Abstract
Aims: To describe the collective costs of vitamin K antagonist (VKA) treatment for stroke prevention in non-valvular atrial fibrillation (NVAF). VKA drug costs are relatively low, but they necessitate frequent international normalized ratio (INR) monitoring. There are currently minimal data describing the economic impact of this in Mexico.
Materials and methods: Cardiologists provided data on their NVAF patients (n = 400) to quantify direct medical costs (INR testing, appointments, drug costs). A sub-set of patients (n = 301) completed a patient questionnaire providing data to calculate direct non-medical costs (travel and other expenses for attendance at VKA-associated appointments) and indirect costs (opportunity cost and reduced work productivity associated with VKA treatment).
Results: Estimated annual direct medical costs totaled $753.6 per patient. Annual direct non-medical and indirect costs were USD$149.8 and $132.1, respectively.
Limitations: Recruited patients were those who consulted with a cardiologist during the study period and selected due to inclusion criteria. All had received uninterrupted treatment for 12–24 months. Consequently, the results are not fully generalizable to all VKA treated NVAF patients.
Conclusions: The true cost of VKA treatment cannot be appreciated by a consideration of drug costs alone. Ongoing monitoring appointments incur additional expenses for both patients and the healthcare system.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia, occurring in an estimated 33 million people worldwideCitation1. The prevalence of AF in Mexico has been estimated at 1.58% in people aged ≥40 yearsCitation2. AF can be valvular or non-valvular, occurring in the presence or absence, respectively, of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repairCitation3. Non-valvular AF (NVAF) comprises the majority of AF casesCitation4, with NVAF estimated as accounting for 86% of AF cases in MexicoCitation2.
Patients with AF have a 5-fold increased risk of strokeCitation5, which is a leading cause of mortalityCitation6 and disabilityCitation7. Furthermore, the costs associated with stroke are considerable, both for the patientCitation8 and for healthcare systems across the globeCitation9. For example, an international comparison study estimated that 3% of all healthcare costs could be attributed to stroke careCitation10. Stroke is a major issue in Mexico, with cerebrovascular disease being the third leading cause of death here. In 2013, among an estimated 31,999 deaths in Mexico related to cerebrovascular disease, 5,333 could be attributed to AF-related strokeCitation11.
Guidelines recommend treatment with oral anticoagulants to reduce the risk of stroke in at-risk patients with NVAFCitation12. Historically, this has been with vitamin K antagonists (VKAs) such as warfarin. However, more recently two new classes of oral anticoagulation agents have been approved: factor Xa inhibitors and direct thrombin inhibitors (DTIs). Although VKA therapy is effective in the prevention of strokeCitation13, it has a narrow therapeutic rangeCitation14. Consequently, the international normalized ratio (INR) of prothrombin time should be closely monitored, necessitating frequent blood tests and dose adjustments. Challenges associated with achieving and maintaining VKA patients within therapeutic range include genetic differences in response, poor patient adherence, and numerous drug–drug and drug–food interactions associated with VKAsCitation15–17. VKA treatment may, therefore, result in a considerable healthcare resource-use burden as a result of these challenges and the need for INR monitoring.
When determining the economic impact of VKA treatment for stroke prevention in NVAF, direct and indirect costs should be considered, as drug costs alone will under-estimate the collective burden. The direct and indirect costs of using VKAs for stroke prevention in patients with AF have previously been assessed in some regions, primarily North America and Western EuropeCitation18–21. Little information is available regarding the collective cost of warfarin use in other regions, despite recognition that there is considerable geographical variation in anticoagulation managementCitation22. We undertook the present study to assess the cost of VKA use in Mexican patients with NVAF using real-world data from patients consulting a cardiologist in Mexican urban areas.
Methods
Study population
A sample of 40 cardiologists was recruited across different urban regions in Mexico. Screening criteria mandated that all cardiologists were routinely responsible for stroke prevention in AF including (but not limited to) treatment with VKA therapy, seeing at least 15 of these patients per month. Physicians also had to have qualified between 1979 and 2012. Each cardiologist completed a record form for the next 10 eligible patients they saw according to the following criteria: patient was ≥18 years and had received VKA treatment for a minimum of 12–24 months with no interruptions within the considered period to ensure sufficient data. Eligible patients were invited to complete a patient questionnaire on a voluntary basis, which could be filled in with the assistance of a caregiver if required.
Data collection
Physician-reported data
The record form completed by physicians was online and captured information on patient demographics and clinical characteristics including comorbidities and risk factors. Physicians also reported the number of INR tests the patient had in the first 12 months of treatment as well as INR test results and VKA dose over the first 12 months of treatment. Physicians also provided details on the patients typical INR monitoring pathway, for example, whether the patient had separate appointments before and/or after their INR test, the healthcare professional responsible for INR testing, the type of INR test performed, the location where this takes place, and whether any physician appointments were telephone contacts rather than office visits. For each patient, the physician also indicated if the patient was well managed according to their subjective opinion, and supplied reasons for poor management if this was not the case.
Comorbidities and risk factors were reported and were used to derive CHA2DS2-VASc risk scores (congestive heart failure, hypertension, age, diabetes, stroke, vascular disease, age, sex category)Citation23, and HAS-BLED scores (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, age >65 years, drugs/alcohol use)Citation24. The Charlson’s Comorbidity Index was also calculatedCitation25.
Patient-reported data
Patients completed a paper-based questionnaire. Patients were asked to report total overall loss of time for themselves and, if applicable, their caregivers, associated with their last appointment, including travel time and time spent in the hospital. In addition, patients also specifically indicated work time loss for themselves and a caregiver due to appointments. Patients provided travel information for their last appointment including mode of transport, distance traveled, and any travel expenses incurred. Other expenses incurred over the last month (excluding travel costs) due to VKA treatment such as paying for meals/drinks while at appointments were also reported. In order to understand how frequently these costs were incurred, patients indicated the number of site visits relating to VKA treatment in the last 4 months to their main hospital. In addition, some patients reported that they sometimes visit an alternative hospital/clinic for INR testing. If this was the case, patients reported the number of visits to this site over the previous 4 months. For these patients, the same questions relating to loss of time, travel, and other expenses were repeated to collect relevant data associated with use of an alternative site for INR testing, in addition to appointments at the patient’s main hospital.
All patient data were anonymized; however, physician-reported data and patient-reported data were linked via a unique identifier. As completion of the patient questionnaire was voluntary; these data represent a sub-set of the overall sample.
Analysis
Patient demographics and resource use variables for calculating direct medical costs, direct non-medical costs, and indirect non-medical costs are described. Means (SD) are reported for numeric variables and for categorical variables; the percentage of patients is reported.
Direct medical costs
These consisted of three elements, all of which were derived from physician-reported data over the first 12 months of treatment: drug costs (dependent on dose and duration of each dose), INR test costs (dependent on number of INR tests), and cost of appointments surrounding INR testing (dependent on typical INR monitoring pathway and the healthcare professional responsible for conducting blood sampling).
Direct non-medical costs
These were calculated from data provided in the patient questionnaire and included patient reports of travel costs, costs of meals/drinks, and any other expenses. If patients reported travel by car, fuel costs were estimated based on the distance traveled and the cost per litre of fuel in Mexico. Some patients reported sometimes traveling to a different site for INR testing compared to where they usually see their cardiologist. In this instance, travel expenses associated with each site were captured and then amalgamated. Although some patients reported paying for their own medication or to see a healthcare professional as part of their expenses, this was not included in the analysis to avoid double counts with the physician reported data. As the patient reported data was provided for the previous month or previous appointment, an annual estimate was calculated by multiplying by 12 or multiplying by the number of patient reported visits (scaled up to 12 months).
Indirect costs
These were calculated from data provided in the patient questionnaire and included worktime loss and opportunity loss for patients and, if applicable, their caregivers following a similar method to a previous VKA costing study conducted in IrelandCitation19. Employed patients reported lost work time due to VKA treatment over the previous month. Patients similarly indicated any loss of worktime for their caregivers over the previous month due to VKA. In each case, this was multiplied by 12 and the median hourly wage was applied to derive an annual estimate. For patients unemployed due to VKA for a period of greater than 12 months, the median annual wage was applied. If the timeframe was less than 12 months, a proportion of the median annual wage was applied and, for the remaining proportion of the year, work time loss was imputed. The imputation was based on responses supplied by employed patients. For patients who were not working but stated this was not due to VKA, opportunity loss was calculated instead. This was based on patient-reported loss of time for attending their last appointment at their main hospital and an alternative site (if applicable), multiplied by the patient reported number of visits to each site over the past 4 months scaled up to 1 year and multiplied by the national minimum wage to derive an annual estimated cost associated with opportunity loss. The same method was also used to calculate caregiver opportunity loss for instances where the patient said they were accompanied by a caregiver but did not report any associated loss of work time for that person.
Costs per INR test
In addition to the annual cost per patient, costs associated with a single INR test were calculated by simply dividing the estimated annual cost by the number of INR tests in the first 12 months of treatment, as reported by the physician.
Unit costs
Unit cost data were gathered from external, country-specific sources and the literature. Costs were identified in Mexican Peso and converted into USD using an exchange rate of 0.061 (www.xe.com, retrieved 2016).
Generalized linear model
Patient characteristics associated with higher direct medical costs were investigated using a Generalized Linear Model (GLM). A gamma distribution and a log link were used in the model as these options are widely used for modeling cost outcomes. Variables included in the GLM were age, sex, body mass index (BMI), smoking status, disease duration, time to stability, CHA2DS2-VASC score, HAS-BLED score, and Charlson’s comorbidity index.
Analyses were performed using Stata v14.1 or higher (StataCorp, 2015. Stata statistical software: Release 14. College Station, TX, StataCorp LP).
Results
A total of 40 cardiologists were recruited in Mexico (75% male). These physicians were spread across urban centres in the North West (n = 5), North East (n = 1), North Central (n = 12), South Central (n = 21), and South West (n = 1) regions of Mexico. The majority of these physicians (85%) were based in a hospital/clinic setting. Cardiologists provided data on 400 patients, 301 (75%) of whom also completed a patient questionnaire. Physician-reported patient characteristics are summarized in .
Table 1. Patient demographics and baseline characteristics.
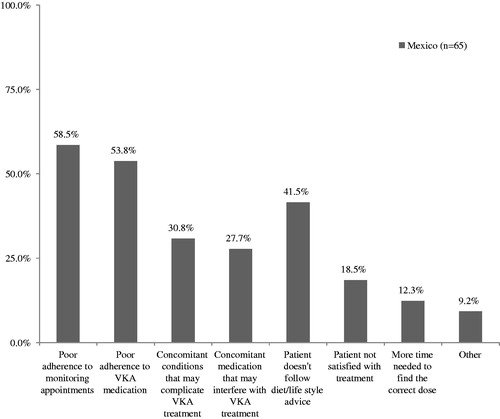
A sub-set of patients were classified by the cardiologist as having not become stable on their VKA treatment or had become stable at some point but were not currently well managed (n = 65). Reasons for this, as reported by the physician, are shown in .
Figure 1. Reported reasons for patients being poorly managed on VKA treatment. VKA, vitamin K antagonist.
Direct medical costs
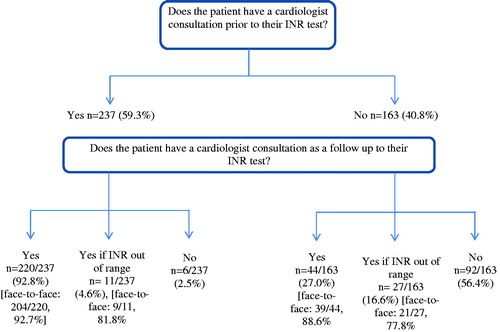
The INR monitoring pathway is summarized in .
Patients in Mexico had an average of 4.9 INR tests over the first 12 months of their VKA treatment, which, according to their monitoring pathway, translated to a mean number of 10.6 face-to-face appointments and 0.9 telephone appointments with their cardiologist. For the majority of patients (85.5%) blood sampling was performed by a lab technician, but it was also performed by a nurse (7.8% of patients) or by the cardiologist (6.8% of patients). Current mean VKA dose for patients was 3.9 mg (SD = 2.6). Direct medical costs are summarized in .
Table 2. Direct medical costs associated with the first 12 months of VKA treatment.
Estimated direct medical costs per INR test were $150.9 (SD = 50.4). If physicians/patients adhered to one appointment per month as per guideline recommendationsCitation26, the direct medical costs for a 12-month period would’ve been notably higher, at $1,810.4.
Direct non-medical costs
Patient expenses due to attending appointments are shown in . Patient incurred expenses associated with paying for medication and also to see healthcare professionals were also reported but are not included in the analysis to avoid double counting with the physician-reported data.
Table 3. Direct non-medical costs.
Estimated direct non-medical costs per INR test were $39.5 (65.4). If physicians/patients adhered to one appointment per month as per guideline recommendationsCitation26, the direct non-medical costs for a 12-month period would have been notably higher, at $473.5.
Indirect non-medical costs
Patients reported their current employment status. A total of 97 patients (32.2%) were either in full or part-time employments, 201 (66.8%) were either retired or unemployed, and three patients (1.0%) did not respond. Employed patients reported taking an average of 5.6 hours (SD = 7.5) off work due to VKA treatment over the previous month. Of the 201 patients who were out of work, two (1.0%) stated that this was due to their VKA treatment (duration of 3 months for one patient and not specified for the other patient). From the total patient sample, based on the last appointments, 213 (70.8%) patients reported being accompanied by a caregiver to their usual hospital and, of the 67 patients who stated that they sometimes visit a different hospital, 40 patients (59.7%) had been accompanied by a caregiver. Mean patient reported worktime loss for caregivers (n = 252) was 2.6 hours (SD = 6.3).
On average, patients reported 80.3 min (SD = 54.1) for traveling to and from appointments and an additional 59.8 min (SD = 35.0) were spent at the hospital. In addition, from the 67 patients stating that they sometimes went to a different hospital for INR testing, a loss of 55.1 min (SD = 41.5) was reported due to travel and 29.1 min (SD = 19.1) spent at the hospital. Indirect non-medical costs are shown in .
Table 4. Indirect non-medical costs.
Estimated indirect costs per INR test were $51.7 (SD = 148.1). If physicians/patients adhered to one appointment per month as per guideline recommendationsCitation26, the indirect costs for a 12-month period would have been notably higher, at $620.3.
Cost drivers
Patient characteristics associated with direct medical costs in the first 12 months of treatment were investigated using GLMs. Results are shown in .
Table 5. GLM exploring variables associated with direct medical costs.
For each unit increase in the CHA2DS2-VASc score, this increases the direct medical costs in the first 12 months of treatment by a factor of 1.14 or 14% (p = .008). Patients with CHA2DS2-VASc scores between 0–3 (n = 269) had a mean number of 4.6 INR tests in the first year of treatment compared to a mean of 5.6 INR tests for patients with scores between 4–6 (n = 119) and 5.8 INR tests for patients with scores between 7–9 (n = 12).
Discussion
This study examined the mean annual cost per patient associated with VKA treatment for the first year of treatment for stroke prevention in Mexican patients with NVAF. Mean direct medical costs incurred by the healthcare system were $753.60 per patient per year, the majority of which were attributable to INR monitoring and physician consultations; only $13.10 (<2%) of the total direct cost was attributable to drug costs. In addition to direct medical costs, more broadly, direct non-medical and indirect costs are also of relevance, which has sometimes been overlooked in previous research.
Mean direct non-medical costs, estimated at $149.80 per patient per year, were incurred in the form of travel to and from appointments and meals/other expenses while at appointments. Mean indirect non-medical costs incorporating work time and opportunity loss for both patients and their caregiver where applicable were estimated at $132.1 per patient per year. Direct and indirect non-medical costs reflect the burden to patients and caregivers, but can also be of relevance to the healthcare system if, for example, they drive poor adherence to treatment.
Additional costs, over and above drug acquisition costs, have been shown by others to impact on the cost of VKA treatment for patients at risk of stroke. In a modeling analysis of direct and indirect costs of warfarin treatment in Canada, Schulman et al.Citation21 estimated that the cost of drugs accounted for 2–6% of total costs in three models of care. In their analysis of the cost of anticoagulation in patients with AF in a single-center study, Ali et al.Citation20 estimated that drug price accounted for 14% of the total cost of warfarin treatment when drug costs, INR monitoring, and bleeding costs were taken into consideration. The cost of INR monitoring was shown to vary widely, from $6.19 to $145.70 per test depending on the setting and the perspective, in a systematic review of studies reporting the direct cost of INR monitoringCitation27. These data support the findings of the present study that the cost of VKAs are only a minor component of the overall economic burden associated with this treatment option in NVAF.
Further analysis aimed to identify significant variables associated with greater direct medical costs. A GLM found CHA2DS2-VASc scores were a significant driver, which may suggest that higher-risk patients are monitored more closely. Consistent with this, INR tests were more frequent for patients with higher CHA2DS2-VASc scores. However, it is not clear from the data presented here whether it is the treating cardiologists driving increased monitoring or if patients themselves choose to attend monitoring appointments more frequently. Also significant in this model was smoking status; patients who had never smoked were associated with higher direct medical costs. One potential explanation for this is that non-smokers are more health conscious and are, therefore, more likely to attend monitoring and physician appointments. However, it is perhaps surprising that age and the Charlson’s comorbidity index scores were not significant drivers of direct costs. Older age and a greater number of comorbidities would be expected to contribute to a patient’s overall health and could, therefore, make control of their stroke prevention treatment more complex and consequently more expensive. We suggest that age becomes increasingly relevant when combined with other risk factors, as is the case for the CHA2DS2-VASc scores. Similarly, the Charlson’s Comorbidities Index includes a range of different conditions, only some of which may be viewed as of relevance in stroke prevention and NVAF.
A key finding from this study that was somewhat unanticipated was that INR monitoring occurred less frequently than might be expected, with physicians reporting that their patients had a mean of 4.9 tests in the first 12 months of treatment. In contrast, a Quest survey of physician practices in the US revealed that patients had a median of 19.4 INR tests per yearCitation28. Guidelines have typically advised testing at least every 4 weeksCitation26. However, while guidelines describe the optimal scenario, the reality is often somewhat different, as demonstrated in this real world study. Significant geographic variability in INR testing frequency has been reported in previous researchCitation29, and this has even been noted in clinical trials where treatment is protocol drivenCitation22. For example, analysis of data in the ROCKET AF study identified that the interval between INR tests ranged from 19 days in Canada/US to 22 days in Latin America, and 23 days in Eastern Asia and Eastern EuropeCitation22.The authors suggest geographical variation in medical practice as the reason for these between-country differences in anticoagulation controlCitation22. In real world studies such as this one, that examine routine clinical practice, variation in INR testing frequency will be even more marked, reflecting differences in the procedures at individual hospitalsCitation29, preferences of individual physicians, and/or patient adherence to test appointments.
Two implications of this research should be considered. As the costs generated in the present study were for patients undergoing sub-optimal INR testing, the potential costs of INR testing as recommended in guidelines would be substantially higher, placing a higher burden on the Mexican healthcare system, although this might be offset by a reduction in complications. Second, the low frequency of INR testing identified in this study suggest that Mexican patients with NVAF are at an increased risk of stroke and other complicationsCitation29–31. Further research identifying the factors contributing to INR test frequency and the implications this has on patient safety would be of benefit for addressing this issue.
Some limitations of this study should be considered. Physician reported data were collected from cardiologists only; patients treated by other physician types, such as primary care physicians, are not represented in this study. Patient-reported data were collected using a pen and paper questionnaire; such data collection is reliant on accurate and honest reporting by participants. Missing data occurred, resulting in a variable number of patients contributing to some analyses. In addition, to reduce the impact of recall bias, the maximum retrospective recall period for patients was 1–4 months. Estimations of annual direct non-medical and indirect costs per patient are, therefore, based in part on recent data that was scaled up to 12 months and may not be representative of the entire preceding year or indeed the first year of treatment for patients who had been in receipt of VKA treatment for 12–24 months.
Conclusion
Comprehensive data regarding the direct and indirect cost of VKA treatment in patients with NVAF have been lacking to date. This study has shown that the true cost of VKA treatment for stroke prevention in patients with AF cannot be appreciated by consideration of the drug costs alone, as these accounted for less than 2% of the direct medical costs and, in addition, patient’s themselves experience an economic burden in terms of expenses associated with appointments and loss of time for themselves and often also for a caregiver. When determining the best course of treatment for stroke prevention in a patient with NVAF, the broader healthcare and patient cost implications should be considered.
Transparency
Declaration of funding
The study was sponsored by Bayer Pharma AG.
Declaration of financial/other interests
JBB and KB are employed by Bayer Pharma AG. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
The authors would like to thank Deirdre Carman for assistance with medical writing, and our Mexican colleague Jack Garcia Uranga who provided local support. Some of the data presented in this manuscript was presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) conference 2016, Vienna.
References
- Chugh SS, Havmoeller R, Narayanan K, et al. worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2013;25:837-47
- Cubillos L, Haddad A, Mould J, et al. Burden of disease from atrial fibrillation in adults from seven countries in Latin America. Int J Gen Med 2014;7:441-48
- Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for practice guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:e257-e354
- You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation Chest 2012;141:e531S-75S
- Members WG, Lloyd-Jones D, Adams RJ, et al. Heart disease and stroke statistics—2010 update a report from the American Heart Association. Circulation 2010;121:e46-e215
- Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke 2011;42:2351-5
- Mayo N, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil 1999;21:258-68
- Gonzalez-Zuelgaray J. The burden of stroke and atrial fibrillation. ISPOR 3rd Latin American Conference; Mexico City, Mexico: September 2011
- Christensen MC, Valiente R, Sampaio Silva G, et al. Acute treatment costs of stroke in Brazil. Neuroepidemiology 2009;32:142-9
- Evers SMAA, Struijs JN, Ament AJHA, et al. International comparison of stroke cost studies. Stroke J Cereb Circ 2004;35:1209-15
- Alcocer L. Challenges and treatment for stroke prophylaxis in patients with atrial fibrillation in Mexico: a review. Am J Cardiovasc Drugs Drugs Devices Interv 2016;16:171-82
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:2246-80
- Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003;290:2685-92
- Boulanger L, Kim J, Friedman M, et al. Patterns of use of antithrombotic therapy and quality of anticoagulation among patients with non-valvular atrial fibrillation in clinical practice. Int J Clin Pract 2006;60:258-64
- Jaffer A, Bragg L. Practical tips for warfarin dosing and monitoring. Cleve Clin. J Med 2003;70:361-71
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005;165:1095-106
- Witt DM, Delate T, Clark NP, et al. Nonadherence with INR monitoring and anticoagulant complications. Thromb Res 2013;132:e124-30
- Hidalgo-Vega A, Askari E, Vidal R, et al. Direct vitamin k antagonist anticoagulant treatment health care costs in patients with non-valvular atrial fibrillation. BMC Health Serv Res 2014;14:46
- Walsh C, Murphy A, Kirby A, et al. Retrospective costing of warfarin. Ir Med J 2014;107:133-5
- Ali A, Bailey C, Abdelhafiz AH. Stroke prophylaxis with warfarin or dabigatran for patients with non-valvular atrial fibrillation-cost analysis. Age Ageing 2012;41:681-4
- Schulman S, Anderson DR, Bungard TJ, et al. Direct and indirect costs of management of long-term warfarin therapy in Canada. J Thromb Haemost 2010;8:2192-200
- Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF Clinical Trial. J Am Heart Assoc 2013;2:e000067
- Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Clin Epidemiol 1987;40:373-83
- Hirsh J, Fuster V, Ansell J, et al. American Heart Association/American College of Cardiology Foundation Guide to Warfarin Therapy. Circulation 2003;107:1692-711
- Chambers S, Chadda S, Plumb JM. How much does international normalized ratio monitoring cost during oral anticoagulation with a vitamin K antagonist? A systematic review. Int J Lab Hematol 2010;32:427-42
- Dlott JS, George RA, Huang X, et al. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation 2014;129:1407-14
- Dolan G, Smith LA, Collins S, et al. Effect of setting, monitoring intensity and patient experience on anticoagulation control: a systematic review and meta-analysis of the literature. Curr Med Res Opin 2008;24:1459-72
- Giugliano RP, Ruff CT, Rost NS, et al. Cerebrovascular events in 21 105 patients with atrial fibrillation randomized to edoxaban versus warfarin: effective anticoagulation with factor xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48. Stroke J Cereb Circ 2014;45:2372-8
- White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007;167:239-45