Abstract
Objective: This analysis estimated the cost-effectiveness of intravitreal aflibercept injection(s) (IAI) for wet age-related macular degeneration (wAMD) compared with other treatments in Japan.
Methods: This was a cost-utility analysis based on published data. A state-transition cohort model was constructed with six health states based on best-corrected visual acuity in the better-seeing eye. The cycle time was 4 weeks, and the time horizon was 12 years. The model compared IAI 2 mg every 8 weeks (2q8) for 2 years after three initial monthly injections, ranibizumab as needed, ranibizumab 0.5 mg every 4 weeks (0.5q4), pegaptanib sodium 0.3 mg every 6 weeks, verteporfin photodynamic therapy (PDT), and best supportive care, assumed to include medical management and monitoring, but no active therapy. Costs (expressed as Japanese yen [JPY]) and quality-adjusted life years (QALYs) gained were estimated for each treatment and discounted at 2.0%. Input data were obtained from clinical studies, the Japanese drug tariff and social insurance reimbursement schedule, and expert opinion. The analysis was conducted from the societal perspective, including medical costs as well as costs of blindness.
Results: IAI 2q8 was dominant (i.e. more effective in terms of QALYs and less costly) to all other comparators (ranibizumab as needed, ranibizumab 0.5q4, pegaptanib sodium, PDT, and best supportive care), as shown by the incremental cost-utility ratio (i.e. cost per QALY gained).
Limitations: The strengths of the analysis include the wide range of comparators evaluated and the use of Japanese-specific utility data. The limitations include the use of one eye, inclusion of published data up to 2 years only, and assumptions on disease course over 5 years.
Conclusions: IAI 2q8 was more effective in terms of QALYs and less costly compared with other treatments for wAMD in Japan.
Introduction
Current estimates from population-based studies in Japan suggest that the prevalence of early wet age-related macular degeneration (wAMD), a leading cause of blindness in developed countries, is comparable to that observed in white populationsCitation1,Citation2. In 2007 it was estimated (based on survey data of persons with disability, national census materials, and official population projections) that 1.64 million people in Japan had significant visual impairment, and 10.9% of this was attributable to AMDCitation3. The prevalence of wAMD is also predicted to increase dramatically to 113 million in Asia by 2040Citation4. It is, therefore, of utmost importance to identify cost-effective approaches to wAMD treatment throughout this region.
Over the past decade, anti-vascular endothelial growth factor (VEGF) agents have become a mainstay of treatment for patients with wAMDCitation5–8. These agents are known to target VEGF, a key factor associated with the angiogenic cascade that is critically linked to choroidal neovascularization in wAMDCitation9,Citation10.
The anti-VEGF agent intravitreal aflibercept (VEGF Trap-Eye; Eylea [Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA]) was approved for the treatment of wAMD in Japan in 2012. Two large-scale, international, randomized studies (VIEW 1/2), which were undertaken in 2419 patients with wAMD, showed that intravitreal aflibercept injection(s) (IAI 0.5 mg or 2 mg every 4 weeks, or 2 mg every 8 weeks [2q8] after three initial monthly injections) were clinically equivalent and non-inferior to intravitreal ranibizumab (0.5 mg every 4 weeks [0.5q4]) based on the primary efficacy outcome (loss of <15 letters at week 52)Citation11–13. Ocular and non-ocular adverse events were comparable across treatment groupsCitation11–13. These findings were also observed in the Japanese sub-population of the VIEW 2 study; the mean changes in best-corrected visual acuity (BCVA) were 10.0 letters (IAI 2q8; n = 24) and 9.4 letters (ranibizumab; n = 25) at week 52Citation14. However, there is a lack of studies examining the cost-effectiveness of IAI compared with ranibizumab or other treatments approved for use in wAMD in Japan.
The objective of the current study was to perform a comprehensive cost-effectiveness analysis of IAI 2q8 in the better-seeing eye of patients with wAMD in Japan compared with other treatments, including ranibizumab 0.5q4 or as needed, pegaptanib sodium, photodynamic therapy (PDT) with verteporfin, and best supportive care.
Methods
Overview
The analysis used a state-transition cohort model with six health states. Patients could move between health states at each cycle according to transition probabilities, which were based on outcomes observed in major studies. The model was an adaption of one submitted to the National Institute for Health and Care ExcellenceCitation15 assessment. The analysis was conducted from a societal perspective, and included medical costs as well as costs of blindness.
Population
The patient population included adults with sub-foveal choroidal neovascularization associated with wAMD, which was the population included in the VIEW studiesCitation11–13. The distribution of BCVA in the model was taken from the baseline distribution of BCVA in the study eye in the VIEW studies, and was as follows: no visual impairment, 0.0%; mild visual impairment, 24.0%; moderate visual impairment, 45.0%; severe visual impairment, 31.0%; and blind, 0.0%Citation11–13. The patient starting age in the model was 77 years, which was taken from the mean value in the VIEW studiesCitation11–13.
Model structure and assumptions
The health states used in the model were based on the BCVA (decimal notation) in the better-seeing eye: no visual impairment (BCVA 0.8–1.0); mild visual impairment (BCVA 0.4–0.8); moderate visual impairment (BCVA 0.2–0.4); severe visual impairment (BCVA 0.05–0.2); blindness (BCVA ≤0.05); and death (). The BCVA health states were developed based on a number of simplifying assumptions, which were consistent with previous publicationsCitation16–27. All patients were assumed to have identical visual acuity within each health state. This assumption of homogeneity within a health state is a property of Markov modelsCitation16, and has been previously used in wAMDCitation17. The model assumed that all patients who gained or lost three lines of vision transitioned to an adjacent vision state. Three-line vision changes are generally considered clinically significantCitation18, and are typically reported outcomes in randomized studies of wAMD treatmentCitation19,Citation20. The health states were defined according to vision in the better-seeing eye; this reflects clinical practice and previous models used in wAMDCitation21–27.
Figure 1. Model schema. Visual acuity in each health state was described with decimal notation. The arrows indicate patient movement between any two health states. The cycle was 4 weeks. BCVA, best-corrected visual acuity.
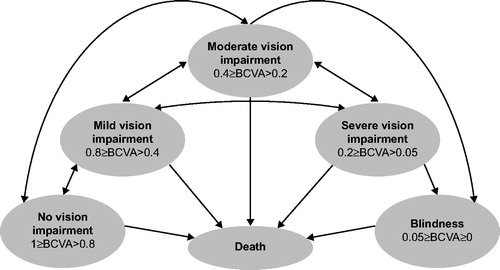
The model was also broadly condensed into three distinct phases: an efficacy phase for the first and second years (i.e. it was assumed patients received active treatment and all visual gains were observed during this phase), a maintenance phase for the third, fourth and fifth years (i.e. patients received less intense treatment to maintain visual acuity) and a rest of life phase at 6 years onward (i.e. patients no longer received active treatment). Within each health state a patient could be on or off treatment. Off treatment was defined as either discontinuation at any phase or no further treatment after the rest of life phase. Patients were also assumed to discontinue treatment when they reached the blindness state; this was consistent with previous modelsCitation18.
The time horizon of the model was 12 years in the base case, based on the difference between the mean age of the base case population (77 years) and the mean age of death for a Japanese person (difference of 10.26 years for men aged 77 years and 13.68 years for women aged 77 years)Citation28, weighted to reflect the sex distribution in the model. Mortality was not incorporated into this time horizon, as this would double the death count. The cycle time was set to 4 weeks, so as to model at a frequency at least as great as any treatment in the VIEW studies. Costs were expressed in Japanese yen (JPY). Costs and outcomes were discounted at 2.0% per year in accordance with the Japanese guideline for health economic evaluationCitation29.
Interventions
The modeled intervention was the IAI-approved dosing regimen of 2q8 after initiation with three monthly injections. The primary comparator was ranibizumab as needed, assumed to be administered by 0.5 mg intravitreal injection monthly until signs of active neovascularization had disappeared. Other comparators were ranibizumab 0.5q4, pegaptanib sodium 0.3 mg every 6 weeks, PDT and verteporfin every 4 months, and best supportive care, which was assumed to include medical management and monitoring but no active therapy. The ranibizumab regimens (as needed or every 4 weeks after the loading phase) reflect the approved indication in Japan.
Outcomes
The model estimated total and incremental costs, total and incremental quality-adjusted life years (QALYs) gained, incremental cost-utility ratio (ICUR) expressed as incremental cost per QALY gained, net monetary benefit (NMB), and blind years (i.e. total time spent with blindness). The NMB is a linear expression of ICUR in that it places a threshold ratio with an assumption of a willingness to pay (WTP) of JPY 5,000,000/QALY (US $48,639/QALY or €43,596 [www.XE.com 2016 values]), which is frequently referred to as a threshold in JapanCitation30,Citation31.
Input data
Transition probabilities, discontinuation rates, and the change in visual acuity associated with the natural history of wAMD used in the model were taken from the VIEW studies and published sources (Supplemental Table 1)Citation11,Citation12,Citation15,Citation20,Citation32–35. Although this study included five comparators, the analyses were always conducted between IAI and one comparator only. Transition probabilities of IAI and ranibizumab 0.5q4 for the first 2 years were derived from a linear interpolation of the outcomes from the VIEW studies. As no direct comparative data on treatment efficacies between IAI and other comparators were available, relative risks from the indirect treatment comparisonCitation36 were used to obtain the efficacies, in terms of transition probabilities, of each treatment with respect to IAI. The transition probabilities of gaining three lines in year 1 were 26.20% (IAI), 25.25% (ranibizumab 0.5q4), 22.01% (ranibizumab as needed), 13.49% (pegaptanib sodium), 1.86% (PDT), and not applicable (best supportive care). The corresponding transition probabilities of losing three lines in year 1 were 4.27%, 5.54%, 5.30%, 17.39%, 26.90%, and 6.52%, respectively.
Given that no direct comparative data on treatment discontinuation rates were available, these were assumed to be equal for all treatments for the first 2 years of treatment, and were taken from the VIEW studies; the discontinuation annual rates were 8.22% (year 1) and 6.24% (year 2). Since study data were not available for discontinuation rates in the maintenance stage (year 3 to year 5), the input values for these years were based on expert opinion, which consisted of feedback from the authors (YY, with 20 years of engaging in clinical research in the field of AMD, and having authored/co-authored more than100 publications) and their colleagues according to their clinical experience, or were taken from published sourcesCitation15. In the base case scenario, it was assumed that, during the maintenance stage (year 3 to year 5), patients remained in the same health state that they were in at the end of year 2, unless they discontinued treatment. At year 6, all patients discontinued active treatment and followed the natural history of visual acuity in wAMD. The values for monthly visual acuity changes (defined as gain or loss of three or six lines and no change) in patients following the natural history of wAMD are presented in Supplemental Table 1. These values were taken from a meta-analysis of 53 studies reporting outcomes in untreated wAMD patients over 36 monthsCitation35. Adverse event rates for each treatment and the associated reduction in utility were taken from published sources (Supplemental Table 2)Citation11,Citation12,Citation19,Citation32,Citation33,Citation37,Citation38. The proportions of patients with intraocular inflammation were 0% (IAI, ranibizumab 0.5q4, pegaptanib sodium), 0.2% (ranibizumab as needed), and 3.5% (PDT), and the proportions of patients with endophthalmitis were 0% (IAI, PDT), 0.40 (ranibizumab as needed), 0.46 (ranibizumab 0.5q4), and 1.30 (pegaptanib sodium).
Table 1. Base case results for costs, quality-adjusted life years, and incremental cost-utility ratio.
The number of injections required for the first 2 years of treatment was taken from studies and published sources (Supplemental Table 3)Citation11,Citation12,Citation32–34. The mean numbers of injections in year 1 were 7 (IAI), 12 (ranibizumab 0.5q4), 6.9 (ranibizumab as needed), 9 (pegaptanib sodium), and 3 (PDT with verteporfin). The corresponding values in year 2 were 4.5, 4.8, 5.7, 9, and 3, respectively. In the absence of data, the number of injections in the maintenance stage and the number of monitoring visits (physician visits and tests) for each treatment regimen were assumed based on expert opinion. Patients who discontinued treatment before the end of the modeled treatment duration moved to best supportive care for the remaining treatment duration. After the end of the treatment period, all patients progressed according to the natural history of the disease, with no further monitoring costs included.
Pharmacy cost data, costs for monitoring, and adverse events were obtained from the Japanese drug tariffCitation39 and the social insurance reimbursement scheduleCitation40 of the fee-for-service system in Japan. The reimbursement schedule covered direct medical costs only. Work productivity loss was not included because most patients were of retirement age. The cost (JPY) of treatment (per vial) was 159,289 (IAI), 176,235 (ranibizumab), 123,457 (pegaptanib sodium), and 182,450 (PDT). The monitoring costs (JPY) ranged from 480 (slit lamp microscopy) to 4476 (fluorescein angiography) (2012 prices).
Societal cost included the annual costs of blindness only and was, therefore, a conservative estimate. These costs were calculated using the methodology of a published study (Supplemental Table 4)Citation39–41. Briefly, the annual costs were calculated as the ratio of support by family members to non-family members using a score of 1 for full care and 0.5 for partial care for each activity of daily living. The ratio was originally calculated as 3.9:1, with a total annual cost of JPY 903,822Citation41. In the current analysis, the ratio was updated to 4.7:1, with an updated annual cost of JPY1,053,618; this was re-calculated using updated dataCitation42.
Utility data for patients with wAMD in Japan were based on a published study, which provided utility values in the better-seeing eye of 0.534 (BCVA: 0.01–0.15), 0.574 (0.2–0.3), 0.613 (0.4–0.6), and 0.653 (0.7–1.0) in a Japanese population; these were derived using a time-trade-off (TTO) approach, which is an established method used in other published studiesCitation43–45. Unfortunately, these data could not be used directly, as the visual acuity categories in the study did not align with those in the current model. The utility estimates were, therefore, derived via two methods (method 1 and 2). In method 1, a predictive model was applied to the data from the previous studyCitation43 to estimate a relationship between visual acuity and utility. Utility values and the midpoint of the visual acuity group for which they were estimated were taken from the study, and a regression model fitted to the utility estimates. Method 2 extended this by conducting a pooled regression analysis of the Japanese data and data from other studiesCitation46–48 to estimate a relationship between visual acuity and utility. An additional variable was included in the regression model to control for unobserved characteristics in the other studies and to predict utility values for a Japanese population. The relationship identified by the regression model was then used to predict utility values for each of the visual acuity health states in the model. The utility estimates derived from method 2 were used in the base case, and the estimates derived from method 1 were used in sensitivity analysis. Supplemental Table 5 shows the utility estimates derived from each method.
Sensitivity analysis
Deterministic one-way sensitivity analysis was used to test the effect of uncertainty in individual parameter values. Parameters considered were: (1) the number of ranibizumab injections; (2) treatment maintenance from year 3 to year 5; (3) estimated utility values (the utility data derived from method 1); (4) discontinuation rate; (5) analysis time horizon; (6) cost of blindness; (7) adverse event rate; (8) patient age; and (9) the number of PDT injections (Supplemental Table 6). To align with the VIEW studies, a life expectancy time horizon of 12 years was used in the base case (as described earlier). However, the model was also capable of extrapolating analyses up to 25 years (lifetime) based on these data inputs. When the analysis time horizon was changed from 12 years (base case) to 25 years (one-way sensitivity analysis), mortality was considered into the analysis. Patients could die in any health state in the model. As mortality data from studies were not available for all treatments, background Japanese mortality was used throughout the model, taken from the full Japanese life tables published in 2010Citation28 and adjusted to cycle length. Probabilistic sensitivity analysis was used to test the effect of simultaneously varying the model inputs. The distributions used are shown in Supplemental Table 7, with standard errors calculated as 20% of the baseline input.
Results
Base case
IAI 2q8 was associated with lower total costs for all cost components than any of the comparators in the model (). IAI 2q8 also resulted in improved outcomes (i.e. higher estimated number of QALYs gained) than any of the alternative treatments considered in the study, although differences among the IAI 2q8, ranibizumab as needed, and ranibizumab 0.5q4 groups were small (). Thus, IAI 2q8 was dominant (i.e. better health outcome at lower cost) over all comparators tested in this analysis. Supplemental Figure 1 shows the total time spent with blindness. As expected, patients receiving best supportive care spent more time blind than those receiving active treatment. Among patients receiving active treatment, ranibizumab (both regimens) and IAI 2q8 were associated with fewer blind years; this ranged from 6.1 (best supportive care) to 2.9 (ranibizumab as needed or 0.5q4) and 2.8 (IAI) blind years.
Sensitivity analysis
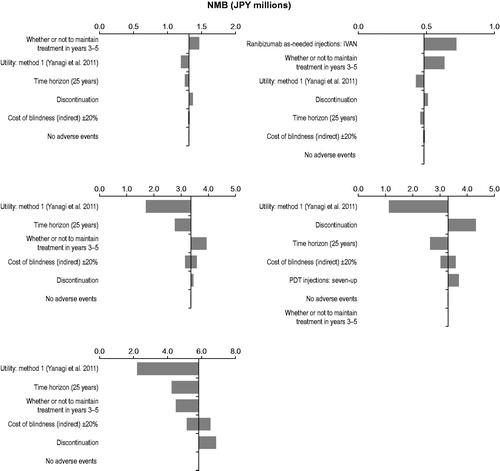
The results of the one-way sensitivity analysis for IAI 2q8 compared with the other five comparators are shown in the tornado plots in . Since IAI 2q8 was either dominant or cost-effective with the assumed WTP value of JPY 5,000,000/QALY to each comparator, NMB was used, instead of ICUR, as a measure in the tornado plots. The parameters with the greatest impact on the results for ranibizumab were the number of as-needed ranibizumab injections, use of active treatments during the maintenance phase (year 3 to year 5), and utility values. Unlike in the two ranibizumab cases, the utility value parameter had the largest impact on results when IAI 2q8 was compared with pegaptanib sodium, PDT with verteporfin, and best supportive care, followed by length of analysis time horizon and discontinuation rate.
Figure 2. Tornado plots of one-way sensitivity analyses for IAI 2 mg every 8 weeks (after three initial monthly injections) compared with (top left) ranibizumab 0.5 mg every 4 weeks, (top right) ranibizumab as needed, (middle left) pegaptanib sodium 0.3 mg every 6 weeks, (middle right) PDT with verteporfin, and (bottom) best supportive care. IAI, intravitreal aflibercept injection(s); JPY, Japanese yen; NMB, net monetary benefit; PDT, photodynamic therapy.
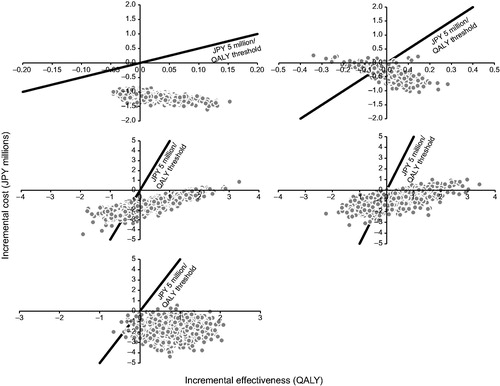
The results of the probabilistic sensitivity analyses for IAI 2q8 compared with the other five comparators are shown as scatter plots in . This analysis showed that IAI 2q8 was highly likely to be cost-saving (range = 88.1–100%). With the assumed WTP value of JPY 5,000,000/QALY as a threshold, IAI 2q8 was also highly likely to be cost-effective (range = 80.4–100%).
Figure 3. Scatter plots of probabilistic sensitivity analyses for IAI 2 mg every 8 weeks (after three initial monthly injections) compared with (top left) ranibizumab 0.5 mg every 4 weeks, (top right) ranibizumab as needed, (middle left) pegaptanib sodium 0.3 mg every 6 weeks, (middle right) PDT with verteporfin, and (bottom) best supportive care. IAI, intravitreal aflibercept injection(s); JPY, Japanese yen; PDT, photodynamic therapy; QALY, quality-adjusted life year.
Discussion
In this analysis, IAI 2q8 dominated all comparators, including ranibizumab as needed or 0.5q4, pegaptanib sodium, PDT with verteporfin, and best supportive care. IAI 2q8 was both more effective in terms of QALYs and less costly than any of these alternative treatments. This is the first economic evaluation of IAI in wAMD in Japan. A previous cost-utility analysis that compared ranibizumab as needed, pegaptanib sodium, PDT, and best supportive care in wAMD in JapanCitation41 reported that ranibizumab and PDT were less costly and more effective than best supportive care, which is consistent with the results presented here. Similar outcomes were observed in other healthcare systems, such as Germany and GreeceCitation49–51. The present analysis enhances our understanding about cost-effectiveness of anti-VEGF treatments in Japan, building on earlier studies by including an additional treatment, IAI. Although previous cost-effectiveness analyses of wAMD treatments are available, published research on cost-effectiveness of IAI is limitedCitation21. From the Dutch societal perspective, which accounted for home care and transportation costs due to the disease, IAI was found to be an equally effective but less costly treatment over ranibizumab as needed and ranibizumab every 4 weeks, which is in line with the results of this analysisCitation52. Another analysis in Sweden, which compared IAI bi-monthly with ranibizumab 0.5q4 or as needed based on a better-seeing eye model using the VIEW population, also found that IAI had similar efficacy to ranibizumab but was more cost-effective, with an incremental cost-effectiveness ratio of 27,000 SEK/QALY gainedCitation44. Similar findings were observed with a Greek model that compared IAI with ranibizumabCitation53.
An important difference between IAI and ranibizumab is the reduction in monitoring visits with IAI, as monthly monitoring is stipulated for patients receiving ranibizumab as neededCitation54. This contributes to the cost savings with IAI, although in the present analysis the lower drug cost for IAI was the largest source of cost savings. With the other three comparators, pegaptanib sodium, PDT with verteporfin, and best supportive care, IAI had lower blindness costs, corresponding with lower number of blind years; these were the main contributors for IAI being a cost-saving treatment option, with the largest QALY value among them.
The tendency of IAI to be either dominant or more cost-effective than each of five comparators did not change when uncertainties in several model parameters were taken into account, indicating that the results were robust. Even if we accounted for the costs of occasional injections to maintain the visual acuity until 5 years after treatment initiation, IAI was a cost-effective treatment choice. This could also encourage patients to maintain their treatment with IAI. Additional strengths of the present study include the wide range of comparators evaluated and the use of Japanese-specific utility data.
However, the analysis has some limitations that are shared by health economics research in the field of ophthalmology. First, the model included only one eye and, as noted in previous cost-effectiveness analyses in wAMDCitation52, this is an important aspect of economic modeling in this disease area. This analysis was based on BCVA in the better-seeing eye; calculating utilities based only on BCVA in the better-seeing eye is likely to under-estimate the impact of vision impairment, particularly when the better-seeing eye has no or little visual acuity loss and the worse-seeing eye is moderately-to-severely visually impaired. Second, availability of published data to populate the model was limited after year 2. Some model inputs in the maintenance phase were also based on expert opinion, which is not an ideal approach; however, given the results of sensitivity analyses, the baseline results are still robust, despite any inherent variability with some input values. Third, this analysis only included societal costs following the transition to blindness. Societal costs were, therefore, conservative for IAI; for example, this analysis did not include direct non-medical and intangible costs related to clinic visits, which could increase transportation costs and the utility burden to ranibizumab with more frequent visits than IAI. Fourth, the model assumed that all patients would no longer receive active treatment and that their visual acuity decreased per natural history with age after 5 years, which may not reflect real-world clinical practice. Last, the current model was adapted from one submitted to the National Institute for Health and Care Excellence assessment, and did not include several large-scale studies (such as HARBOR, IVAN, and GEFAL). It did not include bevacizumab and combination therapy using PDT and anti-VEGF agents for the same reason. Of note, bevacizumab is rarely used in Japan. Future research using real-world data for treatment of wAMD in Japan, such as the evolving treat-and-extend regimen, would be valuable to explore the duration of treatment in routine clinical practice and reflect its impact on costs and outcomes in a cost-effectiveness study. There is also some question about how well BCVA predicts utilities and whether other metrics, such as contrast sensitivity, may be relevant. At this point, there are limited data to populate a model using contrast sensitivity, but this may be interesting to include when more data are available.
In addition to cost savings, the reduction in the number of injections required with IAI, compared with ranibizumab, in the VIEW studiesCitation11–13 may provide an additional benefit to patients by reducing the burden of treatment. In a qualitative study, patients with wAMD regarded intravitreal injections as invasive and problematic treatments, and as something that had to be “endured”Citation55. The present analysis did not capture any beneficial effects to patients of reducing the burden of injections, as the utility values used in the model were based on visual acuity. Exploring the value that patients place on a reduction in injection number could be an interesting area for future research.
Conclusion
In conclusion, IAI 2q8 was more effective in terms of QALYs and less costly compared with other widely available treatments for wAMD in Japan.
Transparency
Declaration of funding
This study was performed by IMS Health, UK, which was funded by Bayer Pharmaceuticals, Japan.
Declaration of financial/other relationships
YY has received honoraria, consultancy, and travel expenses to present preliminary results of this study in the Japan Clinical Ophthalmology Annual Congress from Bayer Pharmaceuticals. AF and KA are employees of Bayer Pharmaceuticals. VB is an employee of IMS, which was contracted by Bayer Pharmaceuticals to perform the analyses. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Presented at the 69th Annual Congress of Japan Clinical Ophthalmology, Nagoya, Japan, October 22, 2015.
Supplemental_material.docx
Download MS Word (43.7 KB)Acknowledgments
Medical writing assistance was provided by Carole Nadin (UK) on behalf of IMS. Editorial assistance was provided by PAREXEL (UK); both were funded by Bayer Pharmaceuticals. The data included in this manuscript are not in the current scope of clinical study data sharing according to Bayer’s transparency policy. This scope was carefully defined based on the EFPIA and PhRMA principles on responsible clinical study data sharing.
References
- Kawasaki R, Wang JJ, Ji GJ, et al. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population: the Funagata study. Ophthalmology 2008;115:1376-81
- Nakata I, Yamashiro K, Nakanishi H, et al. Prevalence and characteristics of age-related macular degeneration in the Japanese population: the Nagahama study. Am J Ophthalmol 2013;156:1002-9
- Yamada M, Hiratsuka Y, Roberts CB, et al. Prevalence of visual impairment in the adult Japanese population by cause and severity and future projections. Ophthalmic Epidemiol 2010;17:50-7
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106-e116
- Kulkarni AD, Kuppermann BD. Wet age-related macular degeneration. Adv Drug Deliv Rev 2005;57:1994-2009
- Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs 2008;68:1029-36
- Kovach JL, Schwartz SG, Flynn HW Jr, et al. Anti-VEGF treatment strategies for wet AMD. J Ophthalmol 2012;2012:786870
- Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:844-51
- Ablonczy Z, Dahrouj M, Marneros AG. Progressive dysfunction of the retinal pigment epithelium and retina due to increased VEGF-A levels. FASEB J 2014;28:2369-79
- Ho QT, Kuo CJ. Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol 2007;39:1349-57
- Heier JS, VIEW 1 Investigators, VIEW 2 Investigators. 96 weeks results from the VIEW 1 and VIEW 2 studies: intravitreal aflibercept injection versus ranibizumab for neovascular AMD shows sustained improvements in visual acuity. Presented at Association for Research in Vison and Ophthalmology (ARVO) Fort Lauderdale, FL; 2012
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537-48
- Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 2014;121:193-201
- Ogura Y, Terasaki H, Gomi F, et al. Efficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 study. Br J Ophthalmol 2015;99:92-7
- National Institute for Health and Clinical Excellence. Macular degeneration (wet age-related) – aflibercept (TA294). Lindon, UK. http://guidance.nice.org.uk/TA294. Accessed October 14, 2016
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford, UK: Oxford University Press, 2005
- Hurley SF, Matthews JP, Guymer RH. Cost-effectiveness of ranibizumab for neovascular age-related macular degeneration. Cost Eff Resour Alloc 2008;6:12
- Colquitt JL, Jones J, Tan SC, et al. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess 2008;12:iii-201
- Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116:57-65
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-31
- Schmier JK, Hulme-Lowe CK. Cost-effectiveness models in age-related macular degeneration: issues and challenges. Pharmacoeconomics 2016;34:259-72
- Hernandez-Pastor LJ, Ortega A, Garcia-Layana A, et al. Cost-effectiveness of ranibizumab compared with photodynamic treatment of neovascular age-related macular degeneration. Clin Ther 2008;30:2436-51
- Hopley C, Salkeld G, Mitchell P. Cost utility of photodynamic therapy for predominantly classic neovascular age related macular degeneration. Br J Ophthalmol 2004;88:982-7
- Meads C, Salas C, Roberts T, et al. Clinical effectiveness and cost-utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess 2003;7:v-98
- Earnshaw SR, Moride Y, Rochon S. Cost-effectiveness of pegaptanib compared to photodynamic therapy with verteporfin and to standard care in the treatment of subfoveal wet age-related macular degeneration in Canada. Clin Ther 2007;29:2096-106
- Javitt JC, Zlateva GP, Earnshaw SR, et al. Cost-effectiveness model for neovascular age-related macular degeneration: comparing early and late treatment with pegaptanib sodium based on visual acuity. Value Health 2008;11:563-74
- Neubauer AS, Holz FG, Schrader W, et al. [Cost-utility analysis of ranibizumab (Lucentis) in neovascular macular degeneration]. Klin Monbl Augenheilkd 2007;224:727-32
- Japanese Government Ministry of Health Labour and Welfare. The 21st Life Tables. Statistics and Information- Department Minister's Secretariat. Tokyo, Japan. 2010 http://www.mhlw.go.jp/english/database/db-hw/lifetb21th/dl/data.pdf. Accessed October 14, 2016
- Guideline for economic evaluation of healthcare technologies in Japan. Tokyo: Research group on economic evaluation for Japanese public medical benefits Website, 2013. http://hta.umin.jp/guideline_e.pdf. Accessed October 14, 2016
- Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 2010;19:422-37
- Shiroiwa T, Igarashi A, Fukuda T, et al. WTP for a QALY and health states: more money for severer health states? Cost Eff Resour Alloc 2013;11:22
- Brown A, Hodge W, Kymes SM. Management of neovascular age-related macular degeneration: systematic drug class review and economic evaluation. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health, 2008
- Gragoudas ES, Adamis AP, Cunningham ET Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-16
- Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-98
- Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 2008;115:116-26
- Lang S, Kleijnen J, Noake C. Indirect comparisons of aflibercept with ranibizumab PRN and bevacizumab PRN in wet age-related macular degeneration: a report for Bayer. York, UK: Kleijnen Systematic Review Ltd, 2012
- Boyer DS, Heier JS, Brown DM, et al. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology 2009;116:1731-9
- Brown GC, Brown MM, Brown HC, et al. A value-based medicine comparison of interventions for subfoveal neovascular macular degeneration. Ophthalmology 2007;114:1170-8
- National Health Insurance Drug Price Standard. Tokyo: Jiho, Inc., 2012
- Social Insurance Research Laboratory. Chiyoda-ku, Tokyo: Reimbursement Schedule of Social Insurance. 2012
- Yanagi Y, Aihara Y, Fukuda T, et al. [Cost-effectiveness of ranibizumab, photodynamic therapy and pegaptanib sodium in the treatment of neovascular age-related macular degeneration in Japanese]. Nippon Ganka Gakkai Zasshi 2011;115:825-31
- Cabinet Office, Governmet of Japan. Chiyoda-ku, Tokyo 2011 http://www8.cao.go.jp/shougai/data/data_h26/zuhyo55.html. Accessed October 14, 2016
- Yanagi Y, Ueta T, Obata R, et al. Utility values in Japanese patients with exudative age-related macular degeneration. Jpn J Ophthalmol 2011;55:35-8
- Panchmatia HR, Clements KM, Hulbert E, et al. Aflibercept vs. Ranibizumab: cost-effectiveness of treatment for wet age-related macular degeneration in Sweden. Acta Ophthalmol 2016;94:441-8
- Czoski-Murray C, Carlton J, Brazier J, et al. Valuing condition-specific health states using simulation contact lenses. Value Health 2009;12:793-9
- Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc 1999;97:473-511
- Brown MM, Brown GC, Sharma S, et al. Utility values associated with blindness in an adult population. Br J Ophthalmol 2001;85:327-31
- Sharma S, Brown GC, Brown MM, et al. Validity of the time trade-off and standard gamble methods of utility assessment in retinal patients. Br J Ophthalmol 2002;86:493-6
- Mitchell P, Annemans L, White R, et al. Cost effectiveness of treatments for wet age-related macular degeneration. Pharmacoeconomics 2011;29:107-31
- Neubauer AS, Holz FG, Sauer S, et al. Cost-effectiveness of ranibizumab for the treatment of neovascular age-related macular degeneration in Germany: model analysis from the perspective of Germany's statutory health insurance system. Clin Ther 2010;32:1343-56
- Athanasakis K, Fragoulakis V, Tsiantou V, et al. Cost-effectiveness analysis of ranibizumab versus verteporfin photodynamic therapy, pegaptanib sodium, and best supportive care for the treatment of age-related macular degeneration in Greece. Clin Ther 2012;34:446-56
- Elshout M, van der Reis MI, Webers CA, et al. The cost-utility of aflibercept for the treatment of age-related macular degeneration compared to bevacizumab and ranibizumab and the influence of model parameters. Graefes Arch Clin Exp Ophthalmol 2014;252:1911-20
- Kourlaba G, Tzanetakos C, Datseris J, et al. Cost-Effectiveness analysis of intravitreal aflibercept in the treatment of neovascular age-related macular degeneration in Greece. Value Health 2015;18:A421
- Iida T, Narimatsu A, Adachi K, et al. Anti-vascular endothelial growth factor outpatient treatment patterns in patients with exudative age-related macular degeneration from a Japanese hospital claims database. JHEOR 2014;2:41-52
- McCloud C, Khadka J, Gilhotra JS, et al. Divergence in the lived experience of people with macular degeneration. Optom Vis Sci 2014;91:966-74
