Abstract
Objective: With a high prevalence of chronic kidney disease (CKD) in type 2 diabetes (T2DM) in Thailand, the appropriate treatment for the patients has become a major concern. This study aimed to evaluate long-term cost-effective of dipeptidyl peptidase-4 (DPP-4) inhibitor monothearpy vs sulfonylurea (SFU) monotherapy in people with T2DM and CKD.
Methods: A validated IMS CORE Diabetes Model was used to estimate the long-term costs and outcomes. The efficacy parameters were identified and synthesized using a systematic review and meta-analysis. Baseline characteristics and cost parameters were obtained from published studies and hospital databases in Thailand. Costs were expressed in 2014 US Dollars. Outcomes were presented as an incremental cost-effectiveness ratio (ICER). One-way and probabilistic sensitivity analyses were performed to estimate parameter uncertainty.
Results: From a societal perspective, treatment with DPP-4 inhibitors yielded more quality-adjusted life years (QALYs) (0.024) at a higher cost (>66,000 Thai baht (THB) or >1,829.27 USD) per person than SFU, resulting in the ICER of >2.7 million THB/QALY (>74,833.70 USD/QALY). The cost-effectiveness results were mainly driven by differences in HbA1c reduction, hypoglycemic events, and drug acquisition cost of DPP-4 inhibitors. At the ceiling ratio of 160,000 THB/QALY (4,434.59 USD/QALY), the probability that DPP-4 inhibitors are cost-effective compared to SFU was less than 10%.
Conclusions: Compared to SFU, DPP-4 inhibitor monotherapy is not a cost-effective treatment for people with T2DM and CKD in Thailand.
Introduction
Chronic kidney disease (CKD) is common in patients with diabetesCitation1. A reported prevalence of CKD in type 2 diabetes (T2DM) in primary care diabetes centers in Thailand was 25–27% for CKD stage 3–5 in 2007Citation2. A higher prevalence was reported in a tertiary care hospital in ThailandCitation3. According to the Thailand Diabetes Registry (TDR) Project, a multi-center, tertiary hospital-based diabetes registry data of 4,875 people with T2DM, the prevalence of diabetic nephropathy was 42.9% (microalbuminuria 19.7% and overt nephropathy 23.2%), while the prevalence of renal insufficiency and end-stage renal disease (ESRD) were 7.7% and 0.5%, respectivelyCitation3,Citation4.
Due to a reduced glomerular filtration rate (GFR) and resultant accumulation of drugs or their metabolites, anti-hyperglycemic treatment options for people with T2DM and CKD are limitedCitation5. Metformin, the most commonly used anti-hyperglycemia agent, is excreted renally and contraindicated in people with T2DM whose creatinine clearance is less than 30 ml/minCitation6. Sulfonylureas (SFU), such as glipizide and gliclazide, can be used; however, these medicines are associated with an increased risk of hypoglycemia and weight gainCitation7,Citation8. Thiazolidinediones are limited in their use among diabetes people with ESRD because they were associated with increased risks of fluid retention and edemaCitation9. Although insulin could be considered as an alternative, its doses are needed to be closely monitored to balance between achieving glycemic control and avoiding risk of adverse events, such as hypoglycemiaCitation7.
A group of anti-hyperglycemia drugs, namely dipeptidyl peptidase-4 (DPP-4) inhibitors, has been recognized as a new avenue for diabetes care in patients with renal impairment because they have been shown to be safe, effective, and well-tolerated. However, the extra benefit gained from the use of DPP-4 inhibitors is needed to justify their additional costs. An economic evaluation, hence, is required to help policy-makers assess the value for money of DPP-4 inhibitor monotherapy compared to SFU monotherapy in people with T2DM and CKD. In Thailand, cost-effectiveness has been recognized as part of the key evidence used for selecting medications to be listed in the National List of Essential Medicine (NLEM), which has been used as a pharmaceutical benefit package for three public health insurance schemes, including Civil Servants Medical Benefits Scheme (CSMBS) for government employees and their dependents, the Social Security Scheme (SSS) for private employees, and the Universal Health Coverage Scheme (UHCS) for the remaining population. This study aimed to assess whether DPP-4 inhibitor monotherapy was cost-effective in people with T2DM and CKD in a Thai context.
Methods
Setting
In Thailand, the health technology assessment (HTA) process used to support the selection of the pharmaceutical reimbursement list involves several steps. The list of HTA topics is initially prioritized by the sub-committee for the development of the NLEM based on the burden of disease and the degree of severity. The sub-committee contracts a Health Economic Working Group (HEWG) to conduct an economic evaluation. The quality of the economic evidence is assured by a comprehensive review of the study report by experts in the field. After the study approval, the findings will be presented to the sub-committee for consideration.
In 2014, DPP-4 inhibitors (saxagliptin, sitagliptin, and vildagliptin) were chosen as one of the listed topics because it is unclear whether these medications should be included in the NLEM for patients with T2DM who had uncontrolled blood glucose and were unable to use insulin. During the study process, the research team undertook two consultation panels with a variety of stakeholders, including policy-makers, health professionals, academics, and private sectors. The aim of the first panel was to reach consensus from participating stakeholders in terms of intervention and comparator, and the scope of the study. At the end of the study, the second panel was held to assess the methodological quality and transparency of input parameters, and the robustness of the study findings. All feedback from stakeholders was taken into consideration in the final analysis. The study had been approved by internal and external reviewers before it was presented to the sub-committee for the development of the NLEM.
Study design
A cost-utility analysis was conducted from the Healthcare System perspective using a Markov model. A validated IMS CORE Diabetes Model (CDM) version 8.5 was used to simulate long-term costs and outcomes associated with each treatment option. A lifetime horizon was used. Both costs and outcomes were discounted at a discount rate of 3% per annum, as suggested by the Thai HTA GuidelineCitation10.
IMS CORE diabetes model
Details of the CDM are described elsewhereCitation11,Citation12. Briefly, the model consists of 15 sub-models of diabetes complications. Each sub-model uses Markov techniques with Monte Carlo simulation in order to simulate accurately the relationship of multiple complications within individual patients. Four main input databases including cohort, clinical, treatment, and economic databases are required for the model. Clinical parameters used in the CDM were obtained from large, longitudinal studiesCitation13–16. Non-specific mortality rate used in the CDM was adjusted with age-specific mortality rate of Thai peopleCitation17. We assumed that 100% of our population were Thai. Other cohort characteristics were derived from the Thai published studiesCitation4,Citation18–24 (). Cost data were mostly based on Thai hospital databases, hospital costs, or published studiesCitation25–30 ().
Table 1. Baseline demographics, risk factors, baseline clinical complications of the population.
Table 2. Cost inputs.
Simulation cohort and interventions of interest
The population of interest in this study was Thai people with T2DM and severe CKD. The severe CKD was defined as patients with an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2. Baseline demographics, risk factors, and clinical complications of the population were derived from Thai data sources ().
The intervention of interest was a monotherapy of DPP-4 inhibitors including saxagliptin, sitagliptin, or vildagliptin. Each DPP-4 inhibitor was compared to glipizide. We used glipizide as a comparator based on the consensus of the first panel consultation. This is because glipizide is the most common SFU used in T2DM with CKD in Thailand. Half of the normal daily dose of DPP-4 inhibitors was used in the analysis, as suggested by a previous studyCitation31 and the consensus of the first panel consultation. Dose was defined as follows: saxagliptin 2.5 mg/day, sitagliptin 50 mg/day, and vildagliptin 50 mg/day, while dose of glipizide was 10 mg/day.
Efficacy of DPP-4 inhibitors and sulfonylurea
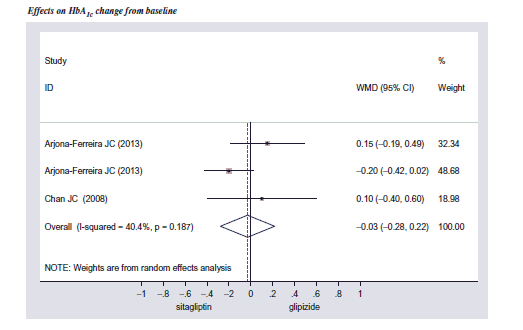
We performed a systematic review and meta-analysis to pool the efficacy of DPP-4 inhibitor monotherapy compared with SFU monotherapy in people with T2DM and CKD (Appendix). The systematic search was performed through MEDLINE, EMBASE, and Clinicaltrial.gov databases from their inception to August 2014. We found three randomized controlled trials (RCTs) that were conducted to determine the efficacy of sitagliptin monotherapy compared to glipizide monotherapy in people with T2DM and CKDCitation32–34. A meta-analysis using a random-effects model was used to pool the effect size of those three RCTsCitation32–34. The weighted mean difference (WMD) of HbA1C reduction between sitagliptin and glipizide was −0.03% (95% CI = −0.28% to 0.22%). Based on the similar efficacy and safety of sitagliptin, saxagliptin, and vildagliptin as treatment for T2DM, either as monotherapy or combination therapyCitation35–38, the pooled effect size of sitagliptin was assumed for other DPP-4 inhibitors (saxagliptin and vildagliptin). The reduction of HbA1c from SFU monotherapy in T2DM with CKD was also pooled from those three RCTsCitation32–34 and was equal to −0.72% (95% CI = −0.52% to −0.92%), compared to placebo. Based on the abovementioned WMD and HbA1c reduction of SFU data, HbA1c reduction of DPP-4 inhibitor was estimated to be −0.75% (95% CI = −0.50% to −1.00%), compared to placebo ().
Table 3. Efficacy and adverse effect of DPP-4 inhibitors and sulfonylurea.
Adverse effects of DPP-4 inhibitors and sulfonylurea
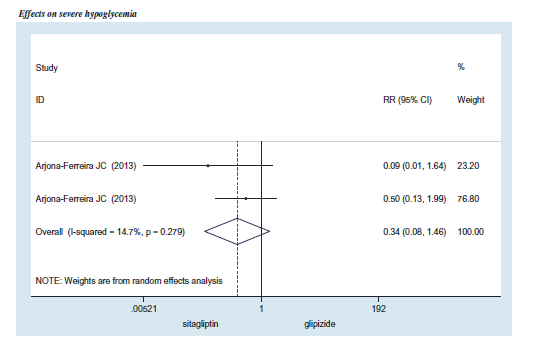
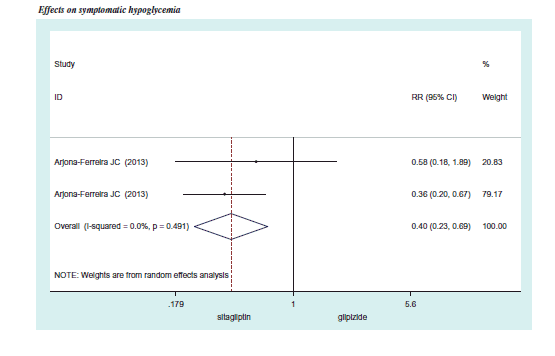
We also pooled the risk ratio (RR) of severe hypoglycemia and symptomatic hypoglycemia from three RCTsCitation32–34. The overall RR of severe hypoglycemia was 0.34 (95% CI =0.08–1.46), while the overall RR of symptomatic hypoglycemia was 0.40 (95% CI =0.23–0.69). A 1-year rate of severe hypoglycemia and symptomatic hypoglycemia of SFU monotherapy in T2DM with CKD was obtained from a retrospective hospital data analysis and the RECAP-DM studyCitation39, indicating 4.06 times/100 person-year and 37.94 times/100 person-year for severe and symptomatic hypoglycemia, respectively. We then applied the RR of severe and symptomatic hypoglycemia to calculate the rate of hypoglycemia of DPP-4 inhibitor monotherapy in T2DM with CKD. The calculation yielded the severe hypoglycemia rate of 1.36 times/100 person-year (95% CI = 0.32–5.99) and symptomatic hypoglycemia rate of 13.50 times/100 person-year (95% CI = 7.54–24.57) ().
Costs
From the Healthcare System perspective, direct medical and direct non-medical costs were supposed to be included in this study according to the Thai HTA GuidelineCitation40. However, the consensus from the first consultation panel suggested that direct non-medical costs, such as food, accommodation, and transportation, of both treatment strategies were not different in general practice for T2DM with CKD. Only direct medical costs, therefore, were included in this study. All costs were adjusted for inflation with the Thailand consumer price index (CPI)Citation41 and presented in year 2014. The costs were converted to USD at a rate of 36.08 THB per USD, as of 30 December 2015Citation42.
Direct medical costs included the cost of intervention, concurrent medications, diabetic screening, management cost, and treatment complication. The complications were composed of cardiovascular disease, renal, acute event (hypoglycemia, ketoacidosis, lactic acidosis, and edema), eye disease, neuropathy, and foot complication. Cost data were derived from the published literature and retrospective hospital database analysesCitation25–30. The details of all cost data are reported in .
The acquisition cost of DPP-4 inhibitors was calculated based on the unit costs that pharmaceutical companies proposed to the sub-committee for the development for the Development of the NLEM. A package of sitagliptin 100 mg for 28 tablets cost 1,271.16 THB or 35.23 USD. As the recommended dose was 50 mg/day, total cost of sitagliptin was then 8,285.24 THB/year (229.64 USD/year). Vildagliptin 50 mg for 56 tables cost 1,219.80 THB (33.81 USD). The recommended dose was 50 mg/day. Thus, the total cost of vildagliptin was 7,950.48 THB/year (220.36 USD/year). The cost of saxagliptin 5 mg for 28 tablets was 1,035 THB (28.69 USD). The recommended dose was 2.5 mg/day. Thus, total cost of saxagliptin was 6,745.98 THB/year (186.97 USD/year).
The cost of glipizide was derived from a national website of healthcare price from the Ministry of Public Health (www.dmsic.moph.go.th)Citation43. Because there were 14 generics of glipizide available in Thailand to date, we followed the Thai HTA GuidelineCitation40 and used a median of the median price of those 14 generics, which was 148.68 THB/year (4.12 USD/year) ().
Sensitivity analyses
To investigate the robustness of the cost-effectiveness results, various types of sensitivity analyses were performed. We conducted a series of one-way sensitivity analysis to examine the parameter uncertainty. These parameters included the change in HbA1c, the rate of hypoglycemia, the cost of DPP-4 inhibitors, and a discount rate. These parameters were varied based on the range of 95% CIs. Annual cost of DPP-4 inhibitors was assumed to be varied by 20% from their mean value. A discount rate was varied from 0–6% based on the recommendation of Thai HTA GuidelineCitation10. A threshold analysis was also conducted to determine the appropriate cost of DPP-4 inhibitors which yielded an ICER within the cost-effectiveness threshold currently used in Thailand (160,000 THB/QALY)Citation44. Finally, a probabilistic sensitivity analysis (PSA) was also performed and presented as the cost-effectiveness acceptability curve (CEAC).
Results
Base-case analysis
displays the summary findings of the study. Compared with SFU, all three DPP-4 inhibitors yielded equal 0.024 QALYs gained, but had different incremental costs. Saxagliptin incurred the lowest incremental costs, followed by vildagliptin, and sitagliptin (66,481 THB (1,842.60 USD), 80,618 THB (2,234.42 USD), and 84,547 THB (2,343.32 USD), respectively). Therefore, saxagliptin yielded the lowest ICER of 2,747,151 THB/QALY (76,140.55 USD/QALY), followed by vildagliptin (3,331,309 THB/QALY or 92,331.18 USD/QALY), and sitagliptin (3,493,661 THB/QALY or 96,830.96 USD/QALY). Compared with the Thai ceiling threshold, all DPP-4 inhibitors were not cost-effective. Drug acquisition cost was a main cost driver of the incremental cost between the DPP-4 inhibitors and SFU.
Table 4. Results of the base case analysis.
Sensitivity analyses
Of those comparisons between DPP-4 inhibitors and SFU, saxagliptin showed the lowest ICER in the base case analysis; hence, the results of one-way sensitivity analysis were based entirely on saxagliptin compared with SFU. shows that the ICER was most sensitive to the effect of DPP-4 inhibitors on HbA1c reduction, discount rate, severe hypoglycemia rate, symptomatic hypoglycemia rate, and annual cost of saxagliptin. It was found that ICERs were still above the ceiling ratio of 160,000 THB/QALY with 80% reduction in the annual cost of DPP-4 inhibitors from the base case analysis (). displays an ICER scatterplot of saxagliptin compared with SFU on a cost-effectiveness plan. Most ICERs fell in the upper left and right quadrants.
Figure 1. Tornado diagram of saxagliptin compared with sulfonylurea in type 2 diabetes with chronic kidney disease.
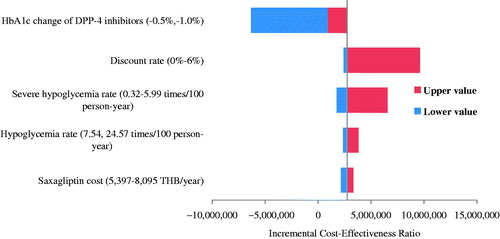
Figure 2. Threshold analysis of DPP-4 inhibitors vs sulfonylurea in type 2 diabetes with chronic kidney disease.
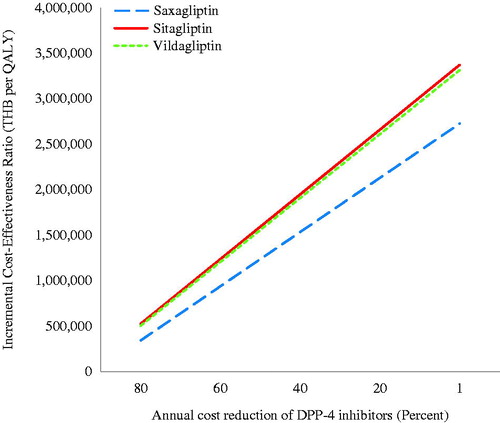
Figure 3. Incremental cost-effectiveness ratio scatterplot of saxagliptin vs sulfonylurea in type 2 diabetes with chronic kidney disease.
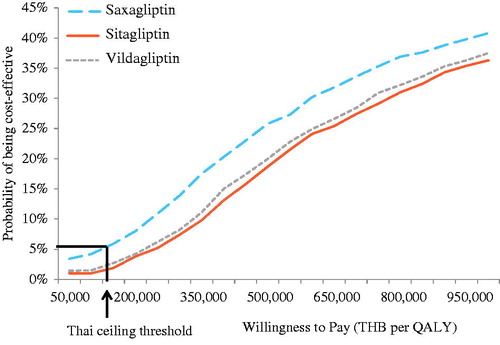
At the ceiling ratio of 160,000 THB/QALY, DPP-4 inhibitors had less than 10% of being cost-effective compared with SFU (). Saxagliptin (5.9%) had the highest probability of being a cost-effective alternative, followed by vildagliptin (2.7%), and sitagliptin (1.9%), respectively.
Discussion
T2DM treatments are costly, especially for individuals with comorbidities, such as renal impairment. This study shows the long-term costs and effectiveness of DPP-4 inhibitor monotherapy compared to SFU monotherapy in people with T2DM and CKD in the Thai context. Our analysis was based on the data obtained from a representative sample of target population of DPP-4 inhibitors in Thailand. Compared to SFU, we found that DPP-4 inhibitors was associated with improved health outcomes, i.e. greater value of 0.024 QALYs and fewer diabetes-related complications relative to SFU (RR of severe hypoglycemia = 0.34, RR of symptomatic hypoglycaemia =0.40). However, therapeutic benefits and associated cost-savings do not outweigh the higher acquisition cost of DPP-4 inhibitors. Compared to SFU, DPP-4 inhibitors were, therefore, not cost-effective at a currently used threshold in Thailand, suggesting that the use of DPP-4 inhibitors for people with T2DM and CKD in Thailand is unlikely to represent an efficient use of finite healthcare budget.
To our knowledge, our study is the first cost-effectiveness study of DPP-4 inhibitors that compared the DPP-4 inhibitor monotherapy with SFU monotherapy in people with T2DM and CKD based on a recently published systematic review of DPP-4 inhibitor for T2DMCitation45. Previous studies focused on the use DPP-4 inhibitors, including saxagliptin and sitagliptin, plus metformin with sulfonylurea plus metformin as second-line therapyCitation46–50. The conclusion of these studies all suggested that the use of DPP-4 inhibitors plus metformin as a second-line add-on therapy was cost-effective when compared with a SFU plus metformin in T2DM.
The reason how this cost-effectiveness question was raised for the CKD population deserves discussion. During a stakeholder meeting for a topic refinement, various target populations were discussed. CKD population was chosen because limited treatment options are available. DPP-4 inhibitors, therefore, have a potential role for this population. In addition, the size of population with CKD is small, making the budget implication potentially affordable from a policy decision perspective.
There are some limitations regarding the availability of data used in the study. First, the study relies on limited evidence for the efficacy parameters from our unpublished systematic review and meta-analysis, which demonstrated no statistically significant difference in terms of HbA1c reduction. Second, although this study had advantages in using hospital data of Thai people with T2DM and CKD to estimate the baseline clinical data, and severe hypoglycemia, the information on the symptomatic hypoglycemia was derived from the RECAP-DM studyCitation39, which included Thai T2DM with or without CKD who treated with SFU monotherapy. We were unable to estimate the rate of symptomatic hypoglycemia from the actual study population because people with T2DM who experienced symptomatic hypoglycemia were less likely to visit or be admitted in a hospital. Third, there were ∼52-times greater acquisition costs of DPP-4 inhibitors compared to SFU used in the study. SFU has many generics available in Thailand, while DPP-4 inhibitors are branded drugs. It is challenging for a new intervention to show value for money when comparing to an alternative that has much lower acquisition cost. However, we believe that our findings would lead to the pharmaceutical companies’ decision to reduce drug price. Moreover, some cost data, such as infected ulcer, were not available, these costs were assumed to be zero. However, we found negligible impact of these cost data on the cost-effective findings. For example, when cost of infected ulcer was assumed to be similar to uninfected ulcer, an ICER of saxagliptin compared to SFU would decrease slightly from 2,747,151 THB/QALY (76,140.55 USD/QALY) to 2,746,400 THB/QALY (76,119.73 USD/QALY). Third, utility values, transition probabilities between different health states within the CDM were mostly based on studies conducted in other countries. Without existing well-established studies in people with T2DM and CKD, we believe that we performed an extensive search in order to seek for the most credible evidence and used very conservative assumptions where information was limited. Although these parameters were not addressed in sensitivity analyses, we believe that our findings would not change due to several reasons. First, the results from the base-case analysis showed an extremely high ICER value (> 2.7 million THB) compared with the threshold value of 160,000 THB/QALY for DPP-4 inhibitor monotherapy compared with SFU monotherapy. Second, the efficacy of DPP-4 inhibitor monotherapy was not significantly different from that of SFU (WMD = −0.03, CI = −0.28 to 0.22); therefore, estimated QALY of both alternatives were not substantially different. As shown in our one-way sensitivity analyses, the main drivers of our study finding were the efficacy of DPP-4 and cost, especially drug cost.
Another major limitation of our study is that we have not taken into account the cardiovascular risk associated with the use of DPP-4 inhibitors and SFU. The concern of increased cardiovascular risk among users of these two groups of products remained unresolved, despite a large number of research attempting to investigate this issueCitation51–53. Based on this limitation, the findings of our cost-effectiveness study should be interpreted with caution.
Conclusion
Compared to SFU monotherapy, DPP-4 inhibitor monotherapy is not a cost-effective strategy for people with T2DM and CKD in the era of limited healthcare resources in Thailand. Given that the increasing prevalence of people with T2DM and CKD and high acquisition costs for DPP-4 inhibitors, the budgetary impact analysis for this population should be considered.
Transparency
Declaration of funding
This study was supported by grants from the Subcommittees of the National List of Essential Medicine, Ministry of Public Health, Thailand.
Declaration of financial/other relationships
The authors and JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors gratefully acknowledge the support of the National Drug Selection Working Group in Endocrinology for their time and suggestions, and the IMS Health team for supporting the IMS CORE Diabetes Model.
References
- Cavanaugh KL. Diabetes management issues for patients with chronic kidney disease. Clin Diabetes 2007;25:90-7
- Narenpitak S, Narenpitak A. Prevalence of chronic kidney disease in type 2 diabetes in primary health care unit of Udon Thani province, Thailand. J Med Assoc Thailand = Chotmaihet thangphaet 2008;91:1505-13
- Deerochanawong C, Ferrario A. Diabetes management in Thailand: a literature review of the burden, costs, and outcomes. Globalization Health 2013;9:11
- Ngarmukos C, Bunnag P, Kosachunhanun N, et al. Thailand diabetes registry project: prevalence, characteristics and treatment of patients with diabetic nephropathy. J Med Assoc Thailand = Chotmaihet thangphaet 2006;89 (Suppl 1):S37-S42
- Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab 2012;38:89-101
- Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes care 2011;34:1431-7
- National Kidney Foundation. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007;49 (Suppl 2):S1-S180
- Krepinsky J, Ingram AJ, Clase CM. Prolonged sulfonylurea-induced hypoglycemia in diabetic patients with end-stage renal disease. Am J Kidney Dis 2000;35:500-5
- Yki-Jarvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106-18
- Permsuwan U, Guntawongwan K, Buddhawongsa P. Handling time in economic evaluation studies. J Med Assoc Thailand = Chotmaihet thangphaet 2014;97 (Suppl 5):S50-S8
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (type 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20 (Suppl 1):S5-S26
- Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin 2004;20 (Suppl 1):S27-S40
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837-53
- Wolf PA, D’Agostino RB, Belanger AJ, et al. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312-8
- Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1989;107:237-43
- World Health Organization. Life tables by country: Thailand; 2014. http://apps.who.int/gho/data/?theme=main&vid =61640. Accessed October 15, 2014
- Aekplakorn W, Srivanichakorn S, Sangwatanaroj S. Microalbuminuria and metabolic risk factors in patients with type 2 diabetes in primary care setting in Thailand. Diabetes Res Clin Pract 2009;84:92-8
- National Statistical Office. Health; 2007. http://web.nso.go.th/en/survey/bts/datafiles/560619_09_Health.pdf. Accessed October 15, 2014
- Thamarangsi T. The situation of alcohol beaverage consumption and impact in Thailand 2013. Center for Alcohol Studies, International Health Policy Program, Bangkok: Ministry of Public Health; 2013
- Nitiyanant W, Chetthakul T, Sang-A-kad P, et al. A survey study on diabetes management and complication status in primary care setting in Thailand. J Med Assoc Thailand = Chotmaihet thangphaet 2007;90:65-71
- Supapluksakul S, Ruamviboonsuk P, Chaowakul W. The prevalence of diabetic retinopathy in Trang province determined by retinal photography and comprehensive eye examination. J Med Assoc Thailand = Chotmaihet thangphaet 2008;91:716-22
- The Endocrine Society of Thailand. Diabetes Registry Project 2003. Bangkok: Health Systems Research Institute; 2004
- Thaneerat T, Tangwongchai S. Prevalence of depression, hemoglobin A1c level, and associated factors in outpatients with type 2 diabetes. Asian Biomed (Res Rev News) 2009;3:383-90
- Maharaj Nakorn Chiang Mai Hospital. Pharmacy and Healthcare Service Fees. Chiang Mai: Maharaj Nakorn Chiang Mai Hospital; 2014
- Pornpinatepong S. Cost-effectiveness analysis of diabetic retinopathy screening in type2 diabetes mellitus [Master Thesis]. Bangkok: Mahidol University; 2005
- Riewpaiboon A. Standard cost lists for health technology assessment. Nonthaburi: Health Intervention and Technology Assessment Program (HITAP); 2011
- Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health 2007;10:61-72
- King Chulalongkorn Memorial Hospital. Pharmacy and Healthcare Service Fees. Bangkok: King Chulalongkorn Memorial Hospital; 2014
- National Health Security Office. Schedule of Health Benefits and Fees. Bangkok: National Health Security Office; 2014
- Russo E, Penno G, Prato SD. Managing diabetic patients with moderate or severe renal impairment using DPP-4 inhibitors: focus on vildagliptin. Diabetes Metab Syndr Obes 2013;6:161-70
- Arjona-Ferreira JC, Corry D, Mogensen CE, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis 2013;61:579-87
- Arjona-Ferreira JC, Marre M, Barzilai N, et al. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes care 2013;36:1067-73
- Chan JCN, Scott R, Arjona-Ferreira JC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab 2008;10:545-55
- Scheen AJ. A review of gliptins in 2011. Expert Opin Pharmacother 2012;13:81-99
- Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab 2011;13:7-18
- Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs 2011;71:1441-67
- Craddy P, Palin HJ, Johnson KI. Comparative effectiveness of dipeptidylpeptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison. Diabetes Ther 2014;5:1-41
- Chan SP, Ji LN, Nitiyanant W, et al. Hypoglycemic symptoms in patients with type 2 diabetes in Asia-Pacific: Real-life effectiveness and care patterns of diabetes management: The RECAP-DM study. Diabetes Res Clin Pract 2010;89:e30-2
- Riewpaiboon A. Measurement of costs for health economic evaluation. J Med Assoc Thailand = Chotmaihet thangphaet 2014;97 (Suppl 5):S17-S26
- Bureau of Trade and Economics Indices, Ministry of Commerce. CPI; 2016. www.price.moc.go.th/price/cpi/index_new_e.asp. Accessed July 8, 2016
- Bank of Thailand. Foreign exchange rates; 2015. https://www.bot.or.th/english/statistics/financialmarkets/exchangerate/_layouts/application/exchangerate/ExchangeRate.aspx. Accessed February 11, 2016
- Drug and Medical Supply Information Center; 2015]. http://dmsic.moph.go.th. Accessed April 15, 2016
- Thavorncharoensap M, Teerawattananon Y, Natanant S, et al. Estimating the willingness to pay for a quality-adjusted life year in Thailand: does the context of health gain matter? Clinicoecon Outcomes Res 2013;5:29-36
- Geng J, Yu H, Mao Y, et al. Cost-effectiveness of dipeptidyl peptidase-4 inhibitors for type 2 diabetes. PharmacoEconomics 2015;33:581-97
- Bergenheim K, Williams SA, Bergeson JG, et al. US cost-effectiveness of saxagliptin in type 2 diabetes mellitus. Am J Pharm Benefits 2012;4:20-8
- Elgart JF, Caporale JE, Gonzalez L, et al. Treatment of type 2 diabetes with saxagliptin: a pharmacoeconomic evaluation in Argentina. Health Econ Rev 2013;3:11
- Erhardt W, Bergenheim K, Duprat-Lomon I, et al. Cost effectiveness of saxagliptin and metformin versus sulfonylurea and metformin in the treatment of type 2 diabetes mellitus in Germany: a Cardiff diabetes model analysis. Clin Drug Investig 2012;32:189-202
- Granstrom O, Bergenheim K, McEwan P, et al. Cost-effectiveness of saxagliptin (Onglyza(R)) in type 2 diabetes in Sweden. Prim Care Diabetes 2012;6:127-36
- Schwarz B, Gouveia M, Chen J, et al. Cost-effectiveness of sitagliptin-based treatment regimens in European patients with type 2 diabetes and haemoglobin A1c above target on metformin monotherapy. Diabetes Obes Metab 2008;10(Suppl1):43-55
- Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2014;24:689-97
- Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2013;15:938-53
- Patil HR, Al Badarin FJ, Al Shami HA, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol 2012;110:826-33
Appendix
Systematic review and meta-analysis of dipeptidyl peptidase-4 inhibitor monotherapy and sulfonyl urea monotherapy for people with type 2 diabetes and chronic kidney disease in Thailand
Method
Data sources and searching strategy
The following databases were systematically searched: MEDLINE, EMBASE, and Clinicaltrial.gov. Databases were searched from their inception to August 12, 2014. For the search strategy, we used
(“new user” OR “drug-naive” OR “Monotherapy”) AND (Sulfonylurea OR gliclazide OR glyburide OR glibenclamide OR glipizide OR glimepiride OR glycopyramide OR glisoxipide OR gliquidone OR glibornuride OR carbutamide OR acetohexamide OR chlorpropamide OR tolbutamide) AND (“Dipeptidyl-Peptidase IV Inhibitors” OR sitagliptin OR saxagliptin OR vildagliptin OR linagliptin OR anagliptin OR teneligliptin OR alogliptin)
References of initially identified articles will be further examined to identify additional studies that met the selection criteria.
Study selection
We included studies that: (i) compared monotherapy of sulfonylurea vs DPP-4 Inhibitors, (ii) reported the number (or percentage with total number of patients) of outcomes, and (iii) studied humans. There was no language restriction. Abstracts/titles and full-text articles were reviewed by two independent reviewers. We excluded studies that were not original articles such as comments, letters, reviews, meta-analyses, guidelines, case reports, surveys, or editorials. Studies from the same population (duplicate studies), and studies not reporting effect-estimates or with insufficient information to compute effect estimates were also excluded.
Results
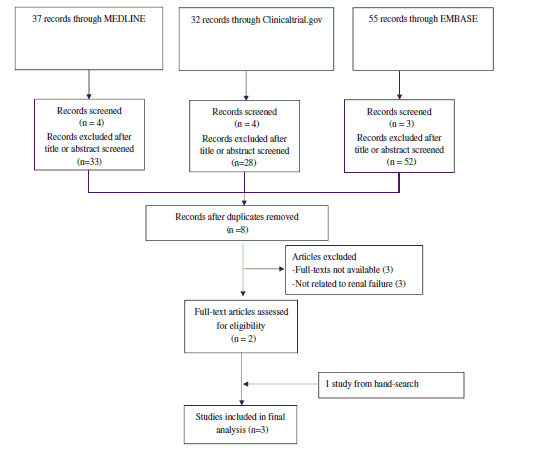
Flow diagram of study selection.