Abstract
Objective: To assess the economic impact of urinary tract infections (UTIs) and genital mycotic infections (GMIs) among patients with type 2 diabetes mellitus (T2DM) initiated on canagliflozin.
Methods: Administrative claims data from April 2013 through June 2014 MarketScan® databases were extracted. Adults with ≥1 claim for canagliflozin, T2DM diagnosis, and ≥90 days enrollment before and after canagliflozin initiation were propensity score matched to controls with T2DM initiated on other anti-hyperglycemic agents (AHAs). UTI and GMI healthcare costs were evaluated 90-days post-index and reported as cohort means.
Results: Rates of UTI claims 90 days post-index were similar in patients receiving canagliflozin for T2DM (n = 31,257) and matched controls (2.7% vs 2.8%, p = .677). More canagliflozin than control patients had GMI claims (1.2% vs 0.6%, p < .001) and antifungal utilization (5.3% vs 2.6%, p < .001). Mean post-index costs to treat UTIs were lower but not significantly different for canagliflozin patients vs matched controls ($27.61 vs $37.33, p = .150). GMI treatment costs were higher for the canagliflozin cohort ($3.68 vs $2.44, p = .041). Combined costs to treat either UTI and/or GMI averaged $31.29 per patient for the canagliflozin cohort v $39.77 for controls (p = .211). Rates and costs of UTIs and GMIs were higher for females than males, but the canagliflozin vs control trends observed for the overall sample were similar for both sexes. There were no significant cost differences between the canagliflozin and control cohorts among patients aged 18–64. Among patients aged 65 and above, GMI treatment costs were not significantly different, but costs to treat UTIs and either UTI and/or GMI were significantly lower for canagliflozin patients vs controls.
Conclusions: In a real-world setting, the costs to payers of treating UTIs and GMIs are generally similar for patients with T2DM initiated on canagliflozin vs other AHAs.
Introduction
Type 2 diabetes mellitus (T2DM) is a major public health problem that affects 387 million adults worldwideCitation1. Currently, more than 29 million adults (9.3% of the population) have diabetes in the USCitation2, and T2DM accounts for ∼95% of new cases of diabetesCitation3. If the projected growth trend holds, by 2025, nearly 53.1 million adults in the US will have diabetes; imposing a substantial economic burden of $514 billion on the US healthcare systemCitation4. Patients with T2DM are prone to an array of complications including macrovascular and microvascular damage and increased susceptibility to infectionCitation5–7. Macrovascular and microvascular complications are the major drivers of the healthcare costs in patients with T2DM, but infections such as urinary tract infections (UTIs) and genital mycotic infections (GMIs) also contribute to the overall medical costsCitation8–14. The cost of each case of lower urinary tract infection or vulvovaginal candidiasis in this population was estimated at $105 and $111, respectively (2012 US dollars) in one studyCitation15. Another study estimated the annual cost associated with treating UTIs—including cystitis, acute and chronic pyelonephritis, and UTIs with unspecified site—among patients with T2DM was $2,300Citation12. The lower costs identified by Ward et al.Citation15, as compared to Yu et al.Citation12, can be explained by Ward et al.’s focus on only lower UTIs (e.g. cystitis), which are generally less severe and easier to treat than upper UTIs (e.g. pyelonephritis)Citation16.
SGLT-2 inhibitors are a novel class of diabetic agents with an insulin-independent actionCitation17. These drugs reduce hyperglycemia in patients with T2DM by increasing urinary glucose excretionCitation18,Citation19. Randomized controlled clinical trials and retrospective studies of canagliflozin (Invokana®), the first such agent approved by the US Food and Drug Administration, have shown canagliflozin either as monotherapy and in combination improves hemoglobin A1c, as well as body weight and systolic blood pressure outcomesCitation20–26. As anticipated because of SGLT-2’s mode of action, adverse events such as osmotic diuresis, UTIs, and GMIs have been associated with canagliflozin, but these adverse events were generally mild and rarely led to treatment discontinuationCitation20–22,Citation26–29. In clinical studies, most patients with UTI and GMI adverse events had them early in the course of treatment and did not experience a subsequent UTI or GMI recurrenceCitation30,Citation31.
At the time this study was undertaken, the authors were unaware of published studies that assessed the costs of GMI or UTI among SGLT-2 inhibitor-treated patients with T2DM. Therefore, the study described herein, which utilized a large integrated administrative database of paid healthcare claims from US payers, was undertaken to evaluate the rate and economic impact of UTIs and GMIs among patients with T2DM initiated on the SGLT-2 inhibitor canagliflozin.
Methods
Study design and data source
This retrospective observational cohort study was based on claims data from two Truven Health MarketScan® Research Databases—the Commercial Claims and Encounters (Commercial) Database and the Medicare Supplemental and Coordination of Benefits (Medicare) Database—between April 1, 2013 and June 30, 2014. These databases profile the healthcare experience (inpatient and outpatient) of employees and dependents with employer-sponsored health insurance and Medicare-eligible active employees, retirees, and dependents from employer-sponsored supplemental plans. Claims in the Medicare Database were submitted to the supplemental plan after Medicare processing, but include both the Medicare and supplemental plan payment amounts for these services. All database records were de-identified in compliance with the Health Insurance Portability and Accountability Act (HIPAA) of 1996, so this study was exempt from institutional review board (IRB) approval.
Patient selection criteria
Canagliflozin cohort
Adult patients (≥18 years) with a principal diagnosis of T2DM (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 250.x0, 250.x2) in the 90-day pre-index period or on the index date, and having at least one claim for canagliflozin between April 1, 2013 and June 30, 2014, were included. The date of the first canagliflozin claim was considered as the index date. All study patients were required to have continuous health plan coverage with medical and pharmacy benefits for 90 days before (pre-index period) to 90 days after (follow-up period) the index date. (Any patients who became Medicare-eligible during the study period were followed across both the Commercial and Medicare databases to assess continuous enrollment.) The study period length was selected in order to maximize the sample size, given data available at the time of the study. Patients with a diagnosis indicative of pregnancy or childbirth, nursing home admission, or hospice service on the index date or in the previous 90 days were excluded from the initial sample pool. Patients with a urosepsis diagnosis during an inpatient admission in the previous 90 days were excluded from the analysis prior to matching because follow-up care for this condition may have been erroneously attributed to the index medication if the treatment extended into the post-index period.
Control cohort
This cohort comprised patients with T2DM with at least one claim for any anti-hyperglycemic agent other than a SGLT-2 inhibitor, of which canagliflozin was the only available agent during the years of data included in this study, or short-acting insulins between April 1, 2013 and June 30, 2014. The date of the earliest claim for such an agent was used as the index date. Patients with claims(s) for SGLT-2s (canagliflozin) were excluded from consideration for the control pool so that the two study cohorts would be mutually exclusive. Control patients may have used short-acting insulins; however, these agents were not used to establish an index date because they are typically used in conjunction with basal insulin for meal-time glucose control and not alone as maintenance treatmentCitation3. Patients without any claims for the index AHA, pregnancy or childbirth, nursing home admission, or hospice service on the index date or in 90 days pre-index were eligible for inclusion. Continuous enrollment in a health insurance plan for at least 90 days before and after the index date was required of all patients. (As noted above, continuous enrollment was assessed across both the Commercial and Medicare Databases.)
Matching algorithms
Patients in the canagliflozin cohort were propensity score matched 1:1 to control subjects. A match on sex was forced so males and females could be analyzed separately due to the differential risk of UTI and GMI by sexCitation12,Citation16,Citation30,Citation32–34. Propensity score matching was used to control for other factors that could affect healthcare costs in general (e.g. geographic region, primary payer) or the risk of these infections. Potential risk factors were selected based on previous researchCitation12,Citation16,Citation30,Citation32,Citation33,Citation35,Citation36 and limited to characteristics that could be measured in claims data. Propensity scores were computed using a logistic regression model, with the treatment received (canagliflozin or other AHA) as the dependent variable, and relevant demographic and baseline clinical measures (detailed further below) as independent variables. Each patient in the canagliflozin cohort was matched with a respective patient in the control cohort using a nearest neighbor matching technique, without replacement. This method has been found to produce good balance between groupsCitation37, and was deemed appropriate for the current study given the large proportion of potential controls to cases (∼25 to 1) and similar propensity score histograms of the case and control pools. The greedy matching algorithm, which selects treatment group patients in random order and locates the best available match for each patient in order, regardless of whether the match would be a better fit for a subsequent patient, was utilized for matchingCitation38,Citation39. Standardized differences in all measured demographic and clinical characteristics between the canagliflozin and control cohorts were calculated to examine the quality of the match, with standardized differences less than 10 deemed indicative of an acceptable matchCitation40.
Outcome measures
UTI- and GMI-related healthcare costs, the primary outcome of interest, were tallied at the patient level and reported as the mean among all patients in each cohort, including patients with zero costs, in order to assess the overall differential economic impact of UTI and GMI in the canagliflozin and matched control cohorts. Costs were measured from the gross payment information on relevant post-index healthcare claims. Costs on the index date, which was the date of the initial outpatient pharmacy claim for canagliflozin or other AHA, were not included, because UTI and GMI costs occurring on the same date that a new prescription was filled likely represented pre-existing infections, not UTIs or GMIs associated with the index medication.
UTI-related costs were defined as costs from inpatient and outpatient claims with a diagnosis of UTI (ICD-9 590.0x, 590.1x, 590.2, 590.8x, 590.9, 595.0, 595.9, 597.8, 599.0) in any position on the claim, and outpatient pharmacy claims for antibiotics commonly used to treat UTIs (fluoroquinolones, cephalexin, trimethoprim/sulfamethoxazole, and amoxicillin-clavulanic acid). GMI-related costs were defined as costs from inpatient and outpatient services claims with a diagnosis of GMI (ICD-9 112.1, 112.2, 607.0, 616.10) in any position on the claim, and outpatient pharmacy claims for oral and topical antifungals commonly used to treat GMIs (itraconazole, fluconazole, ketoconazole, and voriconazole). UTI/GMI-related costs included costs that met either the UTI-related or GMI-related criteria as described above. Relevant antibiotic and antifungal pharmacy costs were included regardless of their temporal relationship to a claim with a UTI or GMI diagnosis. This approach was used so relevant pharmacy claims would not be inadvertently excluded if clinicians prescribed antibiotics or antifungals from patient-reported symptoms of UTI or GMI, as opposed to an examination resulting in a claim with a relevant diagnosis.
Healthcare costs can be highly skewed, so univariate review of patient-level cost data was performed to assess for outliers. No a priori rules were established for outlier trimming but, in light of one extreme GMI cost outlier in the control cohort that would have artificially inflated the mean for the cohort, the authors decided to exclude the outlier control and matched canagliflozin patient from the analysis. The excluded control had GMI costs of $354,640, which was almost 60-times higher than the GMI costs of any other patient in the control cohort and almost 44-times higher than the GMI costs of any patient in the canagliflozin cohort.
Incidence of UTIs and GMIs was measured based on the presence of one or more claims in the post-index period, not counting the index date, that carried a relevant diagnosis code in any position on the claim. Because not all UTIs and GMIs may result in a diagnosis listed on a healthcare claim, rates of filled prescriptions for antibiotics and antifungals commonly used to treat these conditions also were measured from outpatient pharmacy claims. The proportion of patients who filled prescriptions for these agents during the entire 90-day post-index period, excluding the index date, was the primary measure. Similar measures within 3, 7, and 14 days of an observed UTI or GMI diagnosis also were created to assess the potential rates of pharmacy utilization temporally associated with claims evidence of the conditions. (See above for relevant ICD-9 diagnosis codes and medication classes.)
UTI and GMI claims observed post-index could have represented follow-up treatment of UTIs or GMIs diagnosed pre-index. To help ensure this did not affect study findings, all post-index UTI and GMI cost and incidence measures described above were also assessed after excluding patients with any claims for the conditions pre-index.
Covariates used in matching
A match on sex was forced and other patient demographic variables including age in years, geographic region, and primary payer type (Commercial vs Medicare), measured as of the index date, were included when deriving propensity scores. The following UTI risk factors that could be measured in claims data were also included: benign prostate hypertrophy (men only); kidney disorder diagnosis; and inpatient admission based on one or more claim(s) for any primary diagnosis in an inpatient setting of care. GMI risk factors included outpatient prescription claim(s) for any of the following: oral contraceptives or estrogens; immunosuppressant agents other than systemic steroids (i.e. monoclonal antibodies, antineoplastic agents, HIV/AIDS medications and other non-steroidal agents); or antibiotics. In addition, a modified version of the Diabetes Complications Severity Index (DCSI)Citation41, based on claims-based measures only, and the Elixhauser Comorbidity Index, Version 3.7, summary scoreCitation42, were created to serve as general measures of diabetes-specific and overall comorbidity, respectively.
Other covariates
Additional covariates used to characterize the study sample included age group and health plan type, which were measured as of the index date. In addition, various clinical characteristics were measured in the pre-index period, including: number of inpatient admissions; number of dates with a UTI claim; presence of a coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI) procedure code; systemic steroid utilization; presence of diagnosis codes indicative of a variety of specific conditions (e.g. stroke, hypertension, hypercholesterolemia, peripheral vascular disease, congestive heart failure, coronary heart disease, diabetic nephropathy, diabetic retinopathy, diabetic peripheral neuropathy/foot ulcer, chronic kidney disease); and the 29 medical and psychiatric condition indicators that are components of the Elixhauser Comorbidity Index summary score. Some of these clinical measures were considered as possible covariates in the propensity score matching, but not included due to low prevalence in the study sample or weak theoretical association with UTI or GMI.
Statistical analyses
Descriptive analyses were performed to compare the propensity-score matched samples. Categorical measures were summarized as counts and percentages. Continuous measures were summarized as means and standard deviations. Costs were reported as the mean across all patients in each cohort, including patients with no UTI or GMI claims. Incidence was reported as the proportion affected in each cohort, and the corresponding odds ratios were subsequently calculated. Statistical comparisons of UTI and GMI outcomes were evaluated using Chi-square or Fisher’s exact tests for categorical measures and Student’s t-tests for continuous measures. A p-value of <.05 was considered statistically significant. Analyses were conducted for the overall matched samples and were stratified by sex (male, female) and age at index (<65 years, 65 years and above) because UTI and GMI risk may differ by sex and age.
Sensitivity analyses
Because the initial matching revealed slight imbalance on plan type, the analyses were replicated including health plan type as an added covariate in the propensity score matching. In addition, because the core analysis entailed cost outlier trimming, a sensitivity analysis was conducted without cost outlier trimming to assess the impact of trimming on the final study results.
Results
Study population
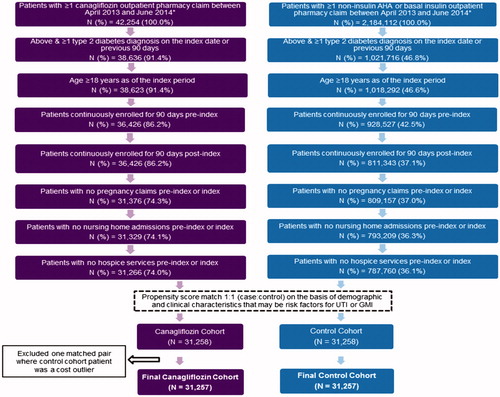
A total of 42,254 patients with at least one claim for canagliflozin and 2,184,112 patients with ≥1 other AHA drug claim (other than canagliflozin) were identified (). Among them, 31,258 (74%) from the canagliflozin cohort met the inclusion criteria and were propensity score matched to an identical number of control patients.
Baseline characteristics
summarizes the demographic characteristics of the unmatched and matched cohorts. The matched cohorts were well balanced with regard to age, sex, geographic location, primary payer and health plan type, as evidenced by most characteristics other than health maintenance organization plan type having a standardized difference less than 10. (The sensitivity analysis [results not shown] that included plan type as a covariate in matching decreased this standardized difference, but did not improve overall sample balance or alter study conclusions.) Mean age of the matched patients in both cohorts was 55.5 years, with a male:female ratio of 56%:44%. The majority of the patients resided in the Southern US Census region (47.4%) and had a commercial insurer, as opposed to Medicare, as their primary payer.
Table 1. Demographic characteristics.
displays the pre-index clinical characteristics for the unmatched and matched canagliflozin and control cohorts. There were no significant differences between the cohorts with regards to the pre-index UTI and GMI risk factors used in propensity score matching. Moreover, clinically relevant covariates that were included to help characterize the study sample were also well balanced (standardized differences <10), as were all other baseline clinical characteristics that were not included in the propensity score regressions. A comparison of the matched samples on the same clinical measures in the post-index period (results not shown) revealed that the balance between cohorts continued post-index. Stratifying by sex and/or age group (results not shown) did not alter the cohort balance.
Table 2. Baseline clinical characteristics.
UTI-related treatment utilization and costs
The proportion of patients with a claim carrying a UTI diagnosis was similar in the canagliflozin cohort and the matched control cohort (2.7% vs 2.8%, p = .667; odds ratio [OR] = 0.98, 95% confidence interval [CI] = 0.89–1.08). If patients with pre-index UTI were not included in the post-index measures, no numerical difference between cohorts was observed (2.2% vs 2.2%, p = .999). Outpatient pharmacy claims during the post-index period for antibiotics used to treat UTIs did not differ between the cohorts (11.5% vs 11.1%, p = .150), nor did antibiotic claims within 3, 7, or 14 days of a UTI diagnosis, which were observed among only ∼1.0–1.3% of patients in each cohort. Mean post-index costs associated with claims carrying a UTI diagnosis were numerically lower on average, but not significantly different for canagliflozin patients vs matched controls ($27.61 [SD = 771.80] vs $37.33 [SD = 909.95], p = .150) ().
Table 3. Post-index UTI and GMI events and costs.
Results of the sensitivity analyses based on the alternate matching were very similar to the core UTI-related analysis so they are not shown. There were no extreme cost outliers among patients with UTI in either cohort, so no outlier trimming was conducted.
Among males in the core analysis, the post-index diagnosis of UTIs was comparable between the two cohorts (1.1% vs 1.3%, p = .081). The rate of antibiotic prescriptions was also similar in both cohorts (9.8% vs 9.4%, p = .210) ().
Among females, the rate of diagnosis of UTI in the 90 days after the index date was similar in both the canagliflozin and control cohorts (4.7% vs 4.6%, p = 0.587). The proportion of females with antibiotics use in the canagliflozin and control cohorts was similar (13.6% vs 13.3%, p = .428). Compared with the controls, females treated with canagliflozin incurred similar mean costs to treat UTIs ($50.24 [SD = 1,123.83] vs $58.32 [SD = 1,004.53], p = .520) ().
For patients aged 18–65, there were no significant differences between the canagliflozin and matched control cohorts in the costs associated with treatment of UTIs ($28.89 [SD = 804.10] vs $27.02 [SD = 638.16]; p = .984). For patients aged 65 and above, UTI-related costs were lower among canagliflozin patients, as compared to matched controls ($31.72 [SD = 551.80] vs $94.87 [SD = 1,781.05]; p = .021), despite a similar proportion of patients with claims for UTIs in the two cohorts (3.7% in each cohort).
GMI-related treatment utilization and costs
The proportion of patients who had claims with a GMI diagnosis was higher in the canagliflozin cohort than the matched control cohort (1.2% vs 0.6%, p < .001; OR = 2.08, 95% CI = 1.75–2.48). Proportions decreased slightly, but the differential was similar, if patients with a GMI diagnosis in the 90 days pre-index were excluded from the measure (1.1% vs 0.5%, p < .001). The proportion of patients with outpatient pharmacy claims for antifungals used to treat GMIs during the post-index period also was higher for patients treated with canagliflozin vs control patients treated with other anti-hyperglycemic agents (5.3% vs 2.6%, p < .001). Rates of antifungals within 3, 7, or 14 days of a GMI diagnosis were less than 1% in both cohorts, but significantly higher for canagliflozin vs control patients across all time periods. Mean GMI treatment costs were higher among the canagliflozin cohort ($3.68 [SD = 78.92] vs $2.44 [SD = 72.53], p = .041) ().
Results of the sensitivity analyses based on the alternate matching were very similar to the core analysis, so are not shown. In the sensitivity analysis prior to trimming cost outliers, GMI-related costs for the canagliflozin cohort were numerically lower on average, $3.68 (SD = 78.92) vs $13.79 (SD = 2,007.19), with p = 0.374 suggesting no difference. This sensitivity analysis included one control patient with extremely high GMI-related costs (>$300,000) who, along with the patient’s matched canagliflozin patient, were dropped from the core analysis as cost outliers.
Among males in the core analysis, the rate of GMIs was numerically higher in the canagliflozin cohort than the control cohort (0.1% vs 0.02%), but the difference was not significant (p = .109). The use of antifungals, however, differed significantly between males in the canagliflozin and control cohorts (1.3% vs 0.9%; p < .001) ().
Among females, the occurrence of GMIs was significantly higher in the canagliflozin cohort than the controls (2.7% vs 1.3%; p < .001). Pharmacy claims for antifungals also were significantly higher in the canagliflozin cohort compared to the matched control cohort (10.3 vs 4.8, p < .001). Consistent with the utilization findings, compared with the controls, females treated with canagliflozin incurred higher costs to treat GMIs ($7.70 [SD = 117.92] vs $4.42 [SD = 88/09]; p < .009) ().
For patients aged 18–65, there were no significant differences between the canagliflozin and matched control cohorts in the costs associated with treatment of GMIs ($3.75 [SD = 76.08] vs $2.75 [SD = 78.52]; p = .135). The proportion of patients aged 65 and above with GMI-related claims was higher for canagliflozin patients, compared to matched controls (0.9% vs 0.3%, p < .001). Average GMI-related costs were numerically higher; however, the difference did not reach statistical significance ($3.29 [SD = 93.72] vs $0.75 [SD = 14.56]; p = .064).
UTI and/or GMI-related costs
Combined costs to treat UTI and/or GMI averaged $31.29 (SD = 777.54) per patient for the canagliflozin cohort vs $39.77 (SD = 912.90) for controls; these amounts were not statistically different (p = .211) (). Results of the sensitivity analyses based on the alternate matching and cost outlier trimming were similar to the core analysis. Stratifying the sample by male or female sex also revealed that cost differences to treat UTI and/or GMI were not significantly different between cohorts. Stratifying by age suggested no significant difference between cohorts in the combined costs to treat UTI and/or GMI for patients aged 18–65, but combined UTI and/or GMI-related costs were significantly lower for the canagliflozin cohort patients than for controls among patients age 65 and above ($35.02 [SD = 573.72] vs $95.62 [SD = 1,781.11]; p = .027).
Discussion
This large retrospective cohort study assessed the real-world healthcare costs associated with UTIs and GMIs in patients with T2DM treated with canagliflozin compared to similar patients treated with other AHAs from the US payer’s perspective. Results of this analysis suggest costs associated with UTIs and GMIs are generally similar for patients with T2DM initiated on canagliflozin vs other AHAs.
T2DM is a known risk factor for UTIs and GMIs, regardless of treatment regimenCitation12,Citation16,Citation30,Citation32–34,Citation43. The increased healthcare resource use and costs associated with UTIs and GMIs in patients with T2DM has been reported previouslyCitation12,Citation32,Citation44,Citation45. This study adds to the literature by comparing UTI and GMI costs for patients initiated on canagliflozin vs other AHAs. In the 90 days following initiation, mean costs to treat UTI were lower but not significantly different, on average, across a cohort of canagliflozin patients vs matched controls ($27.61 vs $37.33, p = .150). GMI treatment costs were higher for the canagliflozin cohort ($3.68 vs $2.44, p = .041). Combined costs to treat either UTI and/or GMI averaged $31.29 per patient for the canagliflozin cohort vs $39.77 for controls (p = .211). Payers may find this cost data useful as they seek to understand how specific AHAs impact average per-member expenditures.
Due to the mechanism of action of SGLT-2 inhibitors, increases in GMIs have been observed with canagliflozin in most clinical studiesCitation22,Citation27,Citation46, whereas small or no difference in UTIs has been notedCitation21,Citation22,Citation27,Citation28,Citation46. Real-world experience can differ from clinical study findings because the patient population is more varied and treatment patterns less controlled in actual clinical practice than in controlled clinical trials. UTI and GMI rates observed in clinical studies of canagliflozin, however, are consistent with the current retrospective claims database analysis which found that, over a 90-day post-index period, canagliflozin was associated with a non-significant, small numerical difference in the rate of claims related to UTIs (2.7% vs 2.8% for controls, p = .677). The rate of claims related to GMIs was low in both cohorts, although higher rates were observed among patients initiated on canagliflozin than for patients initiated on other AHAs (1.2% vs 0.6%, p < .001).
As anticipated, based on previous researchCitation12,Citation28,Citation29,Citation47, women and older patients in the current study had higher rates of UTI and GMI, and higher associated costs, than men and younger patients. GMI rates and costs among males in the current study were extremely low, such that, although more patients initiating canagliflozin than other AHAs had claims indicative of GMI, the result did not reach statistical significance. This is in contrast to the overall and female populations, in which rates were low overall, but significantly higher among patients initiating canagliflozin than other AHAs. This suggests that projecting GMI-related costs to an overall insured population requires consideration of the male:female split of the population. Patients aged 65 and above initiating other AHAs had significantly higher costs than patients initiating canagliflozin, despite similar UTI incidence rates. Because the cost difference in the overall population was not significantly different, this may represent a less generalizable finding related to a few seniors in the control cohort with high costs due to serious UTI events, and should be interpreted with caution.
The costs of patients in this study were found to be skewed upon univariate review, even after trimming the one extreme cost outlier, resulting in large standard deviations around the mean for all cost variables. The highly skewed nature of the costs, in which most patients had $0 costs, because they did not have any related services, and a small number of patients had very high costs, is typical of healthcare cost data, and not unique to the current analysis.
The propensity score matching method used in this study enabled robust comparison of the outcomes between cohortsCitation37. This method was selected over direct matching, because an exploratory analysis by the authors using an earlier version of the MarketScan Commercial and Medicare Databases (results not shown) indicated direct matching on key demographic characteristics and predisposing medication use would not balance cohorts in terms of the myriad of clinical characteristics that may impact the incidence of UTIs/GMIs. The propensity scores were calculated using variables with theoretical association with UTI and GMI outcomes, identified from previous researchCitation12,Citation16,Citation30,Citation32–36. The propensity score match produced good balance (standardized difference <10) across all key demographic and clinical characteristics, suggesting the study results approximate the UTI and GMI incidence and cost differences associated with initiating canagliflozin vs other AHAs.
The current study followed patients over only 90 days post-initiation, due to data availability at the time the study was conducted. Since payers may like to extrapolate these findings to annual costs, it raises the question of whether the costs reported herein can be expected to be incurred every 3 months during the course of treatment with canagliflozin. Future studies are needed to determine that with certainty, but, because clinical studies of canagliflozin have found most UTIs and GMIs occur relatively early in the course of treatment and do not recurCitation30,Citation31, it suggests payers should not anticipate the costs identified in this study to be incurred in every 3-month period following canagliflozin initiation. Rather, these costs likely represent a high-end estimate of average quarterly costs that is likely to regress towards the mean over time.
In addition, it should be noted that the attribution of all relevant antibiotic and antifungal prescription drug costs as UTI or GMI costs may have over-estimated the associated costs, since these agents have multiple indications, and pharmacy claims do not list diagnosis codes. Similarly, the total costs from claims that carried a diagnosis code of UTI or GMI were attributed to the conditions, even though part of the cost may have been for other services rendered during the same visit or admission, and this also may have inflated the cost estimates. Given the presumed comparable or higher incidence of UTI and GMI in the canagliflozin cohort and the objective of determining the differential costs between patients initiated on canagliflozin and other AHAs, the authors felt this approach was preferable to potentially excluding some relevant costs, which may have artificially minimized cost differences. Given these methodological decisions, the actual costs and cost differences between cohorts may be lower than suggested by study results.
Limitations
The limitations of this study should be considered when interpreting results. The diagnoses of T2DM, UTIs, GMIs, and clinical characteristics were identified using ICD-9-CM diagnosis codes, which are subject to miscoding and may be inexact due to the non-specific nature of some codes included in the analysis. Although the variables used in propensity score matching were drivers of UTI and GMI, the match and study exclusion (e.g. patients with pre-index urosepsis) may not be appropriate for other outcomes. Furthermore, bias due to unknown or unmeasured covariates on the outcomes cannot be ruled out. Data on hygiene practices and hydration status of individual patients enrolled in the study were unavailable, thus it is uncertain whether UTIs/GMIs occurred as a result of poor hydration or hygienic conditions, canagliflozin treatment, or due to diabetes in general. Only healthcare utilization that results in a claim being submitted to a contributing health plan is included in the MarketScan Databases; other healthcare services patients may have received could not be measured. This includes services paid in full by Medicare, since claims for such services are not submitted to the Medicare supplemental plans. In addition, information related to self-pay prescriptions and over-the-counter treatments, which may have had an effect on the outcomes, were not available in the data used for this study. This analysis is limited to those patients with T2DM who utilized healthcare services and were covered by Commercial or Medicare insurance; results may not be generalizable to uninsured patients or those with other types of health coverage. Finally, this study followed patients over only 90 days post-index; UTI and GMI rates and costs may have differed if a longer study period was used.
Despite these limitations, this was a unique study as it provided a contemporary estimate of costs associated with UTIs/GMIs among patients with T2DM treated with canagliflozin and other AHAs from a US payer perspective. This study evaluated a large study population, and the reported costs are reflective of true costs from a payer’s perspective. Additionally, we are unaware of other studies that have used propensity score matching method to quantify and compare the economic burden due to these events among patients with T2DM receiving different AHAs.
Conclusions
The current study suggests that UTI and GMI incidence and related costs among patients with T2DM were low and, thus, likely account for a small proportion of a payer’s budget. GMI-related costs were higher for canagliflozin patients compared to controls, but the incidence and costs of GMI were low across both cohorts. UTI-related costs as well as the combined costs of UTI and GMI did not differ by cohort. Overall, study findings suggest the costs to payers of treating UTIs and GMIs are generally similar for patients initiated on canagliflozin vs other AHAs.
Transparency
Declaration of funding
This study was funded by Janssen Scientific Affairs, LLC, and conducted by Truven Health Analytics, an IBM Company.
Declaration of financial/other relationships
TA and SB are employees of Janssen Scientific Affairs, LLC and stockholders of Johnson & Johnson, the parent company of Janssen Scientific Affairs. LM and PJ are employees of Truven Health Analytics, an IBM Company. Truven Health was paid by Janssen Scientific Affairs in connection with the conduct of the study and development of this manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Jerry Kagan and Santosh Tiwari, both of Truven Health Analytics, an IBM Company, contributed programming and editorial support, respectively. Charmi Patel of Janssen Scientific Affairs, LLC provided editorial support.
References
- International Diabetes Federation. IDF Atlas. Brussels, 2015, http://www.idf.org/diabetesatlas. Accessed June 23, 2016
- Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States; Atlanta: Centers for Disease Control and Prevention; 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed June 23, 2016
- American Diabetes Association. Standards of medical care in diabetes – 2015 Diabetes Care 2015;38(1Suppl):S41-S8
- Rowley WR, Bezold C. Creating public awareness: state 2025 diabetes forecasts. Popul Health Manag 2012;15:194-200
- de Leon EM, Jacober SJ, Sobel JD, et al. Prevalence and risk factors for vaginal Candida colonization in women with type 1 and type 2 diabetes. BMC Infect Dis 2002;2:1
- Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia 2007;50:549-54
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008;26:77-82
- Williams R, Van Gaal L, Lucioni C, et al. Assessing the impact of complications on the costs of Type II diabetes. Diabetologia 2002;45:S13-S17
- Caro JJ, Ward AJ, O'Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care 2002;25:476-81
- Li R, Bilik D, Brown MB, et al. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care 2013;19:421-30
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033-46
- Yu S, Fu AZ, Qiu Y, et al. Disease burden of urinary tract infections among type 2 diabetes mellitus patients in the U.S. J Diabetes Complications 2014;28:621-6
- Alva ML, Gray A, Mihaylova B, et al. The impact of diabetes related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015;32:459-66
- Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med 2013;45:253-61
- Ward A, Alvarez P, Vo L, et al. Direct medical costs of complications of diabetes in the United Stats: Estimated for event-year and annual state cost (USD 2012) J Med Econ 2014;17:176-83
- Nitzan O, Elias M, Chazan B, et al. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes 2015;8:129-36
- Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther 2014;5:355-66
- Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab 2012;14:539-45
- Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care 2013;36:2154-61
- Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013;56:2582-92
- Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract 2013;67:1267-82
- Stenlof K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15:372-82
- Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 2014;16:467-77
- Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care 2015;38:355-64
- Nardolillo A, Kane MP, Busch RS, et al. A clinical perspective of canagliflozin in the management of type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes 2014;7:25-30
- Ji L, Han P, Liu Y, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab 2015;17:23-31.
- Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013;15:463-73
- Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014;30:1109-19
- Nicolle LE, Capuano G, Fung A, et al. Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad Med 2014;126:7-17
- Geerlings S, Fonseca V, Castro-Diaz D, et al. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract 2014;103:3733-81
- Davies MJ, Kushner P, Vijapurkar U, et al. Incidence of GMI and UTI over time in patients with T2DM treated with canagliflozin over 2 years [abstract]. J Gen Intern Med 2015;30:S61-S62
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002;113(1ASuppl):5S-13S
- Grandy S, Fox KM, Hardy E, et al. Prevalence and recurrence of urinary tract and genital infections among adults with and without type 2 diabetes mellitus in the general population: a longitudinal cohort study. J Diabetes Res Clin Metab 2013;2:5
- Hirji I, Andersson SW, Guo Z, et al. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications 2012;26:501-5
- Nyirjesy P, Sobel JD. Genital mycotic infections in patients with diabetes. Postgrad Med 2013;125:33-46
- Grigoriou O, Baka S, Hassiakos et al. Prevalence of clinical vaginal candidiasis in a university hospital and possible risk factors. Eur J Obstet Gynecol Reprod Biol 2006;126:121-5
- Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol 2010;172:1092-7
- D’Agostino R Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statist Med 1998;17:2265-81
- Parsons L. Reducing bias in a propensity score matched-pair sample using Greedy Matching techniques. 26th Annual SAS User Group International Conference, Long Beach, CA, 2001; pp. 214-16, 22-25 April 2001
- Ho DE, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Analysis 2007;15:199-236
- Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15-24
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8-27. Note: For detailed description of Version 3.7, see URL http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed 10 August 2015
- Hirji I, Guo Z, Andersson SW, et al. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD). J Diabetes Complications 2012;26:513-16
- Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in men. J Urol 2005;173:1288-94
- Worley K, Bell KF, Xu Y, et al. Resource utilization and health care costs among diabetics with urinary tract infections in a commercially insured population. J Clin Outcomes Manage 2012;19:539-50
- Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941-50
- Nyirjesy P, Zhao Y, Ways K, et al. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin 2012;28:1173-8