 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: The clinical and economic benefits associated with apixaban treatment have been established in clinical trials and published economic evaluations. The benefits associated with apixaban could extend to improving hospital efficiencies, potentially influencing hospital resource use, and bed days. The objective of this study is to estimate the impact of 6-month treatment with apixaban vs low molecular weight heparin/vitamin k antagonist (LMWH/VKA) on hospital resource use among patients with venous thromboembolism (VTE).
Methods: A model was developed to assess the impact of apixaban vs LMWH/VKA for treatment of VTE and prevention of recurrences on hospital resource use and costs. Resource use items included total hospitalizations, length of stay (LOS), and emergency department (ED) visits, estimated for all incident VTE patients in the UK over a 5-year time horizon. Rates of hospitalizations, ED visits, and LOS associated with recurrent VTE, major, and clinically relevant non-major bleeding were obtained from the AMPLIFY trial; costs were obtained from UK published sources.
Results: Over a 5-year time horizon, the model predicted that, compared to 6 months of LMWH/VKA, 6 months of apixaban led to 3,954 fewer hospitalizations (consisting of 2,341 fewer new admissions and 1,613 fewer re-admissions) and 32,214 fewer bed days, among 332,607 incident VTE patients. ED visits were reduced by 1,582. The reduction in hospital resource use led to a cost saving of ∼£4.5 million in a market of patients treated with apixaban as compared to a market treated with LMWH/VKA. Sensitivity analysis indicated these findings were robust over a wide range of inputs.
Conclusions: 6-month treatment with apixaban for treatment of VTE and prevention of recurrences on hospital resource use led to a reduction in hospitalizations and LOS in comparison to LMWH/VKA. These findings can help the efforts in reducing the growing burden of preventable re-admissions to hospitals.
Introduction
Venous thromboembolism (VTE) is a collective term for deep vein thrombosis (DVT) and pulmonary embolism (PE). It affects approximately 1 in every 1,000 of the UK population and is a significant cause of mortality, long-term disability, and chronic ill-health problemsCitation1. Treatment of non-fatal VTE and its related long-term morbidities represent a large burden to healthcare systemsCitation2. It is estimated that VTE costs the National Health Service in the UK £640 million per yearCitation3. The burden of VTE is large, not only due to initial hospitalization and treatment, but also because of the high number of additional hospitalizations (i.e. re-admissions) and extended hospital stays connected to the conditionCitation4. Approximately half of all cases of VTE are associated with hospitalization, recurrent VTEs, and medication-associated side-effects occurring after the initial admission, precipitating further hospitalizationsCitation1. Studies following large cohorts of patients with VTE have estimated the incidence of hospital re-admission within 30 days of discharge to be 24.9%, and further highlighted that re-admissions for VTE are significantly longer and more costly than index eventsCitation5,Citation6.
Re-admissions that could have otherwise been prevented have been found to place a frequent and significant demand on healthcare resourcesCitation7. In addition to the associated costs, re-admissions are linked with adverse health outcomes to patientsCitation7. Healthcare systems have placed a focus on reducing re-admissions; for example, the Department of Health in England have introduced financial penalties to reduce re-admissions by restricting payments on re-admissions that occur within 30 days of the initial hospitalizationCitation8,Citation9. The introduction of these financial incentives highlights the growing burden of preventable re-admissions and efforts to reduce it.
Appropriate anticoagulant therapy and continuity of care in patients with VTE may reduce the incidence and frequency of hospital re-admissions and have a potential effect on healthcare resources. The National Institute for Health and Care Excellence (NICE) in the UK has issued guidance recommending apixaban in addition to other non-vitamin k antagonist oral anticoagulants (NOACs) for the treatment of VTE and the prevention of recurrencesCitation10, based on clinical and economic evidenceCitation11–16. Treatment with apixaban for the prevention of recurrent VTE was studied in two randomized controlled trials, AMPLIFY and AMPLIFY-EXTCitation12,Citation13. In AMPLIFY, a 6-month randomized, controlled, double-blind trial, apixaban was found to be non-inferior to low molecular weight heparin/vitamin k antagonist (LMWH/VKA) in reduction of recurrent VTE events, and was associated with a significant reduction in major bleeding eventsCitation12. In the placebo-controlled AMPLIFY-EXT trial, conducted in patients with VTE who had previously completed 6–12 months of anticoagulation therapy, apixaban was found to significantly reduce the risk of recurrent VTE and VTE-related death without increasing the rate of major or clinically relevant non-major (CRNM) bleeding, over a 12-month study period as compared to placeboCitation13. In addition to the clinical trials, economic evaluations and NICE guidance have suggested that use of apixaban represents a cost-effective alternative to conventional therapyCitation14,Citation15. The benefits associated with apixaban may further extend to improving efficiency and resource use within hospitals. For example, post-hoc analysis of AMPLIFY and AMPLIFY-EXT found that apixaban was associated with a significant reduction in all-cause hospitalizations and an increase in time to first hospitalizations in comparison to both LMWH/VKA and no treatmentCitation17–20.
The objective of this study was to estimate the effects of treatment for initial VTE and prevention of recurrence for a period of 6 months with apixaban vs LMWH/VKA on number of hospitalizations, bed days, emergency department (ED) visits, and associated costs from the UK hospital perspective over a 5-year time horizon.
Methods
A model was constructed to estimate the effects of treatment for VTE and the prevention of recurrence on hospital resource use among patients who have an incident hospitalized VTE in the UK over a 5-year time horizon. A UK hospital perspective was adopted assessing the impact of alternative treatment strategies on hospitalizations, length of stay (LOS), ED visits, and costs.
Treatment
The analysis compared a market where patients with VTE were treated with apixaban (10 mg twice daily [BID] for 1 week, then 5 mg BID) vs a market treated solely with LMWH/VKA (LMWH initiated for at least 5 days, with dose-adjusted VKA therapy beginning concomitantly and continued for 6 months)Citation12. The analysis considers only patients who were treated as an inpatient at their index event (83% of index PE and 31% of index DVT patients) based on data from earlier NICE submissionsCitation10,Citation21.
Population
The analysis considered UK patients with a recently incident non-fatal VTE. Population estimates were obtained from the Office of National Statistics and assumed to increase by 0.7% per yearCitation22. The annual number of cases of VTE in the UK were estimated by applying the incidence of VTE, expected to be 13.2 per 10,000 person years, to the expected size of UK population in a given yearCitation23. Of the population experiencing VTE, it was assumed that 34.2% has experienced PE, vs 65.8% experiencing DVT, based on the characteristics of patients in the AMPLIFY trialCitation12.
Model structure
The analysis was conducted using a Markov cohort model with three health states: on treatment, off treatment, and dead. The model was developed in Microsoft Excel (), as this is a common platform for most users.
Figure 1. Model structure. CRNM, clinically relevant non-major; DVT, deep vein thrombosis; PE, pumonary embolism; VTE, venous thromboembolism.
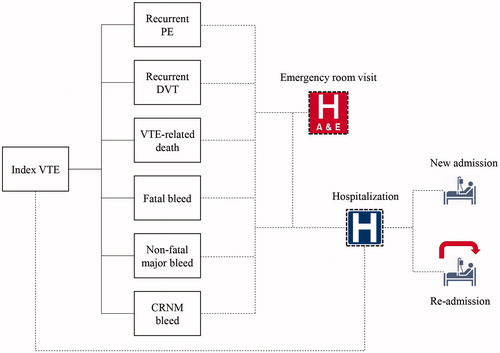
Patients who entered the model each year were assumed to begin with index VTE in the on-treatment state, having just experienced an index VTE event and initiating anticoagulation for the treatment and prevention of further VTE events. Patients remained in the on-treatment health state until the end of their treatment duration (6 months), after which they moved to the off-treatment health state. Patients could transition from the on-treatment or off-treatment health states to death if they experienced a fatal VTE or fatal bleed event. The model used a 3-month cycle length for the first 2 years from index event, and then a cycle length of 1 year thereafter. A shorter cycle length was used in the first period due to the increased risk of clinical events. It was assumed that patients do not prematurely discontinue treatment during the first 6 months.
In each model cycle, patients were subjected to risks of recurrent VTE, major bleeds, and CRNM bleeds, based on analysis of the AMPLIFY and AMPLIFY-EXT trialsCitation14 (risks for those events in patients receiving apixaban and relative risks associated with LMWH/VKA or placebo compared with apixaban are presented in and ). The occurrence of these events could lead to hospitalizations, which could be classified as a new admission or a re-admission. Each hospitalization was associated with a LOS and cost (). In addition to hospitalizations, ED visits were modeled to occur as a consequence of a recurrent VTE or major bleeding event. Hospitalization-related and ED visit costs were calculated based on the unit costs and the total resource use counts estimated in the model. Drug costs were not separately included, as apixaban, LMWH, and VKA are currently covered under Healthcare Resource Group (HRG) payment system, thus considered non-incremental for the purposes of this analysisCitation9.
Table 1. Risks of clinical events.
Table 2. Relative treatment effects for LMWH/VKA and placebo over apixaban.
Incident VTE patients entered the model at the start of each year and were followed for the remainder of the model time horizon. Patients from previous years in the model continued to accrue costs and hospital resource use until the end of the model time horizon.
Hospital resource use ()
Hospital resource use data, as a direct consequence of VTE and anticoagulation treatment, are considered as detailed in . Resource use related to recurrent VTE and bleeding events was obtained from post-hoc analysis of the AMPLIFY trialCitation12. Using the recorded cause of hospitalization or ED visits, means, and 95% confidence intervals (CI) for the rates of hospitalizations, rates of ED visits, and LOS associated with hospitalization for each event (e.g. recurrent VTE, major bleed, and CRNM bleed) were computed. In estimating LOS, consideration was made of potential outlier records and data was winsorized at the top 10%Citation24.
Table 3. Hospital resource use.
All hospital resource use items were included as rates and were multiplied by the number of events occurring in each cycle.
To distinguish between new admissions and which were re-admissions among the hospitalizations, re-admissions were classified as any admission occurring within 30 days of a previous hospitalization, consistent with the definition used in the UKCitation9. The number of hospitalizations classified as re-admissions were determined using the following equation:
where γ is the number of hospitalizations occurring within 30 days of the start date, φ is the proportion of patients who were an inpatient at index event, λ is the number of hospitalizations within 30 days of any previous hospitalization, and δ is the total number of hospitalizations.
Each hospitalization was assigned a corresponding LOS to estimate the total number of bed days and a cost per day to estimate the total costs to hospital.
Costs ()
Hospitalization associated costs and sources are detailed in and reflect 2014/2015 valuesCitation25. When prices for these years were not available, inflation rates were applied using the Pay & Prices indexCitation26.
Table 4. Hospital event costs.
Table 5. Resource use results.
Table 6. Hospital costs results.
Analyses
Base-case analyses
The base-case analysis assessed the total population of incident VTE patients in the UK over a period of 5 years, comparing 6-month treatment of apixaban vs 6-month treatment with LMWH/VKA. The analysis followed patients who were hospitalized due to recurrent VTE or major bleeding following index VTE; patients who were treated as outpatients for these complications were not considered. Outcomes considered included total hospitalizations, new admissions, re-admissions, ED visits, number of bed days, and related costs for each treatment. Incremental reductions or increases in resource use as well as costs were assessed for apixaban vs LMWH/VKA.
Sensitivity analyses
One-way sensitivity analyses were conducted to evaluate the robustness of the model results and conclusions in relation to uncertainties in all model inputs, and to assess how the model outcomes varied in relation to changes in model parameters. The model inputs varied included: clinical event rates (e.g. recurrent VTE, major bleeding, CRNM bleeding), event-related resource use (e.g. rates of hospitalizations, ED visits, LOS), and costs. The one-way sensitivity analyses were performed by changing all these model inputs, one by one, according to their 95% CIs, while keeping all others constant and recalculating results. The results are illustrated diagrammatically as tornado diagrams. The tornado diagrams include the top 15 parameters by the order of influence on hospitalizations, ED visits, hospital costs, and bed days.
Scenario analyses
In addition to the above, and due to the uncertainty associated with the optimal duration of treatment, scenario analysis was conducted to assess the impact of extended treatment (18 months) with apixaban in comparison to 6-month LMWH/VKA on hospital resource use. Clinical event rates for the extended period (beyond 6 months) were obtained from the AMPLIFY-EXT trial ()Citation14. Treatment effects for placebo compared with apixaban for the extended period in the form of relative risks (RRs) were estimated from the secondary analysis of the AMPLIFY-EXT trial ()Citation14.
Results
Base-case analysis ( and )
Over a 5-year time horizon, the model predicted hospital resource use and cost outcomes following a total of 162,259 index hospitalizations for all 332,607 incident VTE patients in the UK. Compared to 6 months of LMWH/VKA, 6 months of apixaban led to 3,954 (−10.0%) fewer hospitalizations, including 2,341 (−9.9%) fewer new admissions and 1,613 (−10.1%) fewer re-admissions. The reduction in number of hospitalizations led to 32,214 (−10.0%) fewer bed days. There were 415 (−1.4%) fewer hospitalizations due to recurrent VTE, 2,273 (−40.8%) fewer hospitalizations due to major bleed, and 1,266 (−30.4%) fewer hospitalizations due to CRNM bleed. The majority of the reduction (∼78%) in new admissions was observed in patients with a DVT event at index, whilst the majority of the reduction in re-admissions (∼52%) was observed in patients with a PE event at index.
In addition to hospitalizations, treatment with apixaban was associated with 1,582 (−3.1%) fewer emergency department visits, 365 (−1.1%) of which would have led to hospitalization under treatment with LMWH/VKA.
The reduction in hospital resource use led to an overall cost saving of £4.49 million in a market where patients with VTE were treated solely with apixaban instead of LMWH/VKA.
Sensitivity analysis
present the results of the sensitivity analyses of the estimation of hospitalizations, ED visits, hospital costs, and bed days when comparing 6 months’ treatment with apixaban vs LMWH/VKA. These figures show the 15 parameters that had the greatest effect on the hospitalizations, ED visits, hospital costs, and bed days arranged in descending order of such influence. In our analysis, no scenario tested in the sensitivity analysis resulted in apixaban increasing total hospitalizations, ED visits, costs, or bed days when compared to LMWH/VKA.
Figure 2. One-way sensitivity analysis of long-term treatment with apixaban vs LMWH/VKA. CRNM: clinically relevant non-major; DVT: deep vein thrombosis; ED: emergency department; LMWH: low molecular weight heparin; LOS: length of stay; PE: pumonary embolism; VKA: vitamin K antagonist; VTE: venous thromboembolism.
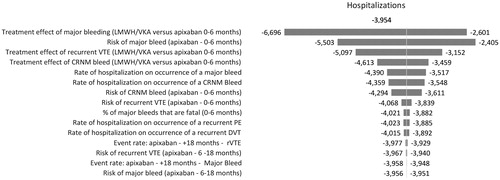
Figure 3. One-way sensitivity analysis of long-term treatment with apixaban vs LMWH/VKA. CRNM: clinically relevant non-major; DVT: deep vein thrombosis; ED: emergency department; LMWH: low molecular weight heparin; LOS: length of stay; PE: pumonary embolism; VKA: vitamin K antagonist; VTE: venous thromboembolism.
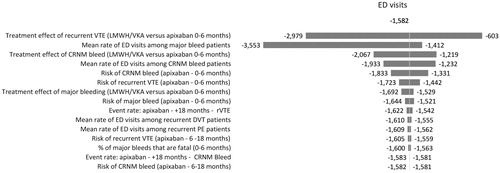
Figure 4. One-way sensitivity analysis of long-term treatment with apixaban vs LMWH/VKA. CRNM: clinically relevant non-major; DVT: deep vein thrombosis; ED: emergency department; LMWH: low molecular weight heparin; LOS: length of stay; PE: pumonary embolism; VKA: vitamin K antagonist; VTE: venous thromboembolism.
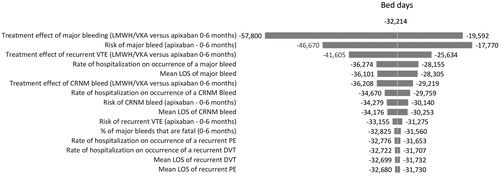
Figure 5. One-way sensitivity analysis of long-term treatment with apixaban vs LMWH/VKA. CRNM: clinically relevant non-major; DVT: deep vein thrombosis; ED: emergency department; LMWH: low molecular weight heparin; LOS: length of stay; PE: pumonary embolism; VKA: vitamin K antagonist; VTE: venous thromboembolism.
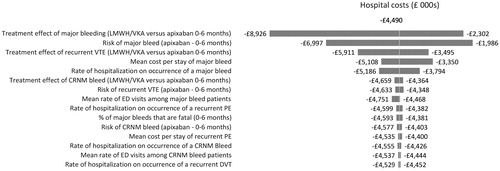
For hospitalizations, hospital costs, and bed days, the most influential parameters were the same—the relative risk of major bleeding vs apixaban for patients treated with LMWH/VKA for the initial period, the event rate of major bleeding for patients treated with apixaban and the relative risk of recurrent VTE vs apixaban for patients treated with LMWH/VKA for the initial period. For ED visits the most influential parameters were the relative risk of recurrent VTE vs apixaban for patients treated with LMWH/VKA for the initial period, the mean rate of ED visits among major bleed patients and the relative risk of CRNM bleed vs apixaban for patients treated with LMWH/VKA for the initial period.
Scenario analysis
Scenario analysis extending the duration of treatment with apixaban to 18 months resulted in a greater decrease in resource use. Specifically extending treatment to 18 months resulted in 12,064 (−30.5%) fewer admissions, 40% of which would have been classified as re-admissions. This reduction resulted in 99,922 fewer bed days (−31.0%), and cost savings of ∼£14 million.
Discussion
This study assessed the effects of apixaban for 6-month treatment and prevention of recurrent VTE vs LMWH/VKA on hospital resource use and costs. Over a 5-year time horizon, our analysis highlighted that 6-month anticoagulation with apixaban vs LMWH/VKA led to reductions in the total number of hospitalizations and bed days. Re-admissions were significantly reduced with greater benefit in reducing re-admissions observed in patients with an index PE event. This matches a priori expectations, as patients with PE are more likely to be hospitalized than patients with DVTCitation27. As a consequence of a reduced number of hospitalizations, the overall hospital costs were reduced in patients treated with apixaban. The gains increased with increasing treatment durations as extended treatment with apixaban (18 months) led to greater reductions in overall hospitalizations and associated hospital costs.
The results of this analysis add to the comprehensive assessment of the effects and costs of apixaban from the cost-effectiveness analyses and the clinical trialsCitation11–14. This is the first study to attempt to quantify the resource use implications of treatment with apixaban in patients with VTE in the UK hospital setting. Although our study focused on a 6-month treatment duration, due to uncertainty surrounding the optimal duration of anticoagulation, scenario analyses to determine the impact of apixaban over an extended treatment duration was conducted. This analysis highlighted even more favorable results in reducing hospital resource use, thus highlighting potential further benefits of extended anticoagulation.
There are some limitations associated with our analysis. First, clinical and resource use data to inform the model were obtained from AMPLIFY and AMPLIFY-EXT, which were multinational trials. Thus, rates of hospitalizations were based on hospitalizations across multiple countries rather than a UK population. This is an important limitation, as resource use and treatment patterns may vary significantly between countriesCitation28. Nonetheless, our estimates are comparable to those found by studies conducting surveys with physicians and emergency departments on the management of VTE in the UKCitation29,Citation30, as well as solicited clinical opinion, which highlighted that ∼30% of patients with DVT and 50% of patients with PE are managed as inpatientsCitation15. Moreover, LOS estimates are within the lower range reported in systematic reviews of resource use associated with VTECitation28, indicating that any bias associated with the use of multinational data would be unfavorable to apixaban.
Furthermore, the risks of recurrent VTE and bleeding events applied in our analysis do not necessarily reflect a population of VTE patients treated solely in an inpatient setting, and may include a proportion of patients treated as outpatients. Risks of clinical events could not be obtained by treatment setting as this information was not collected in the AMPLIFY trial; however, studies have recommended that treatment in the outpatient setting should generally be restricted to patients with a lowered risk of adverse clinical outcomesCitation31,Citation32. This implies that patients treated in an inpatient setting may have a higher risk of recurrent VTE or bleeding. Whilst we find lack of adjustment in the risk level of patients treated as inpatients to be a limitation of our analysis, the implications of adjustment would likely be favorable for apixaban. With an increased risk of recurrent VTE or bleeding, due to the relative treatment effect of LMWH/VKA over apixaban in recurrent VTE and major bleeding (1.20 and 3.30, respectivelyCitation12), apixaban would be anticipated to avert even more clinical events in absolute terms
A further limitation to our analysis is that we did not differentiate LOS by treatment. Data to inform LOS was based on recurrent VTE events and bleeds occurring after the initial VTE event. The number of hospitalizations occurring as a result of recurrent PE, recurrent DVT, and major bleeding from the AMPLIFY trial were not high enough to allow analysis of LOS for each event by treatment. We were, therefore, unable to detect whether the LOS for each event varied depending on treatment received. Previous research suggests that use of NOACs may be associated with a lower LOS as compared to LMWH/VKACitation33, which can be attributed to lack of need to bridge initiation of VKA with a LMWH. This suggests that treatment with apixaban may have a further effect reducing LOS for each event beyond that attributed to a reduced number of events, which was not captured in the current analysis.
Further uncertainty stems from assumptions made when analyzing LOS. For example, data was winsorized at the top 10% to account for outliers. In addition, LOS was not varied by type of bleeding event; thus, the potential increased LOS due to intracranial bleeds may not have been sufficiently captured as only two events occurred in AMPLIFY. However, varying LOS for each event by their 95% CI in the sensitivity analysis showed that increasing LOS would have caused a larger reduction in bed days avoided with apixaban. Further uncertainty stems from assumptions made when analyzing LOS. For example, data was winsorized at the top 10% to account for outliers. In addition, LOS was not varied by type of bleeding event; thus, the potential increased LOS due to intracranial bleeds may not have been sufficiently captured as only two events occurred in AMPLIFY. However, varying LOS for each event by their 95% CI in the sensitivity analysis showed that increasing LOS would have caused a larger reduction in bed days avoided for apixaban.
In addition, the data used within our analysis was based on an international population rather than data from the UK directly, as data from the UK were not available in AMPLIFY. Data from the UK NHS reference costs on the mean average LOS for DVT and PE patients fell within the range varied within the sensitivity analysis (6.9 and 7.3 days, respectively). Although the estimate from the UK NHS reference costs is lower than that included in the base case analysis, these estimates are not based on patients with recurrent VTE, but also patients with a first index VTE. The LOS associated with a recurrent event have been found to be significantly longer than the LOS associated with the initial event; thus, estimates derived from patients with recurrences were preferredCitation6. Nonetheless, lowering the LOS associated with these events in sensitivity analysis did not alter the conclusions of our analysis.
Finally, several simplifying assumptions were included in the model that could have influenced results. The model only included mortality due to fatal VTE and fatal bleeding; other-cause mortality was not considered; however, over a 5-year time horizon other-cause mortality was assumed to be negligible and equivalent between the two treatments and, thus, non-incremental in terms of number of hospitalizations and bed days avoided. We also assumed that patients do not prematurely discontinue treatment in the first 6 months, for consistency with efficacy data used which were based on the intention-to-treat population. This assumption is likely to be conservative for apixaban, as findings from the AMPLIFY trial suggest that 52.7% of patients discontinue treatment after experiencing a major bleed eventCitation14. Had we incorporated premature discontinuation due to bleeding, more patients with LMWH/VKA would have discontinued to the off treatment health state and been exposed to the inferior VTE prevention of no treatment, thus potentially resulting in higher resource use for the LMWH/VKA arm
Despite limitations and simplifying assumptions, extensive sensitivity and scenario analysis highlighted that the model conclusions remained unchanged, with the direction of the bias being conservative for apixaban. Thus, the benefits of using apixaban could be even higher than the model estimation in the real world.
Conclusions
The comprehensive assessment of hospital resource use and costs of apixaban in this study predicted that 6-month treatment with apixaban for patients with VTE was a superior alternative to 6-month treatment with LMWH/VKA in terms of hospital resource use and associated costs. The implication of our findings on the reduction of re-admissions and bed days can help the efforts in reducing the growing burden of preventable re-admissions to hospitals.
Transparency
Declaration of funding
This analysis was funded by Bristol-Myers Squibb and Pfizer.
Declaration of financial/other relationships
CB, TL, and TK are employees of Evidera, which received funding from Bristol-Myers Squibb and Pfizer in connection with conducting this study and the development of this manuscript. MH, XL, RH, and JM, are employees of BMS and Pfizer, respectively. AC has received a grant and personal fees from both BMS and Pfizer previously. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- National Health Service (NHS) England. Venous thromboembolism. England: NHS; 2016. https://www.england.nhs.uk/patientsafety/venous-thromb/. Accessed May 2016
- LaMori JC, Shoheiber O, Mody SH, et al. Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States. Clin Ther 2015;37:62-70
- House of Commons Health Committee Report on the Prevention of Venous Thromboembolism in Hospitalised Patients. England: House of Commons Health Committee; 2016. http://www.parliament.the-stationery-office.co.uk/pa/cm200405/cmselect/cmhealth. Accessed May 2016
- Ruppert A, Lees M, Steinle T. Clinical burden of venous thromboembolism. Curr Med Res Opin 2010;26:2465-73
- Bullano MF, Willey V, Hauch O, et al. Longitudinal evaluation of health plan cost per venous thromboembolism or bleed event in patients with a prior venous thromboembolism event during hospitalization. J Manag Care Pharm 2005;11:663-73
- Casciano JP, Dotiwala Z, Kemp R, et al. Economic burden of recurrent venous thromboembolism: analysis from a U.S. hospital perspective. Am J Health Syst Pharm 2015;72:291-300
- Donze J, Lipsitz S, Bates DW, et al. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ 2013;347:f7171
- Department of Health. Payment by results guidance for 2012–13. Gateway reference 17250. London: Department of Health; 2012.
- Kristensen SR, Bech M, Quentin W. A roadmap for comparing readmission policies with application to Denmark, England, Germany and the United States. Health Policy 2015;119:264-73
- National Institute for Health and Care Excellence (NICE). Apixaban for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism. Technology appraisal guidance [TA341] England: NICE; 2015. http://www.nice.org.uk/guidance/indevelopment/gid-tag474. Accessed May 2016
- National Institute for Health and Care Excellence (NICE). Rivaroxaban for treating pulmonary embolism and preventing recurrent venous thromboembolism. NICE technology appraisal guidance [TA287] England: NICE; 2013. https://www.nice.org.uk/guidance/ta287. Accessed May 2016
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799-808
- Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699-708
- Lanitis T, Leipold R, Hamilton M, et al. Cost-effectiveness of apixaban versus other oral anticoagulants for the initial treatment of venous thromboembolism and prevention of recurrence. Clin Therap 2016;38:478-93
- National Institute for Health and Care Excellence (NICE). Edoxaban tosylate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism, Evidence Review Group Report, TA354; England: NICE; 2015 Accessed May 2016
- National Institute for Health and Care Excellence (NICE). Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism. Technology appraisal guidance [TA327] England: NICE; 2014. http://www.nice.org.uk/guidance/ta327/resources. Accessed May 2016
- Liu X, Johnson M, Mardekian J, et al. Apixaban reduces hospitalizations in patients with VTE: an analysis of the AMPLIFY trial. J Am Coll Cardiol 2014;63
- Liu X, Thompson J, Phatak H, et al. Apixaban reduced hospitalizations in patients with venous thromboembolism: An analyisis of the AMPLIFY-EXT trial. Blood 2013;122:3638
- Liu X, Johnson M, Mardekian J, et al. Apixaban reduces hospitalizations in patients with venous thromboembolism: An analysis of the apixaban for the initial management of pulmonary embolism and deep-vein thrombosis as first-line therapy (AMPLIFY) trial. J Am Heart Assoc 2015;4:1-8
- Lee T, Masseria C, Breazna A, et al. Hospitalizations, recurrent venous thromboembolism or venous thromboembolism-related death, and major bleeding, by index event from the AMPLIFY trial. Vasc Med 2016;67:2256
- National Institute for Health and Care Excellence (NICE). Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent venous thromboembolism events. Costing Template: Implementing NICE guidance. NICE technology appraisal guidance TA261; England: NICE; 2012
- Office for National Statistics. National Population Projections: 2014-based Statistical Bulletin England: Office for National Statistics; 2015. http://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/nationalpopulationprojections/2015-10-29. Accessed May 2016
- Martinez C, Cohen AT, Bamber L, et al. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost 2014;112:255-63
- Wilcox RR. Winsorized robust measures. Wiley StatsRef: Statistics Reference Online; 2014. http://onlinelibrary.wiley.com/doi/10.1002/9781118445112.stat06339/full. Accessed May 2016
- National Schedule of Reference Costs Year: 2014–15 - All NHS trusts and NHS foundation trusts - HRG Data. England: National Schedule of Reference Costs; 2014-2015. https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015
- Curtis L. Unit costs of health and social care 2012. Canterbury: Personal Social Services Research Unit, The University of Kent; 2012.
- Lee T, Masseria C, Breazna A, et al. Hospitalizations, recurrent venous thromboembolism or venous thromboembolism-related death, and major bleeding, by index event from the AMPLIFY trial. J Am Coll Cardiol 2016;67:2256
- Fernandez MM, Hogue S, Preblick R, et al. Review of the cost of venous thromboembolism. ClinicoEconom Outcomes Res CEOR 2015;7:451-62
- Sampson FC, Goodacre S, Kelly A-M, et al. How is deep vein thrombosis diagnosed and managed in UK and Australian emergency departments? Emerg Med J 2005;22:780-2
- National Institute for Health and Care Excellence (NICE). Rivaroxaban in the treatment of deep vein thrombosis and prevention of recurrent venous thromboembolic events, Single Technology appraisal, TA261; England: NICE; 2011
- Zondag W, Kooiman J, Klok FA, et al. Outpatient versus inpatient treatment in patients with pulmonary embolism: a meta-analysis. Eur Respir J 2013;42:134-44
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 2011;378:41-8
- van Bellen B, Bamber L, Correa de Carvalho F, et al. Reduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin 2014;30:829-37
