process, and this may be related to their higher mortality at follow-up. The modest number of patients with cardiac resynchronization therapy may reflect the better functional class in this group. Given the significant differences in baseline functional class between the MitraClip and medical management cohorts, NYHA class was not included in the propensity matching. The medical cohort was less symptomatic, with the majority of patients reporting NYHA class II symptoms, compared to the predominantly class IV symptoms in the MitraClip group. As a result, the impact of the MitraClip on improved survival, reduced hospitalization costs, ER costs, and mitral valve surgery costs may have been under-estimated given that higher NYHA functional classes are associated with increased risk of hospitalization and mortality in heart failure patientsCitation20.
The economic study can be criticized for being based on observational data rather than randomized controlled trial data; however, such data is currently unavailable. In addition, this analysis is based on the outcomes of real-world clinical patients as opposed to carefully screened and selected trial subjects. The study was limited by the need to extrapolate survival for both cohorts beyond the follow-up available, resulting in a relatively wide confidence interval for the hazard ratio of MitraClip vs medical therapy. This is reflected by the spread of the data in the probabilistic sensitivity analyses (). However, the mortality rates estimated by the model were consistent with mortality rates observed in existing registries of patients with CHF and functional MR and managed medically or treated with the MitraClipCitation2,Citation7,Citation16. Despite these limitations, the findings of this analysis are in keeping with the aforementioned UK cost-effectiveness analysis of MitraClip in high-risk patients.
Conclusions
The data from this observational study suggest that transcatheter mitral repair with the MitraClip in heart failure patients is associated with improved survival compared to standard medical therapy. The ultimate place for this technology within the armamentarium of heart failure therapy remains to be determined once long-term outcome data become available. However, treatment decisions for these patients will be required prior to the availability of these data, as such clinicians must make such decisions given the best available data. Using such data, this analysis indicates that the MitraClip may represent and clinical effective and cost-effective treatment strategy when compared to medical therapy in patients with functional mitral regurgitation.
Transparency
Declaration of funding
This work was supported by a grant from the Montreal Heart Institute Foundation.
Declaration of financial/other interests
AA, AD, and RB are on the speaker’s bureau for Abbott Vascular. AA, RB, and DC are consultants for Medtronic Vascular and Edwards LifeSciences. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
This was presented at the ISPOR annual meeting in Montreal, QC, Canada, 2014.
Appendix_A_and_B.docx
Download MS Word (82.4 KB)Acknowledgments
The authors would like to acknowledge the work and collaboration of Heather Cameron, Lisa Bernard, and Daniel Grima to the model design, model review, and preparation of this manuscript. We would also like to acknowledge the work of Charaf-Eddine Ahnadi for his assistance with the Heart Failure Clinic database.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11
- Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675-80
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2013;62:e147-239
- Feldman T, Foster E, Glower D, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406
- Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009;54:686-94
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies. 2006. Canadian Agency for Drugs and Technologies in Health, Canada
- Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61
- Gohler A, Geisler BP, Manne JM, et al. Utility estimates for decision–Analytic modeling in chronic heart failure—Health states based on New York Heart Association classes and number of rehospitalizations. Value Health 2009;12:185-187
- Chaplin S, Scuffham PA, Alon M, et al. Secondary prevention after PCI: the cost-effectiveness of Statin Therapy in the Netherlands. Netherlands Heart J 2004;12:331-6
- Marwick TH, Scuffham PA, Hunink MG. Selection for early surgery in asymptomatic mitral regurgitation: a Markov model. Int J Cardiol 2013;165:266-72
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410-20
- Ghatnekar O, Bondesson A, Persson U, et al. Health economic evaluation of the Lund Integrated Medicines Management Model (LIMM) in elderly patients admitted to hospital. BMJ Open 2013;3:1-9
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796-7
- Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol 2014;171:368-76
- Agricola E, Ielasi A, Oppizzi M, et al. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur J Heart Fail 2009;11:581-7
- Nickenig G, Estevez-Loureiro R, Franzen O, et al.; Transcatheter Valve Treatment Sentinel Registry Investigators of the ERPotESoC. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol 2014;64:875-84
- Whitlow P, Feldman T, Pedersen W, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 2012;59:130-9
- Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ; journal de l'Association medicale canadienne 1992;146:473-81
- Mealing S, Feldman T, Eaton J, et al. EVEREST II high risk study based UK cost-effectiveness analysis of MitraClip(R) in patients with severe mitral regurgitation ineligible for conventional repair/replacement surgery. J Med Econ 2013;16:1317-26
- Holland R, Rechel B, Stepien K, et al. Patients' self-assessed functional status in heart failure by New York Heart Association class: a prognostic predictor of hospitalizations, quality of life and death. J Cardiac Fail 2010;16:150-6