Abstract
Objective: The objective of this analysis was to estimate the relative cost-effectiveness of Actikerall1 (5-FU-SA) vs cryotherapy in a secondary care setting in the UK, for lesion-directed treatment in patients with actinic keratoses (AK) of the face and scalp.
Methods: The model was a simple decision tree, with a 6-month time horizon. The perspective was that of the UK National Health Service (NHS). Modeled treatment effects included reported per-patient histological clearance and recurrence rates. Cost inputs comprised professional consultation time and cost of medication. Health-related utility estimation followed previously published methodology. Adverse events were not modeled. The key data and model structural assumptions followed expected UK practice. One-way and probabilistic sensitivity analyses were conducted to assess structural and parameter uncertainty.
Results: 5-FU-SA was found to be less costly (−£204) and more effective (+0.001 QALY) in base case and sensitivity analyses. In the probabilistic analysis there was 100% probability of being cost-effective over cryotherapy at £20,000 willingness to pay. Cost of professional time was a key driver of the model outcome. 5-FU-SA remained dominant across a range of scenario analyses, including exploration of assumptions around setting of care.
Limitations: The time horizon of the analysis was short and data were not extrapolated beyond the duration of the clinical trial; however, this approach is consistent with likely follow-up of an AK patient. The clinical outcomes observed in the trial were based on a large proportion of cryotherapy patients undergoing an additional cycle of treatment; this may not occur or be required in an experienced secondary care setting.
Conclusion: 5-FU-SA could be considered as a cost-effective choice for treatment of AK lesions of the face and scalp in secondary and mixed care settings in the UK. Use of 5-FU-SA in patients who would otherwise be managed with cryotherapy has the potential to result in cost savings.
Introduction
Actinic keratoses (AK) are skin lesions occurring as a result of frequent exposure to ultraviolet radiation. They mostly occur on the face, scalp, hands, and other chronically exposed areas. While usually asymptomatic, AK is recognized as an early stage in the development of squamous cell carcinoma (SCC)Citation1. The risk of progression to SCC increases with the number of lesions, but transformation remains difficult to predict and active treatment is, therefore, usually advised following clinical diagnosis of AKCitation2–4. It is estimated that ∼20% of all individuals over 60 and over 30% of the UK male population over 70 is affectedCitation5,Citation6. Despite the high population impact, there are no recent direct estimates of cost of treatment of AK to the UK NHS, but the expected cost burden of AK and need for cost-sensitive selection of treatments is high.
In the UK, diagnosis of uncomplicated AK is focused in primary care, but in more complex cases where there is diagnostic uncertainty or widespread actinic damage, referral to secondary care is recommendedCitation7,Citation8. Following diagnosis, lesions are managed with different treatment modalities, depending on characteristics such as number, thickness, total lesion area and histological features. Although there are several field and lesion directed treatments available in the UK, not all treatments are appropriate for all patients, and high recurrence rates indicate substantial unmet need. Cryotherapy remains a common treatment in the management of AK, increasingly delivered in a secondary care setting as its use in primary care becomes less common. Currently there are no published cost-effectiveness analyses in this setting.
The combination therapy 5-fluorouracil (0.5%) and salicylic acid (10%) (5-FU-SA or Actikerall) is indicated for the topical treatment of slightly palpable and/or moderately thick hyperkeratotic actinic keratosis, with a total lesion area up to 25 cm2 (SPC). Its efficacy has been confirmed in two randomized controlled trials and it has been available in the UK since 2011Citation9,Citation10. The 5-FU-SA indication is compatible with those patients presenting with multiple lesions and currently treated with cryotherapy (while other available treatments may not be suitable). Until recently only indirect comparisons were available for 5-FU-SA and cryotherapy, with a lack of direct evidence in AK modeling being previously criticized in the literatureCitation11,Citation12. However, trial data published in 2014 reports a direct comparison of 5-FU-SA vs cryotherapy, enabling more robust comparison of the cost-effectiveness of the two modalitiesCitation9. The objective of the current analysis was to conduct a cost-utility analysis of 5-FU-SA vs cryotherapy with a focus on a UK secondary care perspective.
Methods
Model perspective and structure
A cost-utility analysis was undertaken to estimate the relative cost-effectiveness of 5-FU-SA compared to current standard of care in the UK. The analysis was undertaken from the public payer perspective with a focus on a secondary care setting. Only direct costs were included. The population modeled included immunocompetent adult patients with moderately thick hyperkeratotic actinic keratosis (grade II/III, based on the 4-point scale of Olsen et al.Citation25) reflecting the population included in the clinical trial, and was assumed to be representative of a typical patient presenting with multiple lesionsCitation9.
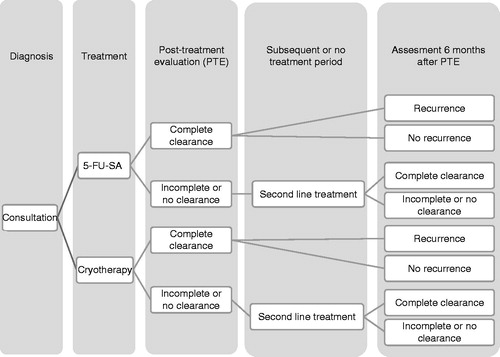
The model was built in MS Excel using a decision tree approach. The approach was in line with previously reported models for AKCitation6,Citation13. The model was populated with data from the 5-FU-SA clinical trial including probability of clearance and recurrence at set time periods. Probabilities were assigned to different courses of action and final model outcomes were determined by the proportion of patients considered clear of AK at 6-month follow-up. The time horizon of the model was limited to 6 months to reflect the available trial data and assumed consistent with expected follow-up in a secondary care setting. The model structure is illustrated in .
The clinical trial
The 5-FU-SA clinical study was a head-to-head open label assessment involving 66 patients randomized to 5-FU-SA or cryotherapy, and has been reported in detail elsewhereCitation9. The primary outcome comprised histological clearance at the post-treatment evaluation (PTE), with patients followed from treatment initiation through to 6-month follow-up. PTE was conducted 14 weeks after completion of the respective therapy (time to PTE differed across treatment groups). At the PTE and at 6 months, all patients were categorized to complete clearance or incomplete/no clearance and/or recurrence. The patients in the study received 5-FU-SA solution once daily for 6 weeks or cryotherapy administered as one or two treatment applications; the first application of cryotherapy was repeated at a 3-week follow-up if this was considered necessary by the trial clinician (88% of patients received two sessions of cryotherapy). Final follow-up was conducted at 6 months’ post-PTE, and assessed continued clearance or recurrence of lesions in those patients rated as completely cleared at the PTE. Only the PTE comprised assessment of histological clearance, with additional follow-up reporting a clinical assessment of outcome (i.e. continued clearance or recurrence). A summary of the 5-FU-SA study characteristics is presented in .
Table 1. Summary characteristics of the phase II trial (Simon et al.Citation9).
Modeled patient pathway
The model reflected the clinical trial protocol with patients followed from PTE until the end of the model time horizon at 6 months. At follow-up points, patients were dichotomized into two groups comprising complete clearance or partial/no clearance and recurrence vs no recurrence. Patients who experienced partial or no clearance at PTE or 6-month follow-up were assumed to undergo additional treatment with cryotherapy. In cases where the treatment outcome was complete clearance followed by recurrence at 6 months, patients were assumed to be re-treated with an additional course of the first line medication. The assumptions were consistent with the authors’ management of AK and were explored in scenario analysis.
In the model, patients could receive a maximum of two discrete treatments over the 6-month time horizon, with treatments truncated at this point to reflect expected UK practice. Within each cycle of cryotherapy, a proportion of patients received two cryotherapy sessions in line with the trial protocolCitation9. The efficacy of subsequent treatment (following recurrence or incomplete clearance) was assumed equivalent to trial-reported first line efficacy (second line efficacy was not assessed in the clinical trial and additional data to support plausible assumptions around this were not uncovered). Discontinuation, compliance, and NMSC were not modeled. Adverse events (AEs) were included in the model structure, but excluded from the base case. The model outcomes comprised assessment of incremental cost-effectiveness ratios based on quality adjusted life years (QALYs) and numbers of successfully treated patients (STPs), where a STP was defined as a patient with complete clearance at PTE and no recurrence at the 6-month follow-up.
Clinical data
The efficacy data used to populate the model are presented in . Initial clearance data were based on per-patient PTE outcomes. Recurrence rates were reported based on patients who experienced complete clearance on initial treatment. All outcomes were patient-based assessments of clearance. Severe, trial-based adverse events rates were used in the scenario analysis, with 3% and 0% rates for 5-FU-SA and cryotherapy, respectively.
Table 2. Model inputs of treatment efficacy from the phase II trial (Simon et al.Citation9).
Cost data
Cost data were taken from the latest available UK sources and included cost of drug (5-FU-SA) or procedure (cryotherapy) and staff costs (). Cost of the professional consultation in the base case was estimated as the cost of a consultation with a dermatologist for both cryotherapy and 5-FU-SA (to reflect the secondary care setting of the analysis). Alternate profiles were assessed in scenario analysis. For cryotherapy, no additional costs on top of consultation were considered; for 5-FU-SA the cost of one pack of medication was added. In order to estimate the cost associated with a GP visit (scenario analysis), a standard duration of GP visit in the UK (11.7 min) was applied and a proportion of patients were assumed to consult the GP rather than the dermatologist (lowering the cost impact of unsuccessful treatment). Cost of treatment of AEs was calculated as cost of single consultation with GP/nurse (20%/80%).
Table 3. Cost inputs.
Table 4. Model inputs of utility values.
Utilities
The health outcomes in the model are expressed in QALYs, which are a function of a health state utility and time spent in this state. QALYs were obtained using methodology published previously by WilsonCitation12 and cited in other CUAsCitation6,Citation12,Citation13. For simplicity, on-treatment time was not explicitly included in the QALY calculation, and estimates were based on PTE to 6-month follow-up. Three health states were assumed with linear progression between them. These were complete clearance, complete clearance with recurrence, and no clearance. AEs utility decrements were applied only in scenario analysis and were based on 8 weeks AE duration and a previously reported utility decrement (0.085)Citation12. The UK utility population norm of 0.785 was also applied ().
Analysis of uncertainty
The parameters of cost, utility, and efficacy were assessed for uncertainty in univariate and probabilistic sensitivity analyses in the model. Key inputs were varied within their given confidence intervals where available, or by 20% of their mean value. In addition, scenario analyses were conducted. These involved scenarios where patients received only one session of cryotherapy, a scenario where GPs managed 50% of AK patients, a scenario where the second session of cryotherapy (after initial treatment by dermatologist) would be carried out by a nurse, and a scenario where patients would switch to comparator after failure in both arms. The latter scenario, where patients failing on treatment with cryotherapy were assumed to be treated with 5-FU-SA, approximates an assessment of 5-FU-SA as a second line treatment (for this reason it is not included in the base case). An additional two scenarios explored the impact of assuming equal efficacy of the treatments, and inclusion of adverse events, respectively.
Results
Base case
In the base case scenario 5-FU-SA was found to be less costly and more effective than cryotherapy (). Treatment with 5-FU-SA resulted in per-patient cost saving of £205, an increased proportion of STP (+16%), and marginal gain in quality of life (+0.001). From a cost-utility perspective, 5-FU-SA dominated cryotherapy.
Table 5. Base case cost-utility results.
Sensitivity analyses
The univariate sensitivity analysis demonstrated that key drivers included: cost of dermatologist visit and efficacy estimates of 5-FU-SA and cryotherapy. Utility values associated with AK had the biggest impact on the incremental cost-effectiveness ratio (when measured in QALYs) due to the small numbers involved (the base case found a utility gain of +0.001, small changes to this denominator result in large changes to the ICER). Choice of 5-FU-SA as a primary treatment proved to be cost-effective and resulted in a positive net monetary benefit (NMB) in all sensitivity analyses. Probabilistic estimates confirmed dominance of 5-FU-SA over cryotherapy, with 99.4% of cost-effectiveness pairs located in the south-east quadrant of the cost-effectiveness plane. 5-FU-SA was found to have a 100% probability of being the optimal choice over cryotherapy at an assumed willingness to pay (WTP) of £20,000. However, given the small utility increments, it may be more plausible to focus on the incremental cost per successfully treated patient.
Scenario analyses
Scenario analyses were conducted to explore the sensitivity of the model to assumptions relating to the cost of consultation and the choice of management strategy. 5-FU-SA remained dominant in all scenario analyses. In the first scenario, where cryotherapy patients were assumed to receive only one session of cryotherapy per cycle, 5-FU-SA generated £11 savings per patient and a marginal QALY gain. The second scenario analysis assumed that 50% of patients were managed by primary care GPs. 5-FU-SA remained dominant, with an estimated per patient cost saving of £120 (QALY gain was not impacted). In the third scenario, where patients were treated by a nurse after initial consultation with a dermatologist, the model estimated an expected cost saving of £103 (QALY gain was not impacted). 5-FU-SA proved to cost significantly less per successfully treated patient, with savings ranging from £176–£403 per successfully treated patient, depending on scenario. In the scenario analysis, where following failure with initial treatment, patients treated with cryotherapy could switch to 5-FU-SA, the model estimated an expected £151 saving per patient and the same QALY gain as in the base case. When equal efficacy was assumed, 5-FU-SA provided £130 savings. Inclusion of AEs in the analysis yielded only marginal differences in QALYs (below 0.001) and costs (less than £1) vs the base case results, with 5-FU-SA remaining dominant.
Discussion
The analysis compared 5-FU-SA against the most relevant comparator for the type of lesions considered in the clinical trial. The results indicated that 5-FU-SA could result in substantial per-patient savings, an increased proportion of STP, and small benefits in quality-of-life when compared against cryotherapy. 5-FU-SA was cost-effective in all scenarios within a wide range of parameter variation. Even in the scenario with assumed equal efficacy, 5-FU-SA provides cost savings. Utilities and cost of professional consultation were found to be key drivers of outcomes, with visit cost as the key cost driver. In exploration of these costs, where we assumed 50% of patients were treated in a primary healthcare setting, 5-FU-SA remained the dominant treatment option, with expected per-patient cost savings greater than GBP100.
The choice of comparator for the analysis was based on the opinion of British clinical experts in AKCitation14, which suggested that the same type of lesions would be treated by 5-FU-SA and cryotherapy. Furthermore, both are lesion directed treatments, whereas comparators not included in this evaluation are predominantly used for field treatment of the lesions.
It is important to highlight that this analysis used per-patient clearance rates, rather than per-lesion clearance rates. Lesion-based assessments of complete clearance consider the proportion of lesions which are completely cleared at follow-up time points, patient-based assessments of clearance are more restrictive, requiring 100% clearance of all lesions in order to meet the metric of complete clearance. The former are usually reported in studies at substantially higher rates than per-patient clearance. For example, Szeimies et al.Citation15 reports 75% lesion-based complete clearance rates for cryotherapy, and Thai et al.Citation16 reports 67% lesion-based complete clearance rates and 57% per-patient rates. Stockfleth et al.Citation10 reports 75% 5-FU-SA clearance rates per lesion and 54% per-patient (broadly consistent with the per-patient clearance rates seen in the current analysis). Per-patient clearance rates were used, as it was assumed that measurement of complete clearance by this metric provides a better reflection of a treatment outcome likely to impact quality-of-life, and may better predict the likelihood of subsequent treatment.
The estimates of effectiveness for this analysis were taken from the recently published head-to-head trial. Despite the fact that the trial involved small sample size (n = 66) it is the most robust evidence available for the comparison. Clearance rates reported in Simon et al.Citation9 for cryotherapy place themselves in the middle of the range seen in other studies (for example Krawtchenko and Thai) and are in line with results of NMA conducted on the similar population as this analysisCitation9,Citation16–19. Please note that the published NMA did not include 5-FU-SA, hence it was not used as a benchmark for these analyses. The patients in the clinical trial had on average more severe AK (grade II/III) than the average AK population (see ). More difficult to treat lesions may be expected to be treated by specialists (e.g. due to higher risk of NMSC or unpredictability of response) which supports the focus on a secondary care setting for the current analysis. While cryotherapy remains a popular choice of treatment for AK, published cost-utility analysesCitation6,Citation13 and evidence synthesis studiesCitation18,Citation20 indicate that cryotherapy is less effective than other available treatments and, hence, could be considered a sub-optimal treatment choice, emphasizing the need for effective treatments in this patient population.
The approach to costing cryotherapy may be considered a study limitation. The estimation of cryotherapy costs was limited to the cost of professional consultation. This was because of difficulties in calculating true costs due to e.g. different storage options, equipment, volume used per single treatment, or liquid nitrogen vaporization over time. These issues have been highlighted previously in the literature and, therefore, a conservative approach was taken to assume that these costs would be incorporated within the cost of a consultationCitation19. In contrast to a potential under-estimate of cycle costs, in line with other published data (e.g. Krawtchenko et al.Citation17), the model included the option of multiple treatments for each cryotherapy cycle, adding to the cost of this treatment arm. The model outcomes were robust to this parameter and 5-FU-SA remained dominant in the scenario where patients received only one session of cryotherapy. This scenario can be considered highly conservative, as effectiveness was not adjusted. The trial-reported clearance rates were based on 88% of patients receiving a second cycle of cryotherapy; it is reasonable to assume that effectiveness rates would have been lower if only one session of cryotherapy was applied, but data does not exist to inform a plausible adjustment.
The treatment protocol modeled was informed by the phase II trial. Cryotherapy treatment protocols vary between practitioners and locations, potentially limiting the genaralisability of the results. Changes in treatment protocol were explored in a sensitivity analysis wherein patients received only one session of cryotherapy per cycle. Dominance was maintained within this scenario.
The current model is simple and reflects the data available. It is difficult to compare our analysis directly to other published CUA models, due to differences such as population, methodology, and assumptions made. However, Soini et al.Citation6 reported in their study that cryosurgery was dominated by all treatments included in the analysis. Tolley et al.Citation13 presented similar results finding 5-FU-SA to be more cost-effective than cryotherapy. Other models have looked at time to clearance, but in this instance we use a simple PTE assessment. Different treatment protocols, especially within cryotherapy, bring additional challenges if the time to clearance is considered. In addition, our analysis used head-to-head trial data of difficult-to-treat face and scalp lesions, whereas other reported studies used data from indirect comparisons which included synthesis of evidence from studies assessing response rate in less severe lesionsCitation6,Citation13.
The model applied a 6-month time horizon, whereas previous models have used up to 2-year time horizons. Based on authors’ opinion, the 6-month time horizon better followed the available data and reflected likely treatment patterns in the UK, with patients unlikely to be followed up actively for more than 6 months.
AEs and discontinuation were not included in our base case analysis (although they were included in scenario analyses) for several reasons. First, there is limited evidence exploring the impact of AEs on QALYs in AK. Second, the definition of AEs, their severity, and reported rates are very different among published studies. Despite the fact that AEs were reported in the phase II trial, based on the arguments above and the fact that AEs would be normally managed in primary careCitation14, the KOLs recommended the base case without AEs. However, they were included in the scenario analysis. We included only severe AEs, which are more likely to impact quality-of-life and incur costs. The costs of AEs were calculated by taking into account only cost of professional consultation, as according to KOLs most of the patients would not be given any medication. Inclusion of AEs yielded only a marginal difference vs the base case results.
Finally, different health professionals are involved in the management pathway of AK including primary care. This has been partially addressed in this analysis by including the scenario where 50% of AK patients are managed by primary care GPs and where second treatment sessions are managed in a practice nurse setting. However, additional analysis would be required to assess the cost-effectiveness of 5-FU-SA in a primary care setting, as different comparators may be applicable.
The impact of compliance and discontinuation was not explicitly modeled in this study; however, plausible variation in efficacy was evaluated in the one-way sensitivity analysis, which would have explored the potential impact of changes in real world effectiveness caused by variations in these parameters. Furthermore, all patients who only partially respond to therapy (or achieve no response) were assumed to switch to the alternative treatment. This conservative assumption is also likely to account for discontinuation arising from lack of efficacy. Further research to evaluate real world effectiveness, and account for the potential impact of compliance and discontinuation in practice, would strengthen future analyses.
The study adds to the evidence base supporting use of 5-FU-SA as a first and second line treatment in AK in a secondary care setting. It supports use of 5-FU-SA as an additional and valuable treatment option alternative to cryotherapy. Furthermore, it suggests cost-effectiveness of 5-FU-SA when considering a secondary care setting and when a mix of primary and secondary care setting is considered. Therefore, the study supports the choice of the optimal and cost-effective treatment option in treatment of AK patients presenting with multiple lesions of the face and scalp.
Conclusion
From a UK perspective, 5-FU-SA is a cost-effective option in treatment of AK lesions of the face and scalp and could result in cost savings when used in patients who would otherwise be managed on cryotherapy.
Transparency
Declaration of funding
This study was funded by Almirall S.A.
Declaration of financial/other relationships
JL and RW are full-time employees of IMS Health (a company providing information and technology services to stakeholders within the healthcare industry), who served as paid consultants to Almirall S.A. during the development of this study and manuscript. SM, DV, and LR are Almirall S.A. employees. CM and JL provided independent clinical expertise and received honoraria for consultancy work. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Note
Notes
1 Actikerall is a registered trademark of Almirall, S.A., Spain.
References
- Fernández-Figueras T, Carrato C, Sáenz X P, et al. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol 2015;29:991-7
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol 2000;42:S23-S4
- Green AC. Epidemiology of actinic keratoses. Curr Probl Dermatol 2015;46:1-7
- Werner RN, Sammain A, Erdmann R, et al. The natural history of actinic keratosis: a systematic review. Br J Dermatol 2013;169:502-18
- de Berker D, McGregor JM. Guidelines for the management of actinic keratoses. British Association of Dermatologists, editor. Br J Dermatol 2006;156:222-30
- Soini EJ, Hallinen T, Sokka AL, et al. Cost-utility of first-line actinic keratosis treatments in Finland. Adv Ther 2015;32:455-76
- Drain C, Dziewulski P. Management of actinic (Solar) keratoses in primary and secondary care. Chelmsford, UK: NHS Mid Essex Locality, 2015
- Keohane S, Kownacki S, Moncrieff G, et l. PCDS Guidlines: Actinic (Solar) Keratosis - Primary care treatment pathway. Hatfield, UK: The Primary Care Dermatology Society, 2007
- Simon JC, Dominicus R, Karl L, et al. A prospective randomized exploratory study comparing the efficacy of once-daily topical 0.5% 5-fluorouracil in combination with 10.0% salicylic acid (5-FU/SA) vs. cryosurgery for the treatment of hyperkeratotic actinic keratosis. J Eur Acad Dermatol Venereol 2015;29:881-9
- Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol 2011;165:1101-8
- De Roos K, Beljaards RC. The Delphi panel in the economic evaluation of photodynamic therapy for actinic keratosis and basal cell carcinoma: poor results? Br J Dermatol 2007;156:1393-4
- Wilson EC. Cost effectiveness of imiquimod 5% cream compared with methyl aminolevulinate-based photodynamic therapy in the treatment of non-hyperkeratotic, non-hypertrophic actinic (solar) keratoses: a decision tree model. Pharmacoeconomics 2010;28:1055-64
- Tolley K, Kemmett D, Thybo S, et al. A cost-utility analysis of ingenol mebutate gel for the treatment of actinic keratosis: a Scottish perspective. Eur J Health Econ 2016;17:287-304
- John Lear CM. Actikerall UK Key Opinion Leaders validation meeting. London, UK: Unpublished source, 2015
- Szeimies R-M, Dirschka T, Prechtl A, et al. Efficacy of low dose 5-fluorouracil/salicylic acid in actinic keratoses in relation to treatment duration. JDDG 2015;13:430-8
- Thai K-E, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol 2004;43:687-92
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol 2007;157(2 Suppl):34-40
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One 2014;9:e96829
- Stamuli E, Cockayne S, Hewitt C, et al. Cost-effectiveness of cryotherapy versus salicylic acid for the treatment of plantar warts: economic evaluation alongside a randomised controlled trial (EVerT trial). J Foot Ankle Res 2012;5:4
- Gupta AK, Paquet M. Network meta-analysis of the outcome 'participant complete clearance'in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol 2013;169:250-9
- MIMS. MIMS online. 2016 http://www.mims.co.uk; Accessed 8/03/2016
- Department of Health, National Schedule of Reference Costs - Year 2014–15, NHS trusts and NHS foundation trusts, 2015, https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015; Accessed 8/03/2016
- PSSRU. Unit Costs of Health and Social Care 2015; University of Kent: Personal Social Services Research Unit, Canterbury, UK, 2015
- Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. Berlin, Germany: Springer, 2014
- Olsen EA, Abernethy L, Kulp-Shorten C, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses of the head and neck. J Am Acad Dermatol 1991;24:738-43