Abstract
Background and aims: Insulin degludec is an insulin analog with an ultra-long duration of action that exhibits less intra-patient variability in its glucose-lowering activity, and reduces nocturnal, overall, and severe hypoglycemia relative to insulin glargine. The aim of the present study was to evaluate the cost-effectiveness of insulin degludec relative to insulin glargine in patients with: type 1 diabetes (T1D), type 2 diabetes receiving basal-only therapy (T2DBOT), and type 2 diabetes receiving basal-bolus therapy (T2DBB) in Denmark.
Methods: A short-term (1 year) cost-utility model was developed to model insulin use, non-severe and severe hypoglycemia, and self-monitoring of blood glucose in patients using insulin degludec and insulin glargine from the perspective of a Danish healthcare payer. Where possible, data were derived from Danish patients with diabetes and meta-analyses of clinical trials comparing insulin degludec with insulin glargine. Using these characteristics, the model estimated costs and quality-adjusted life years (QALYs) gained for the two insulin regimens in each of the three diabetes populations.
Results: Insulin degludec dominated insulin glargine (i.e. reduced costs while improving quality-adjusted life expectancy) in patients with T1D and patients with type 2 diabetes using a basal-only insulin regimen. In the T2DBB cohort, insulin degludec was associated with an incremental cost-effectiveness ratio of DKK 221,063 per QALY gained, which would be considered cost-effective at a willingness-to-pay threshold of EUR 30,000 (∼DKK 224,000) per QALY gained. Sensitivity analysis showed that results were most affected by changes in hypoglycemia rate ratio assumptions, but were broadly insensitive to changes in individual input parameters.
Conclusions: Insulin degludec reduces incidence of hypoglycemia and improves quality-of-life in patients with diabetes. Over a 1-year time horizon, insulin degludec resulted in cost savings relative to insulin glargine in T1D and T2DBOT cohorts, while being cost-effective in T2DBB.
Introduction
The prevalence of all diabetes mellitus in Danish adults is 9.9%, corresponding to ∼406,000 patients, with a further estimated 154,800 adults with undiagnosed diabetesCitation1. A recently-published study estimated that, on average, a patient with diabetes consumes approximately twice the healthcare resources compared to a person without diabetes. The study found that the total attributable cost of diabetes to Danish society was EUR 4.27 billion/DKK 31.8 billion (at an exchange rate of 7.45 Kroner to the Euro) in 2011 corresponding to DKK 106,900 per patient (including societal costs in addition to direct medical expenditure). The total of DKK 31.8 billion included DKK 5.5 billion of healthcare costs and DKK 13.2 billion of lost productivity, while only a small proportion (DKK 1.1 billion or 3.6%) of the attributable diabetes expenditure arose from expenditure on pharmaceutical agents, corresponding to DKK 3,844 per patientCitation2.
Given that hypoglycemic events of any severity have a profound effect on workplace productivity, optimizing diabetes pharmaceutical expenditure to focus on anti-diabetic agents that reduce hypoglycemia while maintaining tight glycemic control should be a key focusCitation3. Insulin degludec is one such basal insulin which received marketing authorization in the EU in 2013 and was fully reimbursed in Denmark as of January 2016. Insulin degludec is a basal insulin analog with an ultra-long duration of action that forms soluble, stable multi-hexamers which slowly release insulin monomers into the blood. This results in a half-life of over 25 hours in patients with type 1 or type 2 diabetes (approximately twice that of insulin glargine) and a flat pharmacokinetic profile in steady state conditions leading to reduced fluctuations in glucose-lowering activity over the course of each dosing intervalCitation4. An outcome of the long half-life and steady state profile is increased flexibility in dose timing with equivalent glycemic control and hypoglycemia incidence, as demonstrated in both the original BEGIN FLEX trials (in which flexibility was enforced per protocol) and a recent study in Japan in which patients were free to dose in a window 8 hours either side of an agreed dosing timeCitation5–7.
The improvements in the pharmacokinetic and pharmacodynamic profile of insulin degludec should translate to tangible clinical improvements in terms of better dose titration and management and reduced hypoglycemiaCitation4. In a post hoc meta-analysis of the BEGIN trial program, Vora et al.Citation8 investigated glycemic control, insulin dosing, and hypoglycemia in patients with type 1 diabetes on a basal-bolus regimen (T1D), and patients with type 2 diabetes on basal-only (T2DBOT) or basal-bolus (T2DBB) insulin regimens. The study found no significant difference in glycemic control (as would be expected from treat-to-target trials), but reported significantly lower insulin doses with insulin degludec than insulin glargine. Specifically, doses were 12% lower in T1D (p < .0001) and 10% lower in patients with T2DBOT (p = .0004), although no significant dose difference was observed in patients with T2DBBCitation8. In terms of hypoglycemia, the analysis demonstrated significantly lower rates of nocturnal non-severe hypoglycemia with degludec than glargine in T1D, T2DBOT, and T2DBB (by 17, 36, and 25%, respectively; all p < .05). Rates of daytime non-severe hypoglycemia were significantly lower with degludec than with glargine in T2DBB (by 17%; p < .05), with no statistical differences in T1D or T2DBOT cohortsCitation8.
All trials in the degludec trial program were based on a treat-to-target design, in which patients were titrated to a fasting blood glucose (FBG) level target of 4–5 mmol/L (70–90 mg/dL). Comparisons based on HbA1c outcomes are, therefore, not informative, as insulin dosing in the trial was simply titrated upwards in patients not at or approaching the FBG target. Most existing models of diabetes mellitus, such as the CORE Diabetes Model and the UK Prospective Outcomes Model (UKPDS) could be described as “glucocentric”, in that they are driven substantially by differences in the achieved level of glycemic control, typically as measured by glycated hemoglobin (HbA1c)Citation9,Citation10. While analyses could be conducted in which no differences in glycemic control were captured in these existing models, a conceptually more straightforward approach would be to exclusively model the differences in insulin administration and hypoglycemia rates observed in treat-to-target trials.
Given the reductions in hypoglycemia reported in the BEGIN trial program and meta-analysis, the aim of the present analysis was to conduct a cost-utility analysis of insulin glargine (Lantus; Sanofi S.A., Gentilly, France) vs insulin degludec (Tresiba; Novo Nordisk A/S, Copenhagen, Denmark) in T1D, T2DBOT, or T2DBB cohorts in Denmark.
Methods
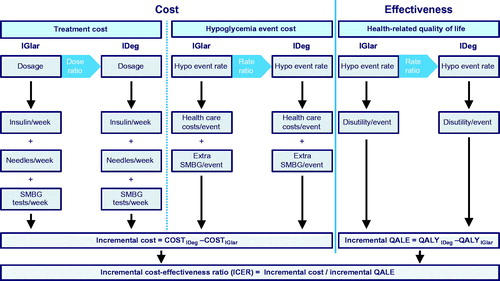
A previously-published short-term cost-utility model was used to evaluate costs and quality-adjusted life expectancy associated with the use of insulin degludec relative to insulin glargine in T1D, T2BOT, and T2DBB cohortsCitation11–13. The model captured basal and bolus insulin doses and dose frequency, incidence of daytime and nocturnal non-severe hypoglycemic and severe hypoglycemic events, and self-monitoring of blood glucose (SMBG) frequency (). Using parameters specific to each insulin, the model estimated costs and quality-of-life (expressed in quality-adjusted life years [QALYs]) with insulin degludec and insulin glargine, and the incremental cost-effectiveness of insulin degludec relative to insulin glargine in the three diabetes populations. Only statistically significant outcomes from the included studies were used to differentiate between the insulin regimens. Cost-effectiveness was assessed relative to a Danish willingness-to-pay (WTP) threshold of EUR 50,810/DKK 378,000 per QALY gained, as derived using a constant “value of prevented fatality” approach in the EuroVaQ studyCitation14. Probabilistic sensitivity analysis (PSA) was built into the model by way of log-normal distribution sampling around the hypoglycemia rate and insulin dose ratios, and normal distribution sampling around the base insulin doses and quality-of-life disutilities associated with each hypoglycemic event ().
Hypoglycemia
Hypoglycemia was modeled using a base rate of hypoglycemia with insulin glargine in each of the three populations and a corresponding rate ratio for insulin degludec (), the latter of which was sampled from a log-normal distribution during PSA. In the reference analysis, hypoglycemia base rates were derived from Danish participants in a 2013 study by Östenson et al.Citation15 that investigated self-reported hypoglycemia outcomes in seven European countries. Of the 3,959 participants (1,631 with T1D and 2,196 with T2D; mean HbA1c of 7.7% and 7.6%, and mean BMI of 26 kg/m2 and 32 kg/m2, respectively), 601 were Danish, in whom 1,894 respondent weeks were recorded. The final derived rates of nocturnal and daytime non-severe hypoglycemia and all severe hypoglycemia employed in the analyses are presented in . The degludec rates were derived from the glargine rates and relative rate data from a meta-analysis of the randomized controlled trials in the BEGIN trial programCitation8. The meta-analysis included six phase 3a trials that compared degludec with insulin glargine and was conducted on a patient-level basis.
The costs associated with non-severe hypoglycemia were assumed to consist of the cost of 2.1, 2.3, and 2.4 additional SMBG tests at a cost of DKK 8.10 in T1D, T2DBOT, and T2DBB cohorts, respectively. These data were derived from a 2013 questionnaire-based study of self-reported hypoglycemia outcomes in Danish patients with diabetesCitation16. In addition to the SMBG test cost, 1.0%, 4.9%, and 6.8% of T1D, T2DBOT, and T2DBB patients reported contacting their general practitioner, which was conservatively assumed to be done via telephone instead of a visit, at a cost of DKK 26.67 per callCitation16. The cost of each severe hypoglycemic event was based on the HypoAna clinical trial which captured resource utilization for Danish T1D patients prone to recurrent severe hypoglycemia (defined as having ≥2 severe episodes in the past year)Citation17. In the study, 63 of 227 (27.8%) severe hypoglycemic episodes required medical assistanceCitation18–24. The full resource utilization data from the trial () was conservatively assumed to apply to patients with T2DM, despite evidence showing a greater proportion of these patients typically require medical assistance for severe hypoglycemiaCitation25.
Insulin dosing and needle use
Insulin doses were specified in a similar manner to hypoglycemia rates, with a reference dose for insulin glargine and a dose ratio from which insulin degludec doses were derived. Dosing data were taken from a recent cost-utility analysis of insulin degludec in Sweden, which in turn was based on a meta-analysis of the BEGIN trials (), and the dose ratios were obtained from a meta-analysis of the randomized controlled trials in the BEGIN trial programCitation8. Patients using insulin degludec were assumed to inject basal insulin once daily, regardless of regimen, while patients with type 2 diabetes using insulin glargine were assumed to use an average of 1.18 needles per day, and patients with type 1 diabetes using insulin glargine were assumed to use an average of 1.36 needles per day due to a proportion of patients splitting their insulin glargine dose into two daily basal injectionsCitation26,Citation27. Whilst the exact proportion of patients dosing insulin glargine twice daily varies between patient groups, bis in die dosing has been observed in numerous previous studies in patients with type 1 or type 2 diabetesCitation28–30.
Routine self-monitoring of blood glucose
It was assumed that, regardless of the basal insulin in use, patients would perform one SMBG test for each injection of either basal or bolus insulin. Costs of individual tests were based on the lowest cost test strips and lancets available in Denmark ().
Quality-of-life
In the reference case analysis, a diminishing marginal hypoglycemia utility model was employed to capture the reduction in quality-of-life associated with non-severe hypoglycemia to reduce the risk of over-estimating the effect of hypoglycemia on quality-adjusted life expectancyCitation31. Disutilities for severe hypoglycemia were taken from an analysis of data from 551 patients with type 1 and 1,603 with type 2 patients published by Evans et al.Citation32. The study used a questionnaire-based time trade-off approach in which respondents were asked to trade-off a portion of their remaining lifespan for an improved health state. In all, 13 such health states were defined, describing diabetes alone or diabetes combined with hypoglycemia of differing event types and frequencies. Participants were randomly assigned to evaluate a sub-set of the available health states to avoid respondent fatigue. The final utility values elicited for the different types of hypoglycemia are presented in .
Table 1. Health-related quality-of-life disutilities associated with severe hypoglycemia in the base case analysis and with non-severe hypoglycemia in sensitivity analysis.
Table 2. Rates of hypoglycemia in the base case analysis. Base rates were from a Danish study of self-reported hypoglycemia, while rate ratios were derived from a meta-analysis of randomized trials in the BEGIN trial program.
Table 3. Unit costs for healthcare resource use for patients required medical assistance for severe hypoglycemia (27.8% of patients).
Table 4. Base insulin dosing and dose ratios in the reference case analysis.
Table 5. Unit costs used in the analysis.
Time horizon and discounting
Costs were captured in 2016 Danish Kroner (DKK) and analyses were run over a 1-year time horizon, mitigating the need to capture discount rates for future cost and effectiveness outcomes. The short time horizon was selected on the grounds that the treat-to-target nature of the trials resulted in no differences in the risk factors that would typically be used as prognostic factors for late onset of complications. No mortality was modeled in the base case analysis, but mortality after severe hypoglycemia was investigated in sensitivity analysis, in which the population size was half-cycle corrected over the first year.
Sensitivity analyses
The reference case was run as a PSA, in which 1000 model iterations were recorded for each of the three analyses sampling from distributions around hypoglycemia rate ratios (log-normally distributed), baseline insulin doses (normally distributed), insulin dose ratios (log-normally distributed), and quality-of-life disutilities (normally distributed). For each model iteration, the incremental cost and quality-of-life was recorded to generate a cost-effectiveness scatterplot and acceptability curve.
In addition to the PSA run in the reference case analysis, a series of one-way sensitivity analyses were conducted to establish the magnitude of effect individual model inputs were having on cost-effectiveness outcomes. Specifically, differences in insulin doses, non-severe daytime, non-severe nocturnal, and severe hypoglycemia were abolished individually. In a separate analysis, a mortality rate of 1.7% was applied after severe hypoglycemia, based on a prospective, population-based study evaluating mortality arising from severe hypoglycemia that resulted in an emergency callCitation33. An analysis was also run in which the unit cost of insulin glargine was switched to that of biosimilar glargine (Abasaglar KwikPen, Eli Lilly and Company, Indianapolis, IN).
Results
The analysis in patients with type 1 diabetes showed that insulin degludec would dominate insulin glargine over a 1-year time horizon, with costs decreasing from DKK 24,712 to DKK 23,219 per patient (saving DKK 1,493), and quality-of-life increasing by 0.0036 quality-adjusted life years (QALYs; ). In type 2 diabetes, analysis of basal-only insulin also resulted in insulin degludec dominating glargine, with a reduction in costs of DKK 139 from DKK 10,117 to DKK 9,978, and an increase in quality-of-life of 0.0085 QALYs. The analysis in T2DBB patients yielded mean outcomes in the north east quadrant of the cost-effectiveness plane with increased cost and quality-adjusted life expectancy. Insulin degludec was associated with an increase in costs of DKK 1,508 over 1 year with an associated increase in quality-adjusted life expectancy of 0.0068 QALYs, resulting in an ICER of DKK 221,063 per QALY gained (), which would fall below the Danish WTP threshold of EUR 50,810/DKK 378,000 per QALY gained.
Table 6. Base case cost-effectiveness outcomes in type 1 and 2 diabetes with basal-bolus insulin, and type 2 diabetes with a basal-only regimen.
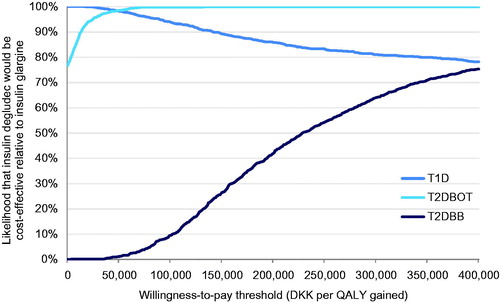
In the probabilistic sensitivity analysis, the results of 1,000 model iterations sampling from all modeled distributions were used to generate cost-effectiveness scatterplots () and acceptability curves () in which the proportion of points falling below a range of willingness-to-pay thresholds were plotted for each analysis. The acceptability curves showed that, at a willingness-to-pay of DKK 250,000, there would be a 83.3% likelihood that insulin degludec would be cost-effective relative to insulin glargine in a T1D cohort, and 99.9% and 54.3% likelihoods that insulin degludec would be cost-effective in T2DBOT and T2DBB cohorts, respectively. In the T1D analysis, all simulations were cost saving, with 65.9% of analyses resulting in increased effectiveness. In the T2DBOT cohort, 76.7% of analyses were cost saving, and 99.9% of analyses resulted in increased effectiveness, while in T2DBB 94.8% of analyses showed increased effectiveness and all resulted in increased costs.
Figure 2. Cost-effectiveness scatterplots for insulin degludec vs insulin glargine in patients with type 1 diabetes on basal-bolus and in patients with type 2 diabetes on basal-only or basal-bolus therapy.
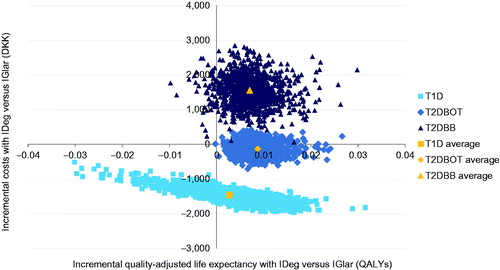
Figure 3. Cost-effectiveness acceptability curves for each analysis over a range of willingness-to-pay values spanning DKK 0–400,000 per QALY gained.
One-way sensitivity analysis showed that the findings of the type 1 diabetes analysis were insensitive to changes in individual model input parameters, with all analyses resulting in insulin degludec dominating insulin glargine (or being equally effective and less costly). In the T2DBOT analysis, only two analyses yielded a mean ICER in the north east scatterplot quadrant, specifically those in which the insulin dose difference was abolished and in which the price of insulin glargine was replaced with the cost of Abasaglar. In the T2DBB analyses, the model was most sensitive to the abolition of differences in the rates of hypoglycemia, with ICERs increasing to DKK 393,886 per QALY gained with the abolition of differences in daytime non-severe rates, and DKK 571,367 with the abolition of differences in nocturnal non-severe rates (). Both analyses would result in degludec falling above a WTP threshold of DKK 378,000 per QALY gained. Conversely, assuming no difference in the insulin dosing in the T2DBB cohort, insulin resulted in an ICER of DKK 121,299 per QALY gained, which would be considered highly cost-effective.
Table 7. One-way sensitivity analysis outcomes.
Discussion
Based on a short-term cost-utility model, insulin degludec was found to dominate insulin glargine in patients on T1D or T2DBOT regimens in the Danish setting over a 1-year time horizon. In patients with type 2 diabetes on a basal-bolus regimen, insulin degludec increased quality-of-life and costs at a ratio of DKK 221,063 per QALY gained. As the majority of basal insulin users in Denmark are patients with either type 1 (28,208; 48.7% of basal insulin users) or type 2 diabetes on a basal-only regimen (25,398; 43.9% of basal insulin users)Citation34–36 where insulin degludec is dominant, the overall weighted impact of insulin degludec is likely to be cost-saving from the healthcare payer perspective when compared with other basal insulin analogues in Denmark. In all analyses, increases in quality-of-life were driven exclusively by reductions in hypoglycemia, while differences in cost were driven by a combination of differences in SMBG test strip utilization, insulin doses, needle use and hypoglycemia treatment costs. The greatest advantage of the present modeling approach is its simplicity and transparency; the model and findings in the present analysis could readily be reproduced without requiring any programming expertise or subscriptions to proprietary models. The approach sits in contrast to the majority of diabetes cost-effectiveness and cost-utility analyses published to date, which typically include modeling microvascular and macrovascular complications over patient lifetimes. With treat-to-target trial data, the differences in the projected incidence of such complications would likely only be minimal, effectively reducing the models to be driven by the differences in dosing and hypoglycemia, as captured in the present analysis.
The reference case presented is likely to be conservative from a Danish societal perspective, in that the societal costs of lost productivity after hypoglycemia were not captured, and neither were the benefits of flexible dosing associated with insulin degludec. Flexible dosing may result in both improved adherence to the prescribed regimen and improved quality-of-life as patients can be less fastidious with the timing of their insulin injectionsCitation37. Furthermore, given that the stable pharmacokinetic profile of insulin degludec results in lower intra-patient glycemic variability than insulin glargine, the frequency of SMBG testing could potentially be reduced, a possibility that was also not captured in the reference case analysisCitation38.
In terms of limitations, the 1-year time horizon precluded capturing any treatment switching or discontinuation, which may materially affect the cost-effectiveness of insulin degludec relative to insulin glargine over longer time horizons. Second, the meta-analysis on which the hypoglycemia rates were modeled was based exclusively on open label studies in the BEGIN trial program. Open-label studies are commonly employed in trials of anti-diabetic agents because of the difficulty in blinding arising from the wide range of insulin delivery devices in use by patients. The open-label nature of the studies may have resulted in biased patient reporting of hypoglycemia, although this was mitigated across the trials by mandating that patients report only confirmed episodes of hypoglycemia.
The EuroVaQ study published three WTP thresholds for undiscounted QALYs in Denmark based on two approaches, the first of which used a constant value per prevented fatality (VPF) aggregated over a group and assuming an average avoidance of life expectancy loss per prevented fatality. The second approach was based on a similar premise, but assumed an inverted U-shaped relationship between age and the VPF, with a peak at 40 years. The three WTP estimates were obtained using approach 1 (EUR 50,810), and approach 2 in patients aged 18 years and over (EUR 23,156) and 40 years and over (EUR 44,452). At an exchange rate of 7.45 Kroner to the Euro, an ICER of DKK 221,063 for the T2DBB cohort would fall above the WTP derived using approach 2 in patients aged 18 years and over, but below the more straightforward constant VPF approach and below approach 2 in patients aged 40 and over. In practice, many different WTP thresholds have been employed in real-world Danish CUAs, and typically reported in pounds sterling or Euros: EUR 40,000–50,000Citation39,Citation40 and GBP 20,000–30,000Citation41,Citation42. At present day exchange rates (7.45 EUR:DKK and 8.85 GBP:DKK), the ICER for degludec vs glargine in the type 2 diabetes basal-bolus cohort would fall under all of these thresholds, with the exception of GBP 20,000, which is the lowest end of the range of commonly-quoted WTP thresholds in the UK.
As hypoglycemia was the primary driver of incremental quality-adjusted life expectancy in the analysis, the rate ratio data formed a critical component of the analysis. The use of a meta-analysis of the BEGIN trials was a robust starting point in terms of the data driving the rate ratios, which has since been corroborated and supplemented by the SWITCH 1 and 2 trials. SWITCH 1 and 2 represent the first ever double-blind, randomized, controlled, cross-over trials of insulin in patients with type 1 or type 2 diabetes. In patients with type 1 diabetes enrolled in SWITCH 1, insulin degludec resulted in significantly lower rates of severe or blood glucose-confirmed hypoglycemia (−11%), severe or blood glucose confirmed nocturnal hypoglycemia (−36%), and severe hypoglycemia alone (−35%) relative to insulin glargineCitation43. Similarly, in the full treatment period of SWITCH 2, patients with type 2 diabetes exhibited significantly lower rates of severe or blood glucose-confirmed hypoglycemia (−23%), severe or blood glucose-confirmed nocturnal hypoglycemia (−25%), and severe hypoglycemia alone (−51%)Citation44. These rates suggest that the present analysis may also have been conservative with regard to the hypoglycemia benefits observed with insulin degludec relative to insulin glargine.
Conclusions
The present analysis represents the first study investigating the cost-effectiveness of insulin degludec relative to insulin glargine in the Danish setting, following on from similar analyses conducted in Sweden and the UKCitation11–13. Based on reductions in the incidence of hypoglycemia and the resultant increase in patient quality-of-life, insulin degludec was found to dominate insulin glargine in patients with type 1 diabetes and patients with type 2 diabetes using a basal-only insulin regimen in Denmark and would likely also be cost-effective in patients with type 2 diabetes using a basal-bolus regimen.
Transparency
Declaration of funding
This manuscript was funded by Novo Nordisk Health Care AG.
Declaration of financial/other relationships
CKT is an employee of Novo Nordisk Health Care AG and owns stocks in Novo Nordisk A/S. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We thank Dr. William Valentine for reviewing the manuscript.
References
- International Diabetes Federation. Diabetes in Denmark. 2015. http://www.idf.org/membership/eur/denmark. Brussels, Belgium. Accessed June 10, 2016
- Sortsø C, Green A, Jensen PB, et al. Societal costs of diabetes mellitus in Denmark. Diabet Med 2016;33:877-85
- Brod M, Christensen T, Thomsen TL, et al. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health 2011;14:665-71
- Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet 2014;53:787-800
- Meneghini L, Atkin SL, Gough SC, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care 2013;36:858-64
- Mathieu C, Hollander P, Miranda-Palma B, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab 2013;98:1154-62
- Kadowaki T, Jinnouchi H, Kaku K, et al. Efficacy and safety of once-daily insulin degludec dosed flexibly at convenient times vs fixed dosing at the same time each day in a Japanese cohort with type 2 diabetes: A randomized, 26-week, treat-to-target trial. J Diabetes Investig 2016;7:711-17
- Vora J, Christensen T, Rana A, et al. Insulin degludec versus insulin glargine in type 1 and type 2 diabetes mellitus: a meta-analysis of endpoints in phase 3a trials. Diabetes Ther 2014;5:435-46
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(Suppl 1):S5-S26
- Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) outcomes model (UKPDS no. 68). Diabetologia 2004;47:1747-59
- Ericsson Å, Pollock RF, Hunt B, et al. Evaluation of the cost-utility of insulin degludec vs insulin glargine in Sweden. J Med Econ 2013;16:1442-52
- Evans M, Wolden M, Gundgaard J, et al. Cost-effectiveness of insulin degludec compared with insulin glargine for patients with type 2 diabetes treated with basal insulin - from the UK health care cost perspective. Diabetes Obes Metab 2014;16:366-75
- Evans M, Wolden M, Gundgaard J, et al. Cost-effectiveness of insulin degludec compared with insulin glargine in a basal-bolus regimen in patients with type 1 diabetes mellitus in the UK. J Med Econ 2015;18:56-68
- Donaldson C, Baker R, Mason H, et al. European value of a quality adjusted life year. Newcastle, UK: Newcastle University, 2010. http://research.ncl.ac.uk/eurovaq/EuroVaQ_Final_Publishable_Report_and_Appendices.pdf. Accessed June 10, 2016
- Östenson CG, Geelhoed-Duijvestijn P, Lahtela J, et al. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med 2014;31:92-101
- Jensen MM, Pedersen-Bjergaard U. Self-reported frequency and impact of non-severe hypoglycemic events in insulin-treated diabetic patients in Denmark. Diabetes Manag 2015;5:67-78
- Pedersen-Bjergaard U, Kristensen PL, Nørgaard K, et al. Short-term cost-effectiveness of insulin detemir and insulin aspart in people with type 1 diabetes who are prone to recurrent severe hypoglycemia. Curr Med Res Opin 2016;20:1-7
- Bilde L. International sammenligning af akut ambulancetjeneste. En foranalyse vedrørende finansieringsmæssige og økonomiske aspekter. Copenhagen, Denmark: KORA. Det Nationale Institut for Kommuners og Regioners Analyse og Forskning, December 2003. http://www.kora.dk/media/529721/dsi-1422.pdf. Accessed February 2016
- Danmarks Statistik. Forbrugerprisindeks. Copenhagen, Denmark: Danmarks Statistik, 2016. http://dst.dk/da/Statistik/emner/forbrugerpriser/forbrugerprisindeks. Accessed February 2016
- The Danish Health Data Authority. DRG-takster 2015: DAGS BG50A charge. Copenhagen, Denmark, 2015. http://sundhedsdatastyrelsen.dk/da/afregning-og-finansiering/takster-drg/takster-2015. Accessed February 2016
- Hatting NP, Mikkelsen S. Treatment of hypoglycaemic diabetics in a prehospital setting is safe. Dan Med J 2015;62:A5044
- The Danish Health Data Authority. DRG-takster 2015: DAGS SP12D charge. Copenhagen, Denmark, 2015. http://sundhedsdatastyrelsen.dk/da/afregning-og-finansiering/takster-drg/takster-2015. Accessed February 2016
- Lyngsie PJ, Lopes S, Olsen J. Incidence and cost of hypoglycemic events requiring medical assistance in a hospital setting in Denmark. J Comp Eff Res 2016;5:239-47
- Praktiserende Lægers Organisation, Honorarium § 50 Grundydelser (dagtid), Telefonkonsultation 0201. Copenhagen, Denmark, 2016. http://www.laeger.dk/portal/page/portal/LAEGERDK/Laegerdk/P_L_O/Overenskomster/Honorartabel/. Accessed February 2016
- Heller SR, Frier BM, Hersløv ML, et al. Severe hypoglycaemia in adults with insulin-treated diabetes: impact on healthcare resources. Diabet Med 2016;33:471-7
- Söderqvist I, Landstedt-Hallin L, Lins P. Byte från 1-dos till 2-dosadministrering av insulin glargin hos patienter med typ 1-diabetes på diabetesmottagningen vid Danderyds sjukhus, en retrospektiv observationsstudie. Abstract presented at the Annual General Meeting for Physicians, November 25–27, 2010, Stockholm, Sweden
- Novo Nordisk. Data on File. Presentation: Afdækning af dosisproblematikken: Levemir – Lantus. Copenhagen, Denmark, 2014
- Allbright ES, Desmond R, Bell DS. Efficacy of conversion from bedtime NPH insulin injection to once- or twice-daily injections of insulin glargine in type 1 diabetic patients using basal/bolus therapy. Diabetes Care 2004;27:632-3
- Garg SK, Gottlieb PA, Hisatomi ME, et al. Improved glycemic control without an increase in severe hypoglycemic episodes in intensively treated patients with type 1 diabetes receiving morning, evening, or split dose insulin glargine. Diabetes Res Clin Pract 2004;66:49-56
- Delgado E, LAUREL Spain study investigators. Outcomes with insulin glargine in patients with type 2 diabetes previously on NPH insulin: evidence from clinical practice in Spain. Int J Clin Pract 2012;66:281-8
- Lauridsen JT, Lønborg J, Gundgaard J, et al. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res 2014;23:2645-50
- Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes 2013;11:90
- Holstein A, Plaschke A, Vogel MY, et al. Prehospital management of diabetic emergencies—a population-based intervention study. Acta Anaesthesiol Scand 2003;47:610-15
- Danish Health Authority (Sundhedsstyrelsen), Tal på diabetes 1996–2012; Copenhagen, Denmark: Danish Health Authority, 2013. http://www.diabetes.dk/
- Bech TH. Diabetikere med pumpe har markant mindre risiko for at dø. 2015. Jyllands-Posten. Copenhagen, Denmark. Available from: http://jyllands-posten.dk/livsstil/familiesundhed/sundhed/ECE7863923/Diabetikere-med-pumpe-har-markant-mindre-risiko-for-at-dø/ Accessed October 2016
- Statens Serum Institute. Dataleverancer og Lægemiddelstatistik [Data Supplies and Medicines Statistics]. Copenhagen, Denmark. 2013. http://medstat.dk/ Accessed October 2016
- Evans M, Jensen HH, Bøgelund M, et al. Flexible insulin dosing improves health-related quality-of-life (HRQoL): a time trade-off survey. J Med Econ 2013;16:1357-65
- Philis-Tsimikas A, Brod M, Niemeyer M, et al. Insulin degludec once-daily in Type 2 diabetes: simple or step-wise titration (BEGIN: once simple use). Adv Ther 2013;30:607-22
- Rolving N, Sogaard R, Nielsen CV, et al. Preoperative cognitive-behavioral patient education versus standard care for lumbar spinal fusion patients: economic evaluation alongside a randomized controlled trial. Spine 2016;41:18-25
- Hastrup LH, Kronborg C, Bertelsen M, et al. Cost-effectiveness of early intervention in first-episode psychosis: economic evaluation of a randomised controlled trial (the OPUS study). Br J Psychiatry 2013;202:35-41
- Oddershede L, Riahi S, Nielsen JC, et al. Health economic evaluation of single-lead atrial pacing vs dual-chamber pacing in sick sinus syndrome. Europace 2014;16:866-72
- Ehlers L, Overvad K, Sørensen J, et al. Analysis of cost effectiveness of screening Danish men aged 65 for abdominal aortic aneurysm. BMJ 2009;338:b2243
- Lane W, Bailey TS, Gerety G, et al. SWITCH 1: reduced hypoglycaemia with insulin degludec (IDeg) vs insulin glargine (IGLar, both U100, in patients with T1D at high risk of hypoglycaemia: a randomized, double-blind, crossover trial. 76th American Diabetes Association Congress, New Orleans, LA, United States. June 10–14, 2016
- Wysham CH, Bhargava AJ, Chaykin LB, et al. SWITCH 2: Reduced hypoglycaemia with insulin degludec (IDeg) vs insulin Glargine (IGlar), both U100, in patients with T2D at high risk of hypoglycaemia: a randomized, double-blind, cross-over trial. 76th American Diabetes Association Congress, New Orleans, LA, United States. June 10–14, 2016