Abstract
Objective: Ulipristal acetate has been found to be non-inferior to other pre-operative treatments of uterine fibroids, particularly leuprolide. The objective of this study was to assess the pharmacoeconomic profile of ulipristal acetate compared to leuprolide for the pre-operative treatment of moderate-to-severe uterine fibroids in women of reproductive age in The Netherlands. The analysis was performed and applied within the framework of the ulipristal acetate submission for reimbursement in 2012.
Methods: A decision model was developed to compare the total costs of ulipristal acetate compared to leuprolide, the standard care in The Netherlands. The target population of this study corresponded to the type of patients included in the PEARL II clinical trial; i.e. women of reproductive age requiring pre-operative treatment for uterine fibroids. Sensitivity analysis was implemented to assess uncertainties. Data regarding costs, effects, and other input parameters were obtained from relevant published literatures, the Dutch Healthcare Insurance Board, and expert opinion obtained by means of a panel of experts from several medical centers in The Netherlands.
Results: In The Netherlands, the total costs of ulipristal acetate and leuprolide were estimated at €4,216,027 and €4,218,095, respectively. The annual savings of ulipristal acetate were, therefore, estimated at €2,068. The major driver of this cost difference was the cost of administration for leuprolide. Sensitivity analyses showed that ulipristal acetate mostly remained cost-saving over a range of assumptions. The budget impact analysis indicated that the introduction of ulipristal acetate was estimated to result in cost savings in the first 3 years following the introduction. The results of this study were used in the decision on reimbursement of ulipristal acetate according to the Dutch Reference Pricing system in 2012.
Conclusion: Ulipristal acetate was cost saving compared to leuprolide and has the potential to provide substantial savings on the healthcare budget in The Netherlands.
Introduction
Uterine fibroids are benign tumors of the smooth muscle cells of the uterus, which are typically found in women of reproductive age. These fibroids can be found in the uterine cavity, on the uterine wall, or on the exterior of the uterusCitation1. Approximately 20–50% of these fibroids cause common symptoms such as menstrual pain and excessive bleeding (hypermenorrhea) that can lead to anemia. Additionally, there are also several less common symptoms of fibroids including infertility, severe abdominal pain, pain and/or bleeding during intercourse, and pain when urinatingCitation2.
The treatment of symptomatic uterine fibroids has conventionally been surgical (hysterectomy or myomectomy), and numerous slightly invasive methods have been developed in addition to these proceduresCitation1. Nevertheless, alternative pharmacological approaches have been proposed to control and manage the symptoms of uterine fibroidsCitation3. However, if pharmaceutical therapies do not suffice or the symptoms are too severe, surgery is still often indicated. The most common therapies for pre-operative treatment for uterine fibroids in The Netherlands are gonadotropin-releasing hormone (GnRH) agonists such as leuprolide, buserelin, histrelin, goserelin, deslorelin, nafarelin, and triptorelin, with leuprolide as the most commonly prescribed GnRH agonistCitation4. However, even though GnRH agonists effectively reduce bleeding as well as fibroid size, they cause hot flashes in some patients, which can be highly inconvenient and potentially affect quality-of-lifeCitation2.
Symptoms and potential surgical procedures have a profound impact on the quality-of-life of the patients. Uterine fibroids are associated with a high societal burden due to the direct costs for the treatment, the indirect costs due to work absence related to both disease and treatment, and the impaired Health Related Quality-of-Life (HRQoL)Citation5. Although the cost of uterine fibroids has not been estimated for the Netherlands, estimation in the US places the total annual cost of illness higher than common cancer treatments such as treatments for breast and colon cancerCitation6, with an associated annual cost of $1,692 per woman due to productivity loss and healthcare costsCitation7.
Recently, an oral selective modulator of progesterone-receptors in the myometrial and endometrial tissues, ulipristal acetate 5 mg has been proposed as a novel and effective option for pre-operative treatment of moderate-to-severe symptoms of uterine fibroids in women of reproductive ageCitation4. Ulipristal acetate inhibits ovulation without affecting the levels of estradiol or the activity of antiglucocorticoidCitation3,Citation5; therefore, the associated incidence of hot flashes is lower compared to leuprolide. The efficacy and safety of ulipristal acetate have been assessed in the PGL4001 Efficacy Assessment in Reduction of Symptoms Due to Uterine Leiomyomata series of PEARL clinical trials. The PEARL I study evaluated the efficacy and safety of oral ulipristal acetate compared to placeboCitation8, and the PEARL II study assessed the efficacy and side-effect profile of ulipristal acetate vs leuprolide for the treatment of symptomatic uterine fibroids before surgeryCitation4. In the PEARL II study, ulipristal acetate was demonstrated to be non-inferior to leuprolide in reducing bleeding associated with fibroids and was found unlikely to cause hot flashes. The results suggested that ulipristal acetate had some benefits over leuprolide regarding the onset and control of the bleeding and side-effectsCitation4.
The trials confirm that ulipristal acetate has a relevantly better tolerability compared to GnRH agonists such as leuprolideCitation3. Due to safety concerns, such as the loss of bone mineral density, the use of GnRH agonists is limited to short-term therapy, up to 3- until 6-months maximum, with an average treatment duration of 3 monthsCitation2,Citation3. In the period of submission, there were limited efficacy and safety data for treatment beyond 3 months for ulipristal acetate; therefore, at that time, ulipristal acetate was registered for a treatment duration of the same period as GnRH agonists in The NetherlandsCitation9,Citation10. However, due to positive sustained effects that were observed in the first trials, additional assessments to further evaluate the possibility of long-term use of ulipristal acetate were assessed in the long-term intermittent administration trials PEARL III and PEARL IVCitation11,Citation12, that led to approval of 5 mg ulipristal acetate for long-term management of uterine fibroids by the European Commission in 2015.
Our analysis was performed within the framework of the manufacturer’s application for reimbursement in the Dutch Reference Pricing System in 2012, therefore excluding the potential use of ulipristal acetate for the long-term. Given the similar performance on the main outcome measures, a cost minimization analysis (CMA) was conducted in order to demonstrate that ulipristal acetate was cost saving compared to the standard of care in the Dutch setting. Additionally, we performed a budget impact analysis (BIA) to assess the financial impact of the introduction of ulipristal acetate to the Dutch healthcare budget. In this analysis, pricing, as suggested by the manufacturer prior to the reimbursement decision (submission to authorities) during the evaluation of the submission, was analysed.
Methods
Modeling
CMA was undertaken based on the non-inferiority of ulipristal acetate vs leuprolide with respect to the main outcomes observed in the PEARL II studyCitation4. The PEARL II study was selected over the PEARL I study for the specific Dutch setting as it reflects active comparator treatment and also because leuprolide is the most commonly prescribed GnRH agonist for pre-operative treatment for uterine fibroids in The Netherlands.
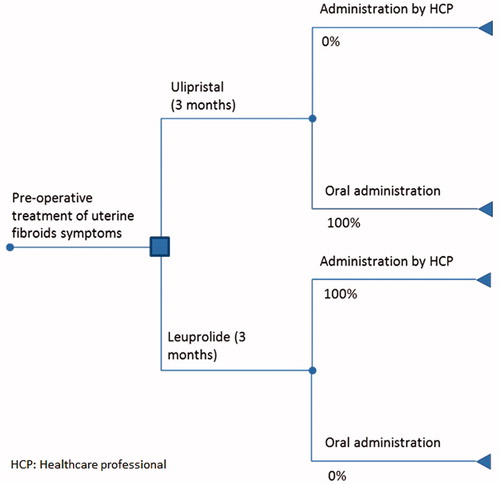
A straight-forward decision tree model was developed to logically link the components influencing the CMA (). The health state in the beginning of the model represents women with uterine fibroids in the pre-surgical state. In addition, according to the treatment guidelines of the Dutch Society of Obstetrics & GynaecologyCitation13 and comparable international advisory bodiesCitation14, treatment with a GnRH agonist is recommended for patients for whom a surgical intervention for their uterine fibroids is scheduled. In the Netherlands, the most frequently prescribed GnRH agonist for the pre-operative treatment of uterine fibroids is leuprolide 3.75 mg (in Dutch: leuproreline, brand name: Lucrin). In the model, leuprolide was defined as the standard treatment and was chosen as the comparator to ulipristal acetate. The time horizon for the base-case we set based on the indicated average treatment period for both drugs involved (3 months).
Patient population
Epidemiological data
The prevalence of uterine fibroids in women of reproductive age within Europe has been estimated to be between 11–24%Citation5. Due to the absence of Dutch prevalence data, we calculated the average prevalence (16.1%) from the estimates of diagnosed uterine fibroids in five surrounding countries (France, Germany, Italy, Spain, UK)Citation5 and applied this to the Dutch population. With 2.95 million women of reproductive age in the NetherlandsCitation15, we estimated that 480,000 women suffer from uterine fibroids. However, these fibroids often appear to be asymptomatic and only ∼15% (∼72,000) are expected to be symptomaticCitation5. Often these symptoms classify as mild and will not escalate into moderate-to-severe. Pharmacological treatment would be adequate in a large proportion of patients, leaving only a relatively small number of patients as candidates for surgeryCitation4.
In another study, the rate of hospital admissions associated with interventions for uterine fibroids per 1,000 women aged 15–55 years in 2005 in the UK, Germany, and France was estimated at 1.34, 2.89, and 2.21 admissions, respectivelyCitation16. We assumed these hospital admission numbers to represent the number of patients with symptomatic fibroids in our analysis. Using data specific to the Dutch population (∼4.4 million women aged 15–55), we estimated 5,950, 9,800, and 12,800 admissions for the Netherlands, when the UK, German, and French admission rates were used, respectively. Based on these numbers, the average estimate of 9,520 patients with uterine fibroids was derived.
Study population
The study population was assumed to be in line with the inclusion criteria in the PEARL II clinical trial. In particular, the study population comprised of pre-menopausal women with:
At least one uterine fibroid with diameter 3 cm or more and no uterine fibroid larger than 10 cm;
Heavy uterine bleeding caused by fibroids, which corresponds to a baseline pictorial blood loss assessment chart (PBAC) score of 100 or more;
Uterine size equivalent to that of pregnancy at 16 weeks of gestation; and
A body mass index between 18–40.
The explicit exclusion criteria in the trial were:
History of uterine surgery;
History of, or current, gynaecological cancer;
Current endometrial hyperplasia; and
Previous, or current, treatment of fibroids with an SPRM or a GnRH agonist.
Perspective and costs
The base-case analysis in CMA applied a healthcare perspective, which was then extended to a societal perspective in the sensitivity analyses. The description regarding all unit costs is presented in . The base-case analysis incorporated only direct medical costs, i.e. cost of leuprolide and cost of ulipristal acetate and its administration, while the societal perspective in the sensitivity analysis incorporated both direct (medical and non-medical) and indirect costs, e.g. productivity loss. Direct non-medical costs included transportation to and from administration site and parking. GP-visits for diagnosis and monitoring as well as the initial prescription costs were plausibly assumed similar for ulipristal acetate and comparator, and were, therefore, not explicitly included in the CMA. The medication cost for ulipristal acetate was set by the manufacturer at €147.62/month (or €5.27/day) at submission. Medication costs for the GnRH agonists were extracted from the Dutch HealthCare Insurance BoardCitation17, with all prices including 6% VAT.
Table 1. Dutch unit costs (€) for resources used.
Administration of leuprolide can be done by the GP or in the hospital outpatient setting (outpatient nurse). In the base-case, conservatively, it was assumed that the “cheapest” administrative option was followed, i.e. during a GP-visit. The costs of a GP visit as well as the transportation costs were based on the Dutch “Manual for Cost Research” (Handleiding voor kostenonderzoek)Citation18.
Additionally, when appropriate, costs were inflated to 2012 prices using the Consumer Price Index (CPI 2009: 105.38 CPI 2012: 111.9), corresponding to the year of submission. Since all costs in the CMA occurred within the first 3 months, discounting was not necessary.
Assumptions
We assumed an average traveling distance of 1.1 km to a GP and 7 km to a hospitalCitation18. In the absence of data for the Dutch situation on work absence due to the administration of a GnRH agonist, we conservatively assumed a duration of 1 h, similar to what has been previously assumed for influenza vaccination of adults at GP-practices in the NetherlandsCitation19. In order to incorporate the labor participation and partial employment for adult reproductive age women, we assumed that 70% of them were employed, where 75% of them worked part-time (28 hours weekly) and the rest worked full time, as reported in the SCP report “Nederland deeltijdland”Citation20. Additionally, we assumed 1 h of work lost due to the administration of leuprolide. Lost productivity was valued according to the Dutch “Manual for Cost Research”Citation18.
Analytical techniques
The total costs related to the pre-operative treatment of uterine fibroids with ulipristal acetate were compared to leuprolide, including direct costs related to administration (oral vs injection). In particular, the base-case considered the costs of medication as well as costs related to administration. The base-case in CMA was based on the conservative and plausible assumptions on administration of leuprolide (100% GP) and oral ulipristal acetate. Moreover, in the absence of effectiveness in the model, transition probabilities between health states were not explicitly modeled. A BIA was also performed to assess the additional burden or the cost savings for the healthcare budget associated with the introduction of ulipristal acetate as a treatment alternative. Cost per year in both the CMA and the BIA was then estimated for the total number of eligible Dutch patients (9,520 patients), based on the assumptions of the aforementioned epidemiological data. In addition, the analysis mimics the reimbursement dossier as submitted to the Dutch authorities.
Budget impact
The cost inputs in the BIA for both drugs were in line with those mentioned previously in the CMA. In the BIA, the estimation was based on comparison of total cost in two scenarios. The first scenario assumed that all eligible patients were treated with leuprolide. The second scenario assumed that ulipristal was introduced as a treatment alternative besides leuprolide in 30%, 40%, and 50% of total patients for pre-operative treatment of uterine fibroids, in the first, second, and third year, respectively.
Sensitivity analysis
In order to substantiate the appropriate estimates on medication administration and comparator, we organized an expert panel (see Supplementary Appendix 1 for expert opinions and Supplementary Table 1 for questionnaire).
Univariate sensitivity analysis was carried out by varying key parameters () of the CMA in accordance with the estimates extracted from the expert panel. Variations considered included the following:
Table 2. Parameter limits used in the univariate sensitivity and scenario analysis.
Duration of treatment. While ulipristal acetate was registered only for treatment duration of 3 months at the time of submission, leuprolide can be administrated for a period of up to 6 months. The expert panel suggested that the treatment duration of GnRH agonists may be extended to 4 months. Therefore, the average treatment duration of leuprolide was varied in the sensitivity analysis from 3–4 months. Additionally, we examined the sensitivity of the CMA to the administration of leuprolide up to 6 months as literature suggested that treatment duration of leuprolide may be extended until 6 months maximumCitation2,Citation3.
Proportion of administration of leuprolide done by the GP and nurse in an outpatient hospital setting. The probability of administration by a GP or a nurse in a hospital outpatient setting was based on expert opinion. Since it was expected to be an influential parameter in CMA, sensitivity analysis was performed in accordance with these specific estimates of the expert panelCitation18.
Societal perspective. We assessed the results where both direct (medical and non-medical) and indirect costs due to productivity losses (with the assumption that 70% of patients were employed, and 75% of them worked part-time)Citation20 were considered in the estimations.
Societal perspective with full-time work assumption. We also assessed the results using a societal perspective, where we assumed that 100% of women worked full-time.
Comparator treatment (mix of GnRH agonists). Leuprolide is the most prescribed GnRH agonist for the treatment of symptomatic uterine fibroids. However, other GnRH agonists are also sometimes given. In a scenario, we compared ulipristal acetate vs a combination of all prescribed GnRH agonists in The Netherlands. To identify the proportion of utilization for each GnRH agonist, we extracted information from the expert panel (see Supplementary Appendix 1, Table A1). We assumed that treatment effectiveness is equivalent across different GnRH agonistsCitation2. Additionally, the hourly cost of a district nurse was based on the Dutch “Manual for Cost Research”.
Results
Base-case
The total treatment costs of daily oral ulipristal acetate 5 mg, for the population of 9,520 Dutch women who are eligible to receive the pre-operative medication for uterine fibroids, was estimated at €4,216,027 per year, in comparison to €4,218,095 for the comparator drug, i.e. once monthly injections of leuprolide. The majority of the costs were attributable to the acquisition costs of the medication (ulipristal acetate: €4,216,027, leuprolide: €3,368,938). However, the incremental cost difference between the two treatments was entirely due to the costs of administrating leuprolide by healthcare professionals such as a GP or nurse (€849,157). Total savings for ulipristal acetate were estimated at €2,068 annually.
Budget impact analysis
With the assumption that ulipristal acetate was being introduced in 30%, 40%, and 50% of Dutch patients in the first, second, and third year, respectively, the total costs were estimated at €4,217,475 in the first year, €4,217,268 in the second year, and €4,217,061 in the third year. Cost savings for the healthcare budget in The Netherlands due to the introduction of ulipristal acetate were estimated at €620, €827, and €1,034 in the first, second and third year, respectively, when compared to the total cost of current treatment without the introduction of ulipristal acetate (estimated to be €4,218,095 in all 3 years), see also .
Table 3. Budget impact analysis*.
Sensitivity analysis
The difference in annual total cost over a range of input parameters was assessed in univariate sensitivity analysis (). Given the fact that daily oral ulipristal acetate was cost-saving compared to leuprolide for almost all alternative levels of the parameters, the base-case result can be considered robust. The sensitivity analysis results also suggested that the parameter that most considerably affected the base-case result was administration of leuprolide in the outpatient setting. The assumption of medication administration of injection leuprolide by either a GP or a nurse in an outpatient clinic resulted in lower annual total costs for leuprolide (€4,128,933), since the administration in an outpatient clinic is less expensive than that in a GP practice. Since there were no administration costs for oral ulipristal acetate, total costs per year for treatment with ulipristal acetate were the same as in the base-case analysis (€4,216,027). Therefore, the annual savings was shifted in favor of leuprolide at €87,094 per year. The assumption of an average of 4 months use for leuprolide resulted in increased annual total costs for leuprolide (€5,624,126). Since the use of ulipristal acetate was only licensed for 3 months by the manufacturer at the time of the submission, the total cost of treatment was the same as in the base-case analysis (€4,216,027). The annual savings was estimated at €1,408,099. Difference in the average treatment duration resulted in favorable results for ulipristal acetate. Even more favorable was when the assumption of 6 months of leuprolide treatment was used: (€8,436,190 vs €4,216,027, with estimated annual savings at €4,220,163).
Figure 2. Incremental annual total costs of ulipristal acetate compared to leuprolide in univariate sensitivity analysis.
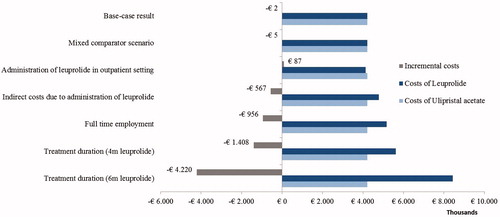
Furthermore, estimation from a societal perspective incorporating direct non-medical (i.e. transportation costs) and indirect costs because of absence from work due to administration of leuprolide, resulted in lower total costs for ulipristal acetate (€4,216,027) than for leuprolide (€4,783,087), which resulted in total annual savings for ulipristal acetate at €567,061. In addition, the assumption of full-time employment of the women with uterine fibroids resulted in increased total costs for leuprolide (€5,171,577) vs ulipristal acetate (€4,216,027).
The variations in comparing ulipristal acetate with other GnRH agonists which are also prescribed in the Netherlands, in the proportion suggested by the expert opinion panel, resulted in virtually similar total costs for both treatment choices, but still higher costs for the GnRH agonists (€4,220,557) compared to ulipristal acetate (€4,216,027).
Discussion
Uterine fibroids are associated with considerably increased healthcare expenses and also place a burden on society in terms of both costs and quality-of-lifeCitation6,Citation21. Women diagnosed with fibroids are also not able to be fully productive at work, with consequent productivity costsCitation5. Before the introduction of daily oral ulipristal acetate, GnRH agonists were the only accessible drugs for pre-operative treatment of uterine fibroidsCitation3.
The results of this study suggested that the use of ulipristal acetate as a pre-operative treatment for uterine fibroids is cost-saving in the Dutch setting. As seen from the base-case results, medication costs of oral ulipristal acetate were substantially higher than leuprolide injection. However, due to direct medical costs associated with the administration of leuprolide, total costs for leuprolide were higher than total costs for ulipristal acetate. An even more favorable result was indicated when a societal perspective was applied in the analysis. A variety of sensitivity analyses was performed, which showed that these cost savings were likely to occur over a range of alternative input values. Additionally, the BIA confirmed that the introduction of ulipristal acetate would result in a positive economic effect for the healthcare budget in The Netherlands. Using a market penetration increasing from 30% to 50% over the first 3 years, an estimated amount of savings in each year were assessed. The results of this cost minimization and BIA are essential to guide healthcare decision-making, e.g. in reimbursement issues.
Our study has contributed to the reimbursement decision for ulipristal acetate within the Dutch Reference Pricing system in 2012. The BIA from this study has been considered as a useful analytical tool for the reimbursement authorities to evaluate the economic efficiency of the novel pre-operative treatment of uterine fibroids compared to the existing ones. Furthermore, two categories exist within this system: (i) groups of basically interchangeable drugs, and (ii) unique non-interchangeable drugs. Alongside eight other applications in 2012, the manufacturer of ulipristal acetate applied for the latter category, for which a pharmacoeconomic dossier was obligatory. Out of nine applications, ulipristal acetate was the only one to receive an unequivocally positive recommendation from the advisory committee of the Dutch National Healthcare Institute (Zorginstituut Nederland - ZiN). Four drugs were recommended to be clustered in corresponding groups of interchangeable drugs (ingenolmebutate, lidocaine, and tetracaine, as well as tapentadol hydrochloride), while for one the pharmacoeconomic dossier was deemed insufficient (ruxolitinib). The other four drugs obtained limited reimbursement with restrictions in the exact indication, prescriber, and/or requiring patient access schemes (dapagliflozin, combination of elvitegravir, emtricitabine, tenofovirdisoproxilfumarate, and cobicistat, fidaxomicin, as well as pirfenidone). Ulipristal was recommended to be labeled as a unique non-interchangeable drug with unlimited access by Zorginstituut Nederland. Generally, the Ministry of Health follows Zorginstituut Nederland’s recommendations, which was also the case regarding these 2012 recommendations. In particular, ulipristal acetate obtained unlimited reimbursement at the price initially requested by the manufacturerCitation9.
There were only very few previous published economic evaluation studies which assess ulipristal acetate for treatment of uterine fibroids. In early 2013, the Scottish Medicines Consortium (SMC) assessed and approved ulipristal acetate and advised the National Health Service (NHS) and Area Drug and Therapeutic Committees (ADTCs) on its use in ScotlandCitation10. For the base-case analysis, the GnRH agonist goserelin was used as a comparator. In 2014, a cost-effectiveness study on ulipristal acetate for pre-operative treatment in moderate-to-severe uterine fibroids in Hungary was publishedCitation22. This study assessed ulipristal acetate as an add-on therapy in pre-surgical treatment and immediate hysterectomy as comparative strategiesCitation22. The results from both studies suggested that ulipristal acetate was a cost-effective strategy relative to the comparators; however, in the Dutch setting, both of the comparators were considered unsuitable, because goserelin was infrequently prescribed in The Netherlands (see expert opinion) and hysterectomy was a procedure that generally did not occur until after pre-operative treatment. The most recent economic evaluation study in this field was a cost-utility analysis to assess ulipristal acetate for the care of symptomatic uterine fibroids in comparison with leuprolide, in Canada. In this study, the model suggested that ulipristal acetate strategy dominated leuprolide over the range of alternative settings as it is provided to Canadian patients, with more quality-adjusted life years (QALYs) at a lower cost from both a healthcare payer and a societal perspectiveCitation23. As the use of ulipristal acetate has been authorized by the manufacturer worldwide, we can expect more research to firmly establish the contribution of ulipristal acetate in the management of uterine fibroids patientsCitation24.
In addition to a cost-analysis, we also assessed budget impact of the introduction of ulipristal acetate in The Netherlands. This analysis gave the overview that increasing the number of patients using ulipristal acetate would lead to greater savings during the first year as well as the subsequent years in the time horizon of 3 years. The budget savings demonstrate that the choice of oral ulipristal acetate as a pre-operative treatment for uterine fibroids in the market would be beneficial, not only clinically, but also financially.
The most recent PEARL III and IV clinical trials suggested that there is a possibility of long-term intermittent treatment of ulipristal acetate for the sustainable management of uterine fibroids instead of the short term 3-month treatment. They also show promise for this strategy to rapidly control bleeding and progressively shrink the fibroids, with a better safety profileCitation11,Citation12. The latest PEARL IV clinical trial confirmed the efficacy and safety of repeated 12-week courses of 5 or 10 mg of ulipristal acetate for treatment of uterine fibroidsCitation11. With the recent approval of long-term intermittent use of ulipristal acetate in the European Union, this option could be promising to provide an effective long-term management for uterine fibroids. In addition to pre-operative treatment for less invasive surgery, ulipristal may offer an alternative to avoid surgical therapy completely, especially for women who want to preserve fertility, although the choice of treatment obviously depends on the symptoms, severity, age, infertility, as well as desire to preserve the uterusCitation25. This possibility might increase the overall cost of ulipristal acetate as a whole; however, it might also eliminate the risk of major surgery that could lead to reduction in health-related quality-of-life for patients.
There are several limitations of this study. First, epidemiological information for the estimation of costs was obtained by synthesizing several sources. Because prevalence data for the Dutch setting were not available, this number was approximated by using the average hospital admission rate attributable to fibroids from three surrounding European countries, i.e. Germany, France, and the UK. These assumptions could have led to an under- or over-estimation of the approximation of both savings and budget impact per year related to the introduction of ulipristal acetate in a Dutch population. Second, we ignored the costs of adverse effects contributable to the use of leuprolide. In order to manage adverse effects from leuprolide, hormonal add-back therapy may be prescribed to the patientsCitation21. The inclusion of these costs would result in a more favorable outcome for ulipristal acetate, implying that current results under-estimate true cost savings. Third, generalizability of these results is limited. While the model represents the pre-operative treatment pathway of uterine fibroids in general, the analysis was performed specifically in the Dutch setting. The treatment patterns of uterine fibroids vary across countriesCitation26, thus the comparator would also be different, in accordance with a country’s specific setting. Although we have performed sensitivity analysis as an attempt to overcome this issue, there are certain cases that are beyond the range of our analysis. Lastly, our analysis applied to the regulatory situation at the time of the submission in 2012, and, therefore, ignores the potential long-term use of ulipristal acetate as an alternative to avoid surgical intervention. Further economic evaluation studies that take into account the results from PEARL III and IV trials need to be done to further confirm the cost-effectiveness of the option for long-term intermittent use of ulipristal acetate for the management of uterine fibroids.
Conclusion
In conclusion, ulipristal acetate is a cost-saving option for the pre-operative treatment of moderate and severe symptoms of uterine fibroids compared to leuprolide, with potential to provide savings on the healthcare budget in the Netherlands; however, because of the restricted treatment duration at the time of analysis, this finding is only valid in the short-term. Given the potential health advantage of long-term treatment of ulipristal acetate, further research in both clinical and economic impact of ulipristal acetate is needed to confirm this possibility.
Transparency
Declaration of funding
This study was funded by a grant from Gideon Richter Benelux (Diegem, Belgium).
Declaration of financial/other relationships
MJP has received grants and honoraria from various pharmaceutical companies, including companies interested in the content of this article, and holds shares in Ingress Health. ADIvA has received a grant from Nestlé for the development of an instrument for infant quality-of-life. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Appendix - Expert Opinions
Download MS Word (19 KB)Table - Example of Questionnaire
Download PDF (293.3 KB)Acknowledgments
The authors would like to thank Petros Pechlivanoglou, PhD (University of Toronto), for his contributions during the initial period of the project and for providing data for this paper.
References
- Stewart EA. Uterine fibroids. Lancet 2001;357:293-8
- Lethaby A, Vollenhoven B, Sowter MC. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids (review). Cochrane Database Syst Rev 2011;2001:CD000547
- Biglia N, Carinelli S, Maiorana A, et al. Ulipristal acetate: a novel pharmacological approach for the treatment of uterine fibroids. Drug Des Dev Ther 2014;8:285-92
- Donnez J, Tomaszewski J, Vázquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012;366:421-32
- Downes E, Sikirica V, Gilabert-Estelles J, et al. The burden of uterine fibroids in five european countries. Eur J Obstet Gynecol Reprod Biol 2010;152:96-102
- Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. Obstet Gynecol 2012;206:211.e1-9
- Côté I, Jacobs P, Cumming D. Work loss associated with increased menstrual loss in the United States. Obstet Gynecol 2002;100:683-7
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012;366:409-20
- Zorginstituut Nederland. Zorginstituut Nederland, 2015. http://www.zorginstituutnederland.nl/. The Netherlands: Ulipristal (Esmya), Accessed January, 2015
- National Health Service Scotland. Scottish medicines consortium ulipristal acetate (esmya). 2016. https://www.scottishmedicines.org.uk/SMC_Advice/Advice/1128_16_ulipristal_acetate_Esmya/ulipristal_acetate_Esmya. Scotland, Accessed: February, 2016
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 2014;103:359-360
- Donnez J, Vázquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate ☆. Fertil Steril 2014;101:1565-73.e1-18
- Thurkow AL, Admiraal CF, Emanuel MH, et al. Submucous myomas: Diagnosis and therapy. Gynecol Surg 2007;5:93-102
- (NICE), National Institute of Health and Clinical Excellence. Heavy menstrual bleeding, clinical guideline no. 44. London: NICE, 2007. https://www.nice.org.uk/guidance/cg44/resources/heavy-menstrual-bleeding-assessment-and-management-975447024325. Accessed June, 2015
- Statistics Netherlands. Statistics Netherlands, 2016. https://www.cbs.nl/en-gb. The Netherlands: StatLine- Population, Accessed
- Fernandez H, Farrugia M, Jones SE, et al. Rate, type, and cost of invasive interventions for uterine myomas in Germany, France and England. J Minim Invas Gynecol 2008;16:40-6
- Zorginstituut Nederland. Medicijnkosten. 2016. https://www.medicijnkosten.nl/. The Netherlands, Accessed January 2014
- Hakkaart-van Roijen I, Tan S, Bouwmans CAM. Handleiding voor kostenonderzoek methoden en standaard kostprijzen voor economische evaluaties handleiding voor kostenonderzoek methoden en standaard kostprijzen voor economische evaluaties. 2010 Instituut voor Medical Technology Assessment, Erasmus MC in opdracht van College voor zorgverzekeringen, The Netherlands: ErasmusUniversiteit Rotterdam
- Hak E, Van Loon S, Buskens E, et al. Design of the dutch prevention of influenza, surveillance and management (PRISMA) study. Vaccine 2003;21:1719-24
- Portegijs W, Keuzenkamp S. Nederland deeltijdland: Vrouwen en deeltijdwerk. Den Haag: Sociaal en Cultureel Planbureau, 2008. http://www.flexworkresearch.org/uploads/publication/document/2693/Nederlanddeeltijdland.pdf. Accessed January, 2014
- Friedman AJ, Daly M, Juneau-Norcross M, et al. Long-term medical therapy for leiomyomata uteri: a prospective, randomized study of leuprolide acetate depot plus either oestrogen-progestin or progestin 'add-back' for 2 years. Hum Reprod 1994;9:1618-25
- Nagy B, Timár G, Józwiak-Hagymásy J, et al. The cost-effectiveness of ulipristal acetate tablets in treating patients with moderate to severe symptoms of uterine fibroids. Eur J Obstet Gynecol Reprod Biol 2014;175:75-81
- Tsoi B, Blackhouse G, Ferrazzi S, et al. Incorporating ulipristal acetate in the care of symptomatic uterine fibroids: a Canadian cost-utility analysis of pharmacotherapy management | CEOR. ClinicoEcon Outcomes Res 2015;7:213-225
- Talaulikar VS, Manyonda IT. Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. Adv Ther 2012;29:655-63
- Donnez J, Dolmans MM. Uterine fibroid management: From the present to the future. Hum Reprod Update 2016;22:665-686
- Zimmermann A, Bernuit D, Gerlinger C, et al. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health 2012;12:6-6874-12-6