Abstract
Objective: To estimate the public health impact of comprehensive hepatitis C virus (HCV) screening and access to all-oral, interferon (IFN)-free direct-acting antivirals (DAAs) in the French baby-boomer population (1945–1965 birth cohorts).
Methods: A sequential, multi-cohort, health-state transition model was developed to assess the impact of different hepatitis C screening and treatment strategies on clinical and economic outcomes in the 1945–1965 birth cohorts. Patients newly-diagnosed with chronic HCV were projected each year from 2016 to 2036 under three screening scenarios (70% [low], 75% [intermediate], and 80% [high] HCV awareness in 2036). Healthcare costs and clinical outcomes (number of liver-related deaths, quality-adjusted life-years [QALYs], life-years [LYs] spent in sustained virologic response [SVR] or with decompensated cirrhosis, hepatocellular carcinoma, or liver transplant) were compared among five treatment strategies (no antiviral therapy; IFN + ribavirin + protease inhibitor for fibrosis stages F2–F4, IFN-based DAAs for stages F2–F4, IFN-free DAAs for stages F2–F4, and IFN-free DAAs for stages F0–F4).
Results: Diagnosis of HCV genotype 1 was projected for 4,953, 6,600, and 8,368 individuals in the low, intermediate, and high screening scenarios, respectively. In the intermediate scenario, IFN-free DAAs for stages F0–F4 had a favorable cost-effectiveness profile vs IFN-based or IFN-free treatment strategies for F2–F4 and offered the greatest return on investment (0.899 LYs gained in SVR and 0.933 QALYs per €10,000 invested).
Conclusion: Comprehensive HCV screening and access to all-oral, IFN-free DAAs is a cost-effective strategy that could help diminish the upcoming burden of HCV in the French baby-boomer population.
Introduction
Chronic hepatitis C (CHC) affects approximately 130–150 million people globally and approximately 700,000 people die each year from CHC-related liver diseasesCitation1. CHC is the most common cause of liver disease (including liver cirrhosis and liver cancer) and the most common indication for liver transplantation in the US, Australia, and most of EuropeCitation2. The burden of CHC in Europe is substantial and, notably, France has among the highest incidence, prevalence, and CHC-related mortality in the World Health Organization European regionCitation3.
The treatment of hepatitis C virus (HCV) infection is rapidly improvingCitation4–6, and the prospects of future HCV treatment innovations are promisingCitation7,Citation8. Until recently, the standard of care (SOC) in France for treatment of HCV genotype 1 (the most common HCV strain in France) consisted of pegylated interferon (IFN) in combination with ribavirin (RBV), often with the addition of direct-acting antivirals (DAAs), such as the protease inhibitors (PIs) telaprevir and boceprevirCitation8,Citation9. However, IFN-based DAA regimens can be difficult to tolerate; serious adverse effects, including anemia, depression, and thyroid dysfunction, occur in 22–65% of patientsCitation8; and treatment is often withheld until moderate-to-severe liver fibrosis is present (i.e. Metavir fibrosis score F2–F4)Citation10. Second-generation, IFN-free, all-oral DAAs have the potential to achieve sustained virologic response (SVR) in >95% of those treated, with substantially improved tolerabilityCitation6,Citation8.
Because chronic HCV infection often remains undiagnosed until serious liver damage has developedCitation1,Citation2, improved screening and earlier diagnosis have been identified as critical components for improving care of patients with HCVCitation11. In France, the strategy of screening based on risk exposure resulted in less than 20% of CHC diagnoses, from 1994–2006, with the majority (57%) of cases being identified through systematic screening (i.e. related to routine office visits or blood donation)Citation12,Citation13. Therefore, France recently adopted recommendations for routine, population-based HCV screening for groups at particular risk of infectionCitation14. The baby-boomer cohort (1945–1965 birth cohorts) is a particularly important focus group for screening and intervention owing to its large size and high CHC prevalenceCitation13,Citation15. For example, baby-boomers comprised approximately 50% of HCV-seropositive individuals in France in 2004Citation16. In addition, this cohort is at increased risk of developing liver diseases that complicate HCV infection, such as cirrhosis and hepatocellular carcinoma (HCC)Citation17,Citation18.
Projections from the Institut National d’Études Démographiques (INED, the official demographic institute of France) and the Institut National de Veille Sanitaire (InVS) indicate that the population of baby-boomers currently exceeds 16.5 million individuals, only 57% of whom are aware of their HCV statusCitation16,Citation19. Routine HCV screening in France, therefore, has the potential to inform millions of individuals in this high-risk group. In addition, the economic burden of HCV in France is high; the total cost of CHC-related inpatient care in France was €65,956,938 in 2009Citation20. The potential costs and benefits associated with comprehensive intervention (e.g. for patients with Metavir fibrosis scores F0–F4), with second-generation, IFN-free DAAs are not yet well understood.
The objectives of the current study are to estimate the public health impact of comprehensive HCV screening in the French baby-boomer population, coupled with access to the current SOC or all-oral, IFN-free HCV treatment at different stages of liver fibrosis.
Methods
Study design
Using Microsoft Excel (Microsoft Corporation, Redmond, WA) we developed a sequential, multi-cohort, health-state transition (Markov) model to assess screening strategies, clinical outcomes, and healthcare costs among French baby-boomers with HCV over a 20-year horizon (2016–2036). Model structure, inputs, and assumptions are summarized in .
Figure 1. Model schematic. Patients enter the model in fibrosis stages F0–F4 and, in the absence of treatment, progress to liver complications and liver-related death according to the annual transition rates derived from the literature (shown next to each arrow). Death owing to all-cause (non-liver-related) mortality can occur during each cycle for patients in F0–F4. SVR12 rates for patients treated with antivirals during stages F0–F4 were derived from clinical trials or published literature. In cases where SVR12 rates were not reported independently for each fibrosis stage, weighted averages of the reported SVR12 rates were calculated, using the fibrosis distribution shown in the model as weighting factors. *Annual transition probability subsequent to Year 1. Sources: aINVS 2004, 2007Citation16,Citation21, bPogorzelska and Flisiak 2016Citation22, cWalker et al. 2015Citation23, dLawitz et al. 2013Citation24, ePoordad et al. 2011Citation25, fThein et al. 2008Citation26, gLiu et al. 2012Citation27, hAlazawi et al. 2010Citation28, iRazavi et al. 2013Citation29, jSingal et al. 2010Citation30, kThuluvath et al. 2010Citation31. CC, compensated cirrhosis (Metavir fibrosis score F4); CHC, chronic hepatitis C; D, all-cause death; DAA, direct-acting antiviral; DCC, decompensated cirrhosis; F, Metavir fibrosis score; HCC, hepatocellular carcinoma; LrD, liver-related death (i.e. death from DCC, HCC, and LT); LT, liver transplant; PI, protease inhibitor; RBV, ribavirin; SOC, standard of care; SVR12, sustained virologic response 12 weeks after treatment.
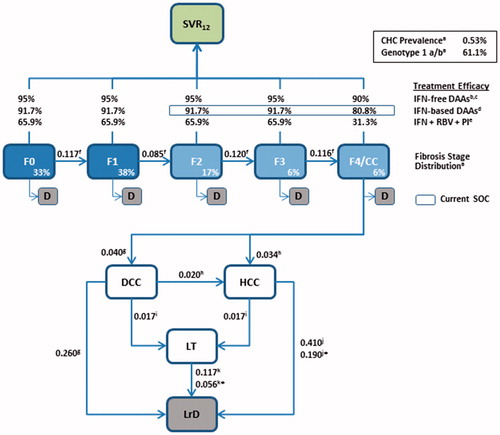
Target population
The target population included all French baby-boomers with genotype 1 HCV previously unaware of their HCV status. The target population size was based on median variant population projections from INEDCitation19. Projections for 20 individual cohorts (corresponding to birth years 1945–1965) were extracted and accounted for all-cause mortality over time. The number of individuals with chronic HCV infection was calculated based on the following epidemiology parameters: first, overall prevalence of HCV (HCV RNA positive, chronic infection) was estimated at 0.53%, according to data from the InVSCitation16, and 61.5% of prevalent cases were estimated to be genotype 1Citation21. The model assumed that 57% of French baby-boomers were aware of their HCV status in 2016 and, hence, excluded from the analysisCitation16. The fibrosis stage distribution of screened patients was assumed to be unchanged, irrespective of calendar year and projected age of individuals in each annual cohort.
Cohort-screening scenarios
The numbers of patients newly-screened and/or diagnosed with chronic HCV from 2016–2036 were projected for three different cohort-screening scenarios, defined as ‘low’, ‘intermediate’, and ‘high’ (corresponding to 70%, 75%, and 80% HCV awareness in 2036, respectively). These three scenarios were used in absence of any published declarations regarding intentions for screening in France over the next 20 years. For simplicity, the model assumed an overall screening rate to account for multi-step screening (which includes initial testing for the presence of HCV antibodies to identify acute or chronic infection followed by RNA testing to identify chronic HCV infection, genotyping to identify HCV strain, and fibrosis staging). The intermediate screening scenario (midpoint between low- and high-screening scenarios) was defined as the base-case for analysis of public health outcomes.
Disease progression
The model incorporated a simplified version of chronic HCV natural historyCitation27,Citation32, in which patients progress through a series of defined health states. The distribution of newly-screened/diagnosed patients among fibrosis states F0–F4 was defined according to epidemiology data from the InVSCitation16,Citation21; these data were complemented by an ad-hoc market research study conducted with HCV reference centers (AbbVie, 2014 data on file) and were further validated by two independent reports from the French public health authoritiesCitation14,Citation33.
Transition probabilities
In the absence or failure of treatment, patients progress to decompensated cirrhosis (DCC), HCC, liver transplant (LT), and liver-related death (LrD), according to annualized transition probabilities determined from the literature. Successful treatment in fibrosis stages F0–F3 would lead to SVR and stop disease progression, but SVR in fibrosis stage F4 would carry residual risk of HCC. Transition probabilities among health states were adjusted for the respective treatment strategy and effectiveness. Four treatment strategies were assessed: IFN + RBV + PI for stages F2–F4, IFN-based DAAs for stages F2–F4 (SOC recommended in France in 2014)Citation9, IFN-free DAAs for stages F2–F4, and IFN-free DAAs for stages F0–F4. Treatment effectiveness was defined as the proportion of patients achieving SVR 12 weeks after treatment (SVR12), based on data from phase 3 clinical trials for IFN + RBV + boceprevirCitation25, IFN + RBV + sofosbuvirCitation24, and paritaprevir/r + ombitasvir + dasabuvir ± RBV (also referred to as 3D ± RBV)Citation34–36.
The model used annual cycles, in which a new cohort of patients diagnosed with genotype 1 CHC was added to the previous cohort each year as screening for the baby-boomer population was increased. Death associated with background (all-cause) mortality for patients in fibrosis stages F0–F4 was informed by the INED population projectionsCitation19; mortality among patients in the DCC, HCC, or LT stages was considered to be LrD.
Costs and health utilities
The model was constructed from the perspective of the French healthcare system. Cost inputs were based on published literature and limited to HCV screening costs and costs associated with drug therapy, management of severe adverse events, direct medical (inpatient and outpatient) spending on treatment of fibrosis and liver complications (including LT), and LrDCitation37 (). Costs associated with HCV screening and drug therapies were taken from Assurance MaladieCitation38. Drug costs were computed based on a 12-week duration of PI- and DAA-based therapies, but doubled for genotype 1a patients in fibrosis stage F4 (assuming that 50% of genotype 1 patients would be genotype 1a in the base case). Costs associated with management of severe adverse events were taken from a modeling study by Deuffic-Burban et al.Citation30. Costs associated with treatment of fibrosis and liver complications, as well as LrD, were taken from a study by Schwarzinger et al.Citation39, updated and detailed at the request of AbbVie France (AbbVie, 2014 data on file). Future costs and life years (LYs) were discounted at an annual rate of 4%Citation40. Quality adjusted life years (QALYs) were estimated using utility weights obtained from a French cost-effectiveness studyCitation41.
Table 1. Direct medical costs associated with HCV screening and treatment (€).
Outcomes
Outcomes were calculated on an annual basis and summarized over the 20-year model horizon for each screening and treatment strategy. Public health outcomes included the projected number of patients diagnosed with CHC (and disease stage); projected number of patients reaching SVR, DCC, HCC, LT, or LrD; and projected number and proportion of LYs spent in each health state. Economic outcomes included total costs, cost per QALY and cost per LY in SVR12. Incremental cost per QALY and incremental cost per LY in SVR12 were also calculated to compare cost-effectiveness among treatment strategies, and return on investment was measured as cost per QALY/LY gained in SVR12 or QALYs/LYs gained in SVR12 per €10,000 invested in treatment.
Sensitivity analyses
One-way sensitivity analyses were carried out on the costs per QALY for each treatment strategy. A more focused sensitivity analysis was conducted on the incremental costs per QALY for all-oral, IFN-free DAAs for F0–F4 vs F2–F4. All inputs parameters were varied within the ±20% range where plausible (i.e. transition probabilities could not be greater than 1 or less than 0). The baseline distribution of fibrosis stages was varied between a “mild” scenario (F0: 45%, F1: 40%, F2: 11%, F3: 2%, F4: 2%) and a “severe” scenario (F0: 2%, F1: 2%, F2: 11%, F3: 40%, F4: 45%) in comparison to the baseline distribution (F0: 33%, F1: 38%, F2: 17%, F3: 6%, F4: 6%; see ). We also assessed outcomes in the “low” and “high” screening scenarios and varied the discount rate from 0–6% for future costs and LYs in SVR12. The impact of the 10 most-influential parameters was plotted on tornado diagrams, as appropriate.
Results
Target population
INED projected a total population of 16,436,663 persons born in 1945–1965 (aged 50–70 years in 2016), declining to 11,903,745 persons in 2036 (aged 70–90 years) owing to all-cause mortality (Supplemental Figure 1). The impact of cohort-based HCV screening within this population is presented in . In the intermediate (base-case) screening scenario (75% of the population screened by 2036), a total of 2,191,313 individuals would be screened for HCV from 2016–2036. Of these, 6,660 were projected to be diagnosed with CHC genotype 1: 3,248 (48.8%) in F0/F1, 2,565 (38.5%) in F2/F3, and 848 (12.7%) in F4 at the time of screening/diagnosis. The low and high screening variants resulted in 4,953 and 8,368 newly-diagnosed individuals (F0–F4), respectively (see ).
Figure 2. Cumulative number of newly-diagnosed HCV cases by fibrosis stage and screening variant. CC, compensated cirrhosis; F, Metavir fibrosis score; HCV, hepatitis C virus.
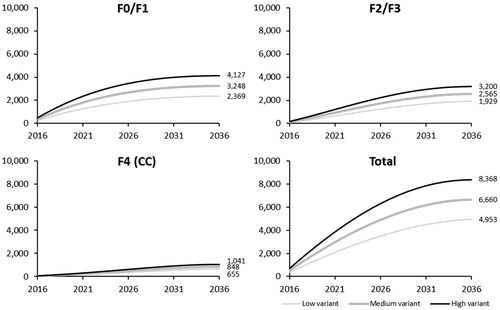
The model projected that among the 3,248 patients diagnosed in F0/F1 at the time of screening, if left untreated 1,774 (54.6%) patients would progress to F2 by 2036. Assuming that these patients were regularly followed up for disease progression, they were considered as eligible at the time of their progression to F2 for the three treatment strategies targeting F2–F4. Therefore, 5,187 patients were eligible for the three treatment strategies targeting F2–F4, and all 6,660 newly-diagnosed patients were eligible for all-oral, IFN-free DAAs for F0–F4 ().
Table 2. Public health impact of HCV treatment strategy: Number of LYs across health states, HCV-related costs, return-on-investment, QALYs, and cost-effectiveness analyses (intermediate screening variant).
Public health and economic outcomes
Compared to no antiviral therapy, all treatment strategies were associated with increased time spent in SVR, reduced time spent with liver complications (DCC, HCC, and LT) and decreased number of LrDs. The total number of patient-years lived in DCC, HCC, or LT during 2016–2036 was projected to be 1,552 without treatment, 370 with IFN + RBV + PI, 118 with the current SOC, and 94 with IFN-free, all-oral DAAs for F2–F4 or F0–F4. The number of LrDs was projected to be 320 without treatment, 75 with IFN + RBV + PI for F2–F4, 23 with the current SOC, and 19 with IFN-free, all-oral DAAs for F2–F4 or F0–F4. Comprehensive treatment with IFN-free DAAs at stages F0–F4 increased by ∼60% the time spent in SVR compared with IFN-based or IFN-free treatment strategies for F2–F4 (proportion of remaining LYs spent in SVR: 89.1% vs 54.7% and 54.2%, respectively). No patients were assumed to achieve SVR in the absence of treatment (see ).
Among treated patients, overall costs per treated patient were projected to be greatest in those receiving IFN-based DAAs for F2–F4, and least among those receiving IFN + RBV + PI (€89,352 vs €68,710 per patient treated). HCV screening and treatment costs were the primary drivers of cost in patients who received therapy; together, they accounted for >95% of total cost. Conversely, healthcare costs were greatest for untreated patients, accounting for 100% of total cost vs 0.5% in patients treated with IFN-free DAAs for F0–F4. Comprehensive treatment with all-oral, IFN-free DAAs was associated with a favorable cost-effectiveness profile and the greatest return on investment (0.933 QALYs and 0.899 LYs gained in SVR12 per €10,000 invested). The incremental cost per LY gained in SVR12 with all-oral, IFN-free DAAs for F0–F4 remained less than €11,000 (range = €3,359–€10,480) vs all other treatment strategies. In addition, the incremental cost per QALY with all-oral, IFN-free DAAs for F0–F4 remained less than €60,000 (range = €22,986–€59,589) vs all other treatment strategies.
Sensitivity analyses
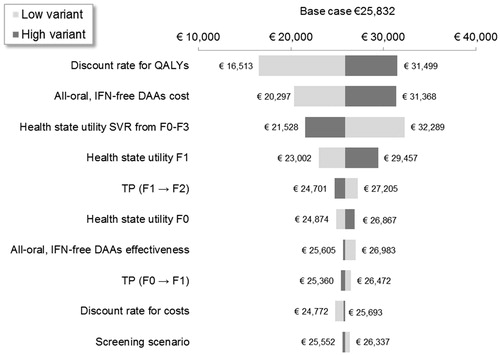
shows the results of sensitivity analyses on the costs per QALY as per-treatment strategies. The discount rate for costs and QALYs (varied between 0–6% from 4%), distribution of fibrosis stages, prevalence of HCV genotype 1 a/b (varied between 0.21–0.47% from 0.32%), and screening cost for HCV (varied between €133–€199 from €166) had the greatest impact on the cost per QALY gained, regardless of treatment strategy. All estimates remained less than €14,000 per QALY and above €5,000 per QALY. shows the impact of parameter variations on the incremental cost per QALY gained with all-oral, IFN-free DAAs for F0–F4 vs for F2–F4 only. The model was most sensitive to the discount rate for QALYs, the cost of INF-free DAAs, and health state utilities associated with F1 and with SVR from F0–F3. All incremental cost per QALY estimates remained between €16,000–€33,000, suggesting that expanding INF-free DAA treatment from F2–F4 to F0–F4 is a cost-effective strategy.
Figure 3. One-way sensitivity analysis on the cost per QALY per treatment strategy. CC, compensated cirrhosis; DAA, direct-acting antiviral; DCC, decompensated cirrhosis; F, Metavir fibrosis score; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon; LY, life-year; PI; protease inhibitor; RBV, ribavirin; SVR12, sustained virologic response 12 weeks after treatment; TP, transition probability.
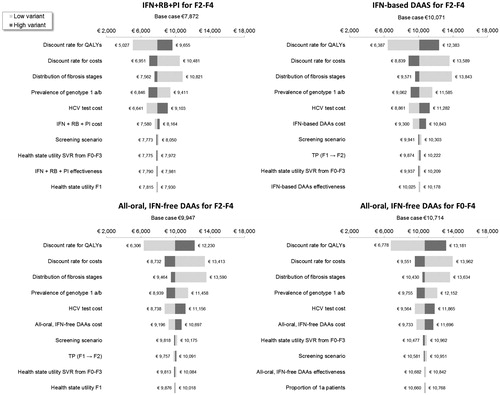
Figure 4. One-way sensitivity analysis on the incremental cost per QALY of all-oral, IFN-free DAAs for F0–F4 vs all-oral, IFN-free DAAs for F2–F4. DAA, direct-acting antiviral; F, Metavir fibrosis score; IFN, interferon; LY, life-year; SVR12, sustained virologic response 12 weeks after treatment; TP, transition probability.
Discussion
We developed a model to describe the potential public health impact of cohort-based HCV screening coupled with new all-oral, IFN-free DAAs for the treatment of CHC in France. The analysis focused on the baby-boomer population and estimates accounted for different HCV screening and treatment scenarios. We found that comprehensive treatment with IFN-free, all-oral DAAs for patients with fibrosis scores F0–F4 has a favorable cost-effectiveness profile and return on investment which could increase by 60% the number of years baby-boomers infected by HCV will live with SVR compared to the current SOC in France (i.e. IFN-based DAAs for F2–F4). Our results suggest that the implementation of a cohort-based screening policy coupled with broader access to IFN-free, all-oral DAAs could considerably diminish the future burden of HCV in the French baby-boomer population.
Our results are consistent with previous studies that have indicated the importance of enhanced screening in the baby-boomer populationCitation11,Citation15,Citation42–45. For example, Rein et al.Citation37 showed that a one-time cohort screening strategy could lead to the identification of 86% of undiagnosed HCV cases in the US, compared with 21% detection with a risk-based screening strategy. In addition, other studies have indicated that the cost-effectiveness of HCV screening and treatment can be enhanced by focusing on higher-risk populationsCitation44,46,47. Given the rate of all-cause mortality in the baby boomer cohort over the 20-year horizon period, our model suggests that screening and treating as many of this cohort as economically viable in a short-time frame would be beneficial from both clinical and economic standpoints.
Our results are also consistent with previous studies showing potential benefits associated with highly effective IFN-free treatment for patients with mild-to-severe fibrosis. Tsochatzis et al.Citation48 recently investigated the cost-effectiveness of non-invasive liver fibrosis testing and concluded that treating all patients, regardless of fibrosis stage, with therapy achieving >90% SVR was more cost-effective than treating patients in stages F2–F4 only. Deuffic-Burban et al.Citation37 showed that awaiting IFN-free regimens and then treating all patients, regardless of their fibrosis stage, was the most efficient strategy (with the exception of F4 patients). Younossi et al.Citation49 determined that all-oral, IFN-free regimens for all HCV genotype 1 patients (regardless of fibrosis stage) were more cost-effective than either IFN-based or IFN-free therapy for stages F0–F2. Hagan et al.Citation50 reported that an all-oral, IFN-free regimen was cost-effective compared with SOC in a cohort of 50-year-old patients with CHC. Deuffic-Burban et al.Citation51 demonstrated how viral eradication with improved antiviral therapies could substantially reduce HCV-related mortality compared to the absence of treatment. Finally, Deuffic-Burban et al.Citation52 projected the impact of newer DAA agents on future needs for liver transplantation in France. They found that, overall, the new regimens would prevent the management of 4,425 potential candidates for LT (1,461 related to HCC and 2,964 related to DCC) from 2013–2022.
Results from this study are complementary and consistent with previous cost-effectiveness and budget impact analyses to inform healthcare decision-making. However, our study is subject to certain limitations. Our modeling approach is a forecasting exercise that depends heavily on assumptions regarding future HCV screening and CHC diagnosis rates. It is naturally unfeasible to foresee with certainty the path of future HCV screening and treatment. Further, the horizon period of 20 years may under-estimate the total burden of disease over time and the cost-effectiveness of comprehensive HCV screening and treatment. In spite of this conservative assumption, a 20-year period is consistent with other models in the literature (e.g. Rein et al.Citation44). The model also made the conservative assumption that fibrosis stage distribution remained unchanged over the 20-year horizon period. Fibrosis stage distribution would likely progress towards more advanced disease over time (i.e. individuals screened later in the horizon period would be of an older age, and would more likely have more advanced fibrosis given increased time from initial infection to screening). The model did not account for treatment compliance or additional risk factors related to chronic HCV infection (e.g. gender, comorbidities, and alcohol abuseCitation2). In addition, the model considered only patients infected with genotype 1 HCV. Although this is the most prevalent strain among individuals in the target group, the potential benefits of screening and treatment in patients with non-genotype 1 infection is an important area of future research. Although costs were discounted in the analysis, drug prices were assumed to remain constant over the next 20 years. Because price erosion is generally observed in the marketplace, this assumption may lead to an under-estimate of the return on investment and cost-effectiveness associated with comprehensive treatment. SVR rates were calibrated based on clinical trials and may not reflect real-world effectiveness. Although the impact of other all-oral regimens aside from 3D ± RBV approved for the treatment of HCV genotype 1 was not explicitly tested in the model, results are not greatly sensitive to SVR rates and, hence, not expected to change substantively. Finally, our analysis did not take into account the fact that barriers to HCV screening and treatment have been consistently reportedCitation53. Assessing the impacts of barriers to care on outcomes may lead to identification of particular areas for improvement to better inform HCV policy.
Conclusions
A cohort-based screening policy, coupled with comprehensive access to newer all-oral, IFN-free DAAs has a favorable cost-effectiveness profile and a high return on investment to diminish the upcoming burden of HCV in the baby-boomer population. Despite the apparent high acquisition cost of these newer agents, the humanistic and economic returns expected from the greater avoidance of CHC liver complications could be substantial.
Transparency
Declaration of funding
The research reported in this manuscript was supported by AbbVie through consulting fees paid to SERFAN innovation.
Declaration of financial/other relationships
AbbVie and SERFAN innovation collaborated to develop the study design and model. Both AbbVie and SERFAN innovation participated in the interpretation of data, review, and approval of the manuscript. OE owns SERFAN innovation and is a consultant for AbbVie Inc. YSG, GJ, and TJ are AbbVie employees and may own AbbVie stock. AD and DM were AbbVie employees at the time of manuscript development. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing assistance was provided by Eric Bertelsen, PhD, an employee of Arbor Communications, Inc. (a member of the Fishawack Group of Companies). This assistance was funded by AbbVie.
References
- WHO (World Health Organization). Hepatitis C fact sheet. Geneva, Switzerland: World Health Organization, 2016. http://www.who.int/mediacentre/factsheets/fs164/en/ [Online, August 2, 2016].
- Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 2006;3:47-52
- Muhlberger N, Schwarzer R, Lettmeier B, et al. HCV-related burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity, and mortality. BMC Public Health 2009;9:34
- Dabbouseh NM, Jensen DM. Future therapies for chronic hepatitis C. Nat Rev Gastroenterol Hepatol 2013;10:268-76
- Soriano V, Labarga P, Fernandez-Montero JV, et al. The changing face of hepatitis C in the new era of direct-acting antivirals. Antiviral Res 2013;97:36-40
- Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies and challenges. Gastroenterology 2014;146:1176-92
- Bourlière M, Khaloun A, Wartelle-Bladou C, et al. Chronic hepatitis C: treatments of the future. Clin Res Hepatol Gastroenterol 2011;35:S84-S95
- Feeney ER, Chung RT. Antiviral treatment of hepatitis C. BMJ 2014;348:g3308
- Bourlière M, Bronowicki JP, Hezode CH, et al. Traitement des hépatites virales C Avis d’experts – mars 2014. Paris, France: Association reconnue d?utilite publique (AFEF), 2014. http://www.afef.asso.fr/ckfinder/userfiles/files/actualites/veille/Avisexperts-AFEF-Mars-2014.pdf. [December 18, 2015].
- Afdhal NH, Lok AS, Di Bisceglie AM. Clinical decisions. Management of incidental hepatitis C virus infection. N Engl J Med 2009;360:1902-6
- Voelker R. Birth cohort screening may help find hepatitis C cases. JAMA 2012;307:1242
- Delarocque-Astagneau E, Meffre C, Dubois F, et al. The impact of the prevention programme of hepatitis C over more than a decade: the French experience. J Viral Hepat 2010;17:435-43
- Brouard C, Le Strat Y, Larsen C, et al. The undiagnosed chronically-infected HCV population in France. Implications for expanded testing recommendations in 2014. PLoS One 2015;10:e0126920
- MASS (Ministère des Affaires Sociales et de la Santé). Prise en Charge des Personnes Infectées par les Virus de l'Hépatite B ou de l'Hépatite C: Rapport de Recommandations 2014. Paris, France: MASSe, 2014. http://social-sante.gouv.fr/IMG/pdf/Rapport_Prise_en_charge_Hepatites_2014.pdf [Online, February 9, 2015].
- Hagan LM, Schinazi RF. Best strategies for global HCV eradication. Liver Int 2013;33(1 Suppl):68-79
- InVS (Institut National de Veille Sanitaire). Prévalence des hépatites B et C en France en 2004. Saint-Maurice cedex, France: InVS, 2004.
- Davis GL, Roberts WL. The healthcare burden imposed by liver disease in aging Baby Boomers. Curr Gastroenterol Reps 2010;12:1-6
- Horne PM, Mills R. Implications of the 2012 Centers for Disease Control and Prevention (CDC) guidelines for screening hepatitis C infection in the United States. Pract Gastroenterol 2013;XXXVII:36-41
- INED (Institut National dÉtudes Démographiques). Population totale. Paris, France: INED, 2014. http://www.ined.fr/fr/publications/population/population-2014-n-3/. [Online, May 16, 2014]
- Rotily M, Vainchtock A, Jouaneton B, et al. How did chronic hepatitis C impact costs related to hospital health care in France in 2009? Clin Res Hepatol Gastroenterol 2013;37:365-72
- InVS (Institut National de Veille Sanitaire). Surveillance nationale de l’hépatite C à partir des pôles de référence. Saint-Maurice cedex, France: InVS, 2007.
- Pogorzelska J, Flisiak R. Real-world experience with ombitasvir/paritaprevir boosted with ritonavir and possibly combined with dasabuvir and ribavirin in HCV infection. Exp Hepatol 2016;2:34-7
- Walker DR, Pedrosa MC, Manthena SR. Early view of the effectiveness of new direct-acting antiviral (DAA) regimens in patients with hepatitis C virus (HCV). Adv Ther 2015;32:1117-27
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878-87
- Poordad F, McCone J, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1195-206
- Thein H-H, Yi Q, Dore GJ, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418-31
- Liu S, Cipriano LE, Holodniy M, et al. New protease inhibitors for the treatment of chronic hepatitis C. A cost-effectiveness analysis. Ann Intern Med 2012;156:279-90
- Alazawi W, Cunningham M, Dearden J, et al. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther 2010;32:344-55
- Razavi H, Elkhoury AC, Elbasha E, et al. Chronic hepatitis C (HCV) disease burden and cost in the United States. Hepatology 2013;57:2164-70
- Singal AG, Volk ML, Jensen D, et al. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol 2010;8:280-8, 288 e1
- Thuluvath PJ, Guidinger MK, Fung JJ, et al. Liver transplantation in the United States 1999-2008. Am J Transplant 2010;10:1003-19
- Siebert U, Sroczynski G; German Hepatitis C Model GEHMO Group; HTA Expert Panel on Hepatitis C. Effectiveness and cost-effectiveness of initial combination therapy with interferon/peginterferon plus ribavirin in patients with chronic hepatitis C in Germany: a health technology assessment commissioned by the German Federal Ministry of Health and Social Security. Int J Technol Assess Health Care 2005;21:55-65
- HAS, Haute Autorité de Santé. Choices in methods for economic evaluation. Haute Autorité de Santé, Department of Economics and Public Health Assessment. Saint Denis La Plaine Cedex, France: HAS, 2012.
- Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1594-1603
- Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370:1973-82
- Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014;370:1983-92
- Deuffic-Burban S, Schwarzinger M, Obach D, et al. Should we await IFN-free regimens to treat HCV genotype 1 treatment-naïve patients? A cost-effectiveness analysis (ANRS N°95141). J Hepatol 2014;61:7-14
- Assurance Maladie. Price of drugs in France. Paris cedex, France: Assurance Maladie, 2015.
- Schwarzinger M, Deuffic-Burban S, Mallet V, et al. Lifetime costs attributable to chronic hepatitis C from the French health care perspective (ANRS N°12188). J Hepatol 2013;58:S21-S2
- HAS (Haute Autorité de Santé). Sovaldi (sofosbuvir), antiviral à action directe. Saint Denis La Plaine Cedex, France: HAS, 2014.
- Deuffic-Burban S, Obach D, Canva V, et al. Cost-effectiveness and budget impact of interferon-free direct-acting antiviral-based regimens for hepatitis C treatment: the French case. J Viral Hepat 2016; doi:10.1111/jvh.12546 [Epub ahead of print]
- Cohen J. Infectious disease. Calling all baby boomers: get your hepatitis C test. Science 2012;337:903
- Roehr B. Screen all baby boomers for hepatitis C, advises US public health agency. BMJ 2012;344:e3707
- Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in US primary care setting. Ann Intern Med 2012;156:263-70
- Asrani SK, Davis GL. Impact of birth cohort screening for hepatitis C. Curr Gastroenterol Rep 2014;16:381
- Tramarin A, Gennaro N, Compostella FA, et al. HCV screening to enable early treatment of hepatitis C: a mathematical model to analyse costs and outcomes in two populations. Curr Pharm Des 2008;14:1655-60
- Sroczynski G, Esteban E, Conrads-Frank A, et al. Long-term effectiveness and cost-effectiveness of screening for hepatitis C virus infection. Eur J Public Health 2009;19:245-53
- Tsochatzis EA, Crossan C, Longworth L, et al. Cost-effectiveness of noninvasive liver fibrosis tests for treatment decisions in patients with chronic hepatitis C. Hepatology 2014;60:832-43
- Younossi ZM, Singer ME, Mir HM, et al. Impact of interferon free regimen on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol 2014;60:530-7
- Hagan LM, Yang Z, Ehteshami M, et al. All-oral, interferon-free treatment or chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat 2013;20:847-57
- Deuffic-Burban S, Deltenre P, Louvet A, et al. Impact of viral eradication on mortality related to hepatitis C: A modeling approach in France. J Hepatol 2008;49:175-83
- Deuffic-Burban S, Mathurin P, Rosa I, et al. Impact of emerging hepatitis C virus on future needs for liver transplantation in France: a modelling approach. Dig Liver Dis 2014;46:157-63
- Papatheodoridis GV, Tsochatzis E, Hardtke S, et al. Barriers to care and treatment for patients with chronic viral hepatitis in Europe: a systematic review. Liver Int 2014;34:1452-63
