Abstract
Objectives: Imatinib (Glivec) and nilotinib (Tasigna) have been covered by critical disease insurance in Jiangsu province of China since 2013, which changed local treatment patterns and outcomes of patients with chronic myeloid leukemia (CML). This study evaluated the long-term cost-effectiveness of insurance coverage with imatinib as the first-line treatment for patients with CML in China from a societal perspective.
Methods: A decision-analytic model based on previously published and real-world evidence was applied to simulate and evaluate the lifetime clinical and economic outcomes associated with CML treatments before and after imatinib was covered by medical insurance. Incremental cost-effectiveness ratio (ICER) was calculated with both costs and quality-adjusted life years (QALYs) discounted at 3% annually. Different assumptions of treatment benefits and costs were taken to address uncertainties and were tested with sensitivity analyses.
Results: In base case analysis, both cost and effectiveness of CML treatments increased after imatinib was covered by the medical insurance; on average, the incremental QALY and cost were 5.5 and ¥277,030 per patient in lifetime, respectively. The ICER of insurance coverage with imatinib was ¥50,641, which is less than the GDP per capita of China. Monte Carlo simulation resulted in the estimate of 100% probability that the insurance coverage of imatinib is cost-effective. Total cost was substantially saved at 5 years after patients initiated imatinib treatment with insurance coverage compared to no insurance coverage, the saved cost at 5 years was ¥99,565, which included the cost savings from both direct (e.g. cost of bone marrow or stem cell transplant) and indirect costs (e.g. productivity loss of patients and care-givers).
Conclusions: The insurance coverage of imatinib is very cost-effective in China, according to the local cost and clinical data in Jiangsu province. More importantly, the insurance coverage of imatinib and nilotinib have changed the treatment patterns of CML patients, thus dramatically increasing life expectancy and quality-of-life (QoL) saving on productivity losses for both CML patients and their caregivers.
Background and objectives
Chronic myeloid leukemia (CML) is a progressive disease which occurs in all age groupsCitation1. In China, CML tends to afflict a younger population than in western countries. The median age of disease onset in China is 45–50 years oldCitation2–5, which is younger than the 67 years old in western countriesCitation6. CML accounts for a frequency of ∼1 in 100,000 and represents nearly 15–20% of leukemia in adultsCitation7. Clinical manifestations of CML include splenomegaly, peripheral blood white blood cells increase extremely, immature granulocytes appear, basophilia, eosinophilia, anemia, and thrombocytosis. CML often leads to fatigue, weight loss, sweating, and abdominal discomfort from an enlarged spleenCitation8. Clinically, CML has three distinct phases of increasing severity and refractoriness to therapy; namely, chronic phase (CP), accelerated phase (AP), and blast crisis (BC)Citation8, the median survival for AP and BC were only 6–12 months and 3–6 months, respectivelyCitation9. The results of an investigation for patients with CML showed that the treatment cost of allo-hemotopoietic stem cell transplantation (HSCT) in CP, AP, and BC patients was ∼¥204,084 ± 107,313, ¥265,778 ± 198,723, and ¥332,605 ± 166,235Citation1. CML bring a heavy financial burden to patients as well as their families if there is no medical insurance available. The quality of patients’ life is severely impaired in the final stages of the diseaseCitation10.
The emergence of imatinib has completely changed the treatment pathway and concept of CML. Imatinib (Glivec, Novartis, Basel, Switzerland) is a small molecule and an oral tyrosine kinase inhibitor (TKI) indicated for the treatment of Philadelphia chromosome-positive CML in all phasesCitation11. The use of imatinib has caused a major impact on extending patients’ lives for many years and improving patients’ quality-of-life (QoL) after the initial diagnosis with CML, transforming CML from a fatal disease to a chronic disease. Seven-year results from the IRIS trial demonstrated an overall survival (OS) rate of 86% for patients randomized to imatinib. The rates of relapse and progression are low for patients treated with imatinib, with an overall estimated progression-free survival of 93% at 7 yearsCitation12,Citation13.
One survey collected and analyzed a total of 1824 cases data of hospitalized CML patients (722 cases) in 2005 and outpatient (1102 cases) information (July 1–September 30, 2006) from 15 hospitals throughout China, and the survey found that, due to limited insurance coverage for imatinib, the proportion of patients treated with hydroxyurea, imatinib, and interferon was 54.00% (985/1824), 37.45% (683/1824), and 25.55% (466/1824), respectively, the proportion of inpatients received HSCT was 22.72% (164/722), and the majority of the patients treated with imatinib were not monitored in timeCitation1. Although imatinib has been recommended as the first line therapy since 2008 by NCCN, interferon or hydroxyurea are still first-line treatment alternatives as well as bone marrow transplant (BMT) due to the affordability, as interferon, hydroxyurea, and BMT are widely reimbursable in ChinaCitation11.
In China, imatinib and nilotinib are recommended by the Hematology Society, Chinese Medical Association as the first-line treatment for newly-diagnosed CML-CP patients according to the current guidelines of CML diagnosis and treatmentCitation14. In Jiangsu province of China, the imatinib and nilotinib have been covered by medical insurance of critical diseases since 2013, which changed the treatment pattern and outcomes of CML in Jiangsu, as most patients start to take imatinib as a first line treatmentCitation15. With the insurance coverage, CML patients only need to co-pay 25% of the drug cost for imatinib/nilotinib; in addition, with the imatinib/nilotinib Patient Assistance Program (GIPAP/TIPAP) through China Charity FederationCitation16, for every purchase of the first 3-month supply of imatinib/nilotinib, there will be a complimentary supply of 9/12 months for eligible CML patients. The First Affiliated Hospital of Soochow University is in Jiangsu province, its department of Hematology is a national treatment center, with 75,000 person-times inpatients per year, and it is a key member of the PCR standardization alliance, with its uniform test providing standardized, reproducible results across laboratory settings. Although the clinical benefits of TKI have been brought to the patients with CML by the insurance coverage, there are no studies so far which have evaluated the societal implications of medical insurance coverage for imatinib as first-line treatment of chronic myeloid leukemia in China. Up to now, a number of imatinib economic evaluations in CML have been conducted from different perspectives in multiple countries including the USCitation17, SwedenCitation18, KoreaCitation19, and ChinaCitation11. However, all of these studies evaluated the cost-effectiveness value of imatinib against non-TKI therapies. Furthermore, the insurance coverage of imatinib and free PCR monitoring have only been piloted as an innovation in several provinces in China, a comprehensive evaluation of the insurance policy outcomes including clinical benefits with direct and indirect costs will help the decision-making in other regions. The current study was to project the long-term clinical and cost-effectiveness of insurance coverage with and without imatinib as the first-line treatment for patients with CML in China and to estimate the incremental cost-effectiveness ratio (ICER) between insurance coverage against a scenario of no insurance coverage for TKI in China from the societal perspective, which allowed the consideration on savings of productivity loss from both caregivers and CML patients treated by imatinib or nilotinib to be included in the evaluation.
Methods
Model design
The cost-effectiveness of insurance coverage of imatinib compared to no insurance coverage was estimated using a decision analytic model, which was designed to assess the differences in direct and indirect costs and effects including life years (LYs) and quality-adjusted life years (QALYs) in newly-diagnosed CML CP patients in China.
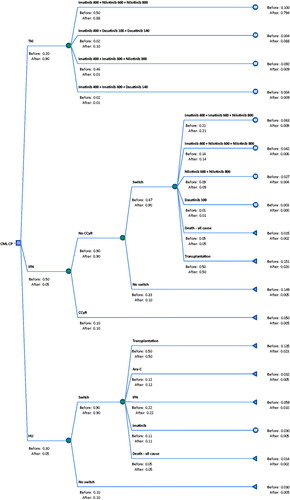
The major treatment options for CML CP patients were TKI, interferon (IFN), hydroxyurea (HU), and bone marrow or stem cell transplant, as summarized in . The patients’ proportion for each option of CML treatment varied before and after insurance coverage for imatinib and nilotinib ().
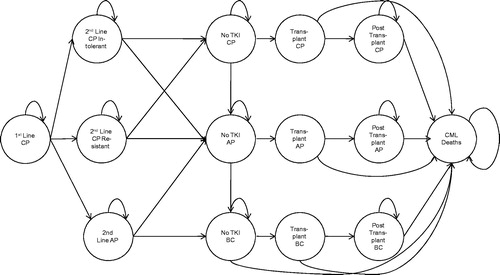
A two-part decision model comprising decision tree and Markov chains was developed in Microsoft Excel to project the long-term outcomes of patients treated with different preference of options before and after the insurance coverage for imatinib ( and ).
Perspective
A clear specification of the study perspective is critical in economic evaluation. The model was developed from the societal perspective in a local clinical and reimbursement setting in China. The costs included in this analysis were drug costs, cost of adverse events, cost of outpatient visit, laboratory tests, and hospital stay, cost of bone marrow or stem cell transplant and productivity loss of patients and caregivers. The productivity loss of patients and caregivers due to bone marrow or stem cell transplant was estimated from the average salary income by age-band.
The ISPOR Drug Cost Task Force recommends that, for CEA of brand name drugs performed from a societal perspective, the analyses should use a cost that more accurately reflects true societal drug costs (e.g. 20–60% of average sales price)Citation20. Accordingly, the applied actual drug costs in the model were only ∼25% of the average sales prices, considering all the relevant factors (e.g. patient assistance program, etc.).
Model data inputs
Chinese epidemiological data for treatment of CML and efficacy data of nilotinib and dasatinib were obtained from a literature search and review, preferably in publications of Chinese; unavailable data from the Chinese publications were referred to international studies. The internationally standardized dosing was applied in the model inputs. The efficacy data of imatinib were collected from a cohort of patients treated locally at the First Affiliated Hospital of Soochow University in Jiangsu province of China, which is a top level and key general hospital in Jiangsu province approved by the Ministry of Health in China. The Department of Hematology at the hospital is a national key discipline of clinical medicine, with national level laboratories for diagnosis, treatment, and research. The Department of Hematology has been a national treatment center with yearly outpatient and inpatient volumes of ∼75,000 person-timesCitation21. The efficacy data of IFNCitation22, and HUCitation23, and the treatment preference or proportion of the treatment options were derived from a Chinese literature study and a panel of local clinical experts (, , and the Appendix).
Table 1. TKI treatment disposition at 12 months: treatment continuations/discontinuations.
Table 2. TKI treatment disposition at 12 months: response levels.
Table 3. Monthly transition probabilities from second line TKI to next states.
The local cost data was derived from the Chinese literature review and the consensus of a panel of local clinical experts from the First Affiliated Hospital of Soochow University, Jiangsu Province in China. Under the current insurance coverage for imatinib, CML patients only need to co-pay 25% of the drug cost for imatinib; in addition, with the imatinib International Patient Assistance Program (GIPAP) through China Charity Federation, for every purchase of the first 3-months supply of imatinib, there will be a complimentary supply of 9 months for eligible CML patientsCitation16. The arrangement was reflected in the cost estimation of imatinib in the model. The published cost data of both treatments and other resource use was employed in the model ( and ). Both estimated costs and QALY were discounted at an annual rate of 3% ().
Table 4. TKI drug costs with PAP.
Table 5. Cost and clinical effectiveness of IFN, HU, and transplant therapy.
Table 6. Discount rates.
For model validation: first, the model structure, assumptions and clinical pathways were verified by the panel of local clinical experts; second, the input parameter values and distributions were tested and confirmed by a local panel of clinical and health economic experts; and, finally, the output values and conclusions were examined by the health economist.
Sensitivity analysis
One-way sensitivity analysis (OWSA)
Several one-way sensitivity analyses were performed to rigorously test the conclusions of the analysis. The parameters included in one-way sensitivity analyses are shown in the tables and all the data inputs were varied by ±25%, which was suggested and confirmed by the panel of local clinical and health economic experts. Results were displayed in a tornado diagram to demonstrate the effect of the variable on the QALY and ICER.
Probability sensitivity analysis (PSA)
Probability sensitivity analyses were performed to test the effect of uncertainty on the base case result for ICER per QALY gained. Measures of distribution were obtained from the literature. We ran 1,000 Monte Carlo simulations to obtain the 95% confidence range of the costs and QALYs for the treatments with and without insurance coverage of imatinib, in order to determine the proportions of simulations that were under cost-effectiveness thresholds. Based on WHO recommendations for cost-effectiveness thresholdsCitation24, we describe an intervention as very cost-effective if the ICER is less than per capita GDP in China (¥51,819 in 2015) and cost-effective if the ICER is less than 3-times per capita GDP in China (¥155,457)Citation25 vs the comparator.
Analysis and results
Base case analysis
In the base case analysis of a life year horizon, a CML CP patient diagnosed after imatinib was included by insurance coverage in Jiangsu province was estimated to cost ¥50,641 per QALY gained compared with the scenario before imatinib was covered by the medical insurance ().
Table 7. Incremental cost-effectiveness ratios (ICER) for patients with insurance coverage.
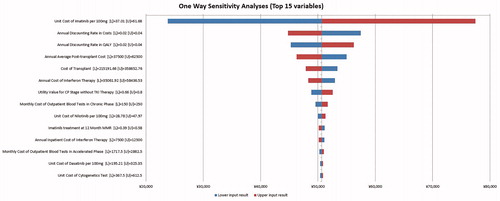
One-way sensitivity analysis
One-way sensitivity analysis results were presented as a Tornado diagram (); the variables with the top five largest impacts on the ICER per QALY were namely unit cost of imatinib, annual average post-transplant cost, annual discounting rate in costs, cost of transplant, and annual discounting rate in QALY.
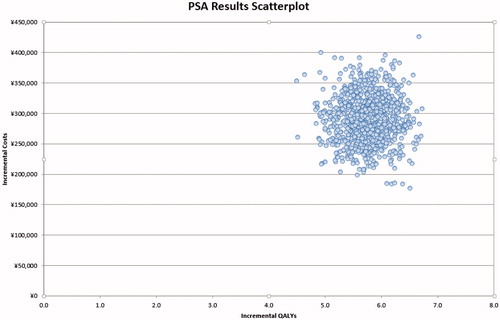
Probability sensitivity analysis
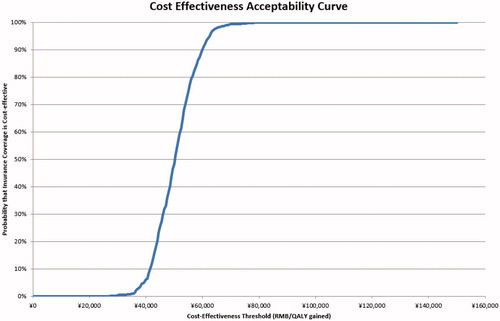
Probability sensitivity analysis projected that, when society was willing to pay ¥74,000 per QALY gained, which is only 1.4-times of GDP per capita of China in 2015 (the GDP per capita was ¥51,819 in China in 2015), there was a 100% probability that the insurance coverage of imatinib would be considered a cost-effective strategy compared to a scenario of no insurance coverage ( and ).
Discussion
CML is a severe and intractable illness as well as one of the most expensive illnesses to treat. Long treatment duration, low cure rate, and expensive treatment costs contribute significantly to the economic burden of patients with CML without medical insuranceCitation28. The present study examined the long-term cost-effectiveness before and after imatinib and nilotinib coverage by public payers using the real-world data in managing CML patients in China, as the efficacy data of imatinib were collected from a cohort of patients treated locally at the Affiliated Hospital of Soochow University in Jiangsu Province.
In the base case analysis of a lifetime horizon, a CML CP patient with insurance coverage in Jiangsu province was estimated to cost ¥50,641 per QALY gained compared with the scenario of no insurance coverage; the sensitivity analyses showed that there will be 100% probability that the insurance coverage of imatinib is cost-effective when the cost-effectiveness threshold is 74,000 RMB per QALY gained. Our results suggested that the insurance coverage of imatinib and nilotinib with PAP is a very cost-effective strategy in China, as concluded in this study that was based on the local real world data collected in Jiangsu province.
The yearly cumulative total discounted costs and discounted productivity loss per CML-CP patient were also calculated in the scenarios of having and no insurance coverage of imatinib. The total amount of medical costs and productivity loss are actually saved with insurance coverage policy, for example, at the 3rd or 5th year, comparing the scenario that patients don’t have insurance coverage of imatinib and nilotinib (), that is to say, the ICER results at the 3rd or 5th year is dominant by the insurance coverage with imatinib and nilotinib.
Table 8. Incremental cost-effectiveness ratios (ICER) for patient with insurance coverage at 3 and 5 years after diagnosis.
Clinically, treatment with TKI has significantly improved the short-term outcomes such as complete cytogenetic response (CCyR) and major molecular response (MMR) among patients with CML. With extensive access to imatinib and nilotinib through insurance coverage in some provinces in China, more patients with CML were treated with imatinib and nilotinib and projected to be in long-term remission, therefore delaying or eliminating the need for bone marrow transplantation, which reduces the quality-of-life, as, for a stem cell or bone marrow transplant, high doses of chemotherapy have to be given, and sometimes a low dose of radiation is also given to the whole body, to kill the leukemia cells. This treatment kills the leukemia cells, but also damages the normal bone marrow cells. Then, after these treatments, the patient receives a transplant of blood-forming stem cells to restore the bone marrow, and subsequent treatments are required after the transplantationCitation29. The patients on imatinib also had a better efficacy and quality-of-life compared with HU and IFN. In addition, the CML patients can take the therapy as an outpatientCitation30, the increased use of imatinib has had a positive impact on the day-to-day life of the CML population and made their lifestyle close to the normal population.
In addition, productivity gained was estimated in the scenario of having insurance coverage for imatinib and nilotinib as more CML patients were projected to be in remission and, therefore, could return to work and the same applies to their caregivers. In contrast, stem cell or bone marrow transplant and relevant pre- and post-procedure treatments limited the ability of working not only for patients with CML but also for their caregivers. In the long-term, insurance coverage of imatinib and nilotinib with PAP was estimated to be cost saving and also improve both the life year expectancy and QALY of CML patients in comparison to a scenario without insurance coverage for imatinib and nilotinib.
Cost-effectiveness of imatinib had been assessed from the perspective of multiple countries including the US, UK, and China in the past few years. Similar to this analysis in which interferon and hydroxyurea were the main therapies for CML for the scenario of no insurance coverage for imatinib, the study in the US assessed the incremental cost-effectiveness of imatinib vs IFN + LDAC as a first-line therapy for newly diagnosed CML-CP patients using data collected in the International Randomized Interferon vs STI571 Study (IRIS), and supplemental data from the literature demonstrated that imatinib is a cost-effective first-line therapy in patients with newly-diagnosed CML-CP patients, with an ICER of $43,300 per QALY gained (95% CI = $38,300–49,100)Citation17. Our findings are also congruent with earlier national studies such as Dalziel K’s 2005 analysis that concluded a Markov model within the setting of the UK NHS and, viewed from a health system perspective, reported an ICER of £26,180 compared with IFN-α per QALY gained, and concluded that imatinib was cost-effective when compared with IFN-αCitation31. In addition, a Chinese study conducted two cost-effectiveness analyses to compare the 1-year responder and lifetime cost-utility of imatinib with interferon monotherapy in newly-diagnosed CML-CP patients from the Chinese public healthcare system perspective (CPHSP). It found that the cost per additional responder was ¥36,545, and the ICER comparing imatinib with interferon was ¥73,674 (95% CI = ¥67,712–79,637) per QALY, which is below the cost-effectiveness threshold recommended by the WHO for developing countries. Thus, imatinib is very cost-effective compared to interferon in newly-diagnosed CML-CPCitation11.
In the UK, the National Institute for Health and Care Excellence (NICE) recommends standard-dose imatinib for the first-line treatment of CML, especially for the patients who have Philadelphia-chromosome-positive CML in the CP and who have not had treatment for CML beforeCitation32. Nilotinib has been recommended for the treatment of imatinib-resistant CML and for people with CML for whom treatment with imatinib has failed because of intoleranceCitation33. In the US, imatinib has been approved for first-line treatment of patients with CML since December 2002 by the National Comprehensive Cancer Network (NCCN)Citation34.
Compared with interferon, hydroxycarbamide, and allo-HSCT, imatinib has a statistically significant advantage as measured by CCyR, MMR, quality-of-life, or progression-free survival for patients with CMLCitation35–41.
There are some limitations which need to be considered in this study. First, the patient level data were collected from Jiangsu province so can only represent Jiangsu province, the varied cost and efficacy data in other regions of China still need to be observed and further studied. More data from the provinces with insurance coverage of imatinib and nilotinib should be included into the analysis, with consideration of the different reimbursement systems. Second, with the application of Tasigna for first-line treatment of CML and the fast development of pharmacy and medication therapy management, the treatment pattern of patients with CML could dramatically change in the future; the long-term cost-effectiveness evaluation could be outdated then. The short-term cost-effectiveness results on 3 and 5 years, however, could be more precise in assessment of the cost savings and efficacy benefits brought by the insurance coverage of imatinib and Tasigna. Third, we didn’t include the predicted medication adherence in the model, which could be a limitation, although we performed sensitivity analyses for the related clinical efficacies. The lack of Chinese patients based utility data could be a further limitation; utility data obtained from the international study were applied in the model as we didn’t identify any relevant study for the Chinese population.
The patient disposition at month 12 is presented in , and the BCR-ABL ratio (%) is presented in . One uncertainty in the analysis came from the discrepancy between the number of patients with disposition data () and the number of patients with available data on BCR-ABL ratio (%) (). It should be noted that the patients for which no BCR-ABL (%) information was available included a mix of patients who progressed, died, discontinued, or had ongoing measurements or atypical baselines ().
To ensure that the above data could be used in the analysis in a reconciled approach, the patient disposition data and response data were adjusted necessarily according to the following assumptions. First, the distribution of BCR-ABL scores was assumed to be conditional on continuing therapy. Although there is an imperfect match between the number of patients continuing and the patient discontinuing at month 12, this discrepancy is likely of very little significance. In addition, it was also assumed that patients with BCR-ABL % ratio >10% had sub-optimal responses and discontinued at month 12.
This study has demonstrated the cost-effectiveness of the insurance coverage with imatinib and nilotinib for CML treatments in China; the insurance coverage of imatinib and nilotinib also changed the treatment pattern and outcomes of the clinical practice for patients with CML in Jiangsu province. From the societal perspective, savings of the average cost can be seen in the following 5 years, and cost-effective at a lifetime horizon, for a diagnosed CML patient with medical insurance covering imatinib as first-line treatment, compared to an insurance policy without imatinib coverage. The stakeholders including public payer, haematologist, and oncologist could have better understanding and an integrated picture of the short- and long-term benefit by covering imatinib and nilotinib in the medical insurance, thus could be assisted and more confident on the shared decision-making for a nationwide insurance policy and clinical guidelines development for CML treatments.
Conclusions
Imatinib has changed the natural history of CML by improving survival and transforming CML from a life-threatening to a chronic disease. The insurance coverage of imatinib with PAP changed the treatment pattern of CML, which is more consistent with the treatment guideline’s recommendations, and was likely a very cost-effective long-term strategy with potential in productivity gains for treating CML in China based on the local clinical and health economics data in Jiangsu province.
Transparency
Declaration of funding
This project was sponsored by Novartis. Funding was not contingent upon publication of the manuscript.
Declaration of financial/other relationships
TX and SCT are current employees of IMS Health, which received funds from Novartis for this study. CL is an employee of Novartis Pharmaceuticals Corporation. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Appendix
Download MS Word (67 KB)Acknowledgment
The authors thank Ms Shuli Qu and Pengzhen Liu from IMS Health China, Dr Jia Chen from the First Affiliated Hospital of Soochow University, and Professor Wen Chen from the School of Public Health at Fudan University for their support in literature review, clinical data collection, and expert opinion for relevant local data inputs in the model.
References
- Wang JX, Huang XJ, Wu DP, et al. [Overview of chronic myelogenous leukemia and its current diagnosis and treatment patterns in 15 hospitals in China]. Zhonghua Xue Ye Xue Za Zhi 2009;30:721-5
- He QT, Shi FT, Yuan ZZ. Epidemiological investigation of leukemia in Baotou. Inner Mongolia Med J 1993;2:3-5
- Hu JL, Mao Z, Dong DP, et al. Epidemiological investigation of leukemia in 15 years in Haian County. Chin Med J Commun 2004;18:114-15
- Tang ZX, Sun QY, Zhang JT, et al. Epidemiological investigation of leukemia in 11 years in the Jinshan district of Shanghai. Zhonghua Xue Ye Xue Za Zhi 1994;15:430
- Zhang XY, Zhang DL, Sun X, et al. Epidemiological investigation of leukemia and aplastic anemia in Shenzhen. Zhonghua Xue Ye Xue Za Zhi 2001;22:347
- Rohrbacher M, Berger U, Hochhaus A, et al. Clinical trials underestimate the age of chronic myeloid leukemia (CML) patients. Incidence and median age of Ph/BCR-ABL-positive CML and other chronic myeloproliferative disorders in a representative area in Germany. Leukemia 2009;23:602-4
- Bian S. Chronic myelogenous leukemia. China Medical Science Press, Beijing. 2003
- D’Antonio J. Chronic myelogenous leukemia. Clin J Oncol Nurs 2005;9:535-8
- American Cancer Society. Leukemia – Chronic Myeloid (Myelogenous). Atlanta, GA: American Cancer Society, 2008
- Hughes TP, Ross DM, Melo JV. Management of patients with chronic myeloid leukemia. Handbook of chronic myeloid leukemia. Switzerland: Springer International Publishing, 2014. pp. 35-51
- Chen Z, Wang C, Xu X, et al. Cost-effectiveness study comparing imatinib with interferon-alpha for patients with newly diagnosed chronic-phase (CP) chronic myeloid leukemia (CML) from the Chinese public health-care system perspective (CPHSP). Value Health 2009;12(3Suppl)3:S85-S8
- Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood 2010;116:3758-65.
- Jiang Q, Xu LP, Liu DH, et al. Imatinib results in better outcomes than HLA-identical sibling transplants in young persons with newly diagnosed chronic-phase chronic myelogenous leukemia. Leukemia 2013;27:2410-13
- Chinese Society of Hematology CMA. [Guideline for management of chronic myeloid leukemia(2016)]. Zhonghua Xue Ye Xue Za Zhi 2016;37:633-9
- Tao Z, Liu B, Zhao Y, et al. EUTOS score predicts survival and cytogenetic response in patients with chronic phase chronic myeloid leukemia treated with first-line imatinib. Leuk Res 2014;38:1030-5
- Novartis. Oncology Patient Assistance Programs. Novartis Global. https://www.novartis.com/about-us/corporate-responsibility/access-healthcare/global-access-programs/oncology-patient. Accessedon 29 Sep 2016
- Reed SD, Anstrom KJ, Ludmer JA, et al. Cost-effectiveness of imatinib versus interferon-alpha plus low-dose cytarabine for patients with newly diagnosed chronic-phase chronic myeloid leukemia. Cancer 2004;101:2574-83
- Ghatnekar O, Hjalte F, Taylor M. Cost-effectiveness of dasatinib versus high-dose imatinib in patients with Chronic Myeloid Leukemia (CML), resistant to standard dose imatinib–a Swedish model application. Acta Oncol 2010;49:851-8
- Shin M, Shin S, Lee JY, et al. Cost-effectiveness of first-line tyrosine kinase inhibitors (Tkis) in newly diagnosed chronic myeloid leukemia (Cml) patients in Korea: comparison of Dasatinib (100mg), Nilotinib (600mg) and Imatinib (400mg). Value Health 2015;18:A458
- Hay JW, Smeeding J, Carroll NV, et al. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report–Part I. Value Health 2010;13:3-7
- Introduction. The First Affiliated Hospital of Soochow University Suzhou First People's Hospital. Suzhou, China. http://fyy.sdfyy.cn/Main/Display_B721_A0.html. Accessed on 29 Sep 2016.
- Wang J, Shen ZX, Saglio G, et al. Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase: ENESTchina. Blood 2015;125:2771-8
- Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of busulfan and hydroxyurea in chronic myelogenous leukemia: prolongation of survival by hydroxyurea. The German CML Study Group. Blood 1993;82:398-407
- WHO. WHO Macroeconomics. Health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization, 2001
- NBS. GDP per capita in China. National Bureau of Statistics of China. Beijing. 2015. http://data.stats.gov.cn/easyquery.htm?cn=C01 Accessed on 29 Sep 2016
- Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010;362:2251-9
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010;362:2260-70
- Jiang Q, Huang X. CML treatment and development in China. Chin J Intern Med 2013;52:803-5
- American Cancer Society. Stem cell transplant for chronic myeloid leukemia. http://www.cancer.org/cancer/leukemia-chronicmyeloidcml/detailedguide/leukemia-chronic-myeloid-myelogenous-treating-bone-marrow-stem-cell. Accessed on 29 Sep 2016
- Hahn EA, Glendenning GA, Sorensen MV. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus lowdose cytarabine: results from the IRIS study. J Clin Oncol 2003;21:2138-46
- Dalziel K, Round A, Garside R, et al. Cost effectiveness of imatinib compared with interferon-alpha or hydroxycarbamide for first-line treatment of chronic myeloid leukaemia. Pharmacoeconomics 2005;23:515-26
- NICE. National Institute for Health and Care Excellence. Dasatinib, nilotinib and standard-dose imatinib for the first-line treatment of chronic myeloid leukaemia. Technology appraisal guidance. London. 2012. http://www.nice.org.uk/guidance/ta251 Accessed on 29 Sep 2016
- NICE. Dasatinib, high-dose imatinib and nilotinib for the treatment of imatinib-resistant chronic myeloid leukaemia (CML) (part review of NICE technology appraisal guidance 70), and dasatinib and nilotinib for people with CML for whom treatment with imatinib has failed because of intolerance (TA241). London. 2012. http://www.nice.org.uk/guidance/ta241 Accessed on 29 Sep 2016.
- NCCN. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Chronic Myeloid Leukemia. London. 2009. http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf Accessed on 29 Sep 2016
- Gao GL, Xu N, Zhou X, et al. Treatment efficacy of imatinib mesylate versus allogeneic hematopoietic stem cell transplantation for patients with chronic myeloid leukemia in chronic phase. Natl Med J China 2013;93:3035-9
- Jin H, Han Y, Zhang Y. Clinical observation of imatinib in treatment of patients with chronic myeloid leukemia. Chin J Trauma Disabil Med 2015;23:23-4
- Meng Y, Liu CS, Li W, et al. Efficacy analysis of imatinib in treatment of patients with chronic myeloid leukemia. J Jilin Uni (Med Ed) 2012;38:563-6
- Wu RJ, Xie HL. Clinical observation of interferon combined with chemotherapy and imatinib in the treatment of chronic myeloid leukemia. Chin J Convalesc Med 2014;5:430-1
- Yang DD, Zhang J. Efficacy analysis of the application of imatinib combined with hydroxyurea and interferon in the treatment of chronic myeloid leukemia. Contemp Med Forum 2015;13:156-8
- Zhou M, Sha XS, Chou HY, et al. Outcomes of imatinib and allogeneic hematopoietic stem cell transplantation in the treatment of chronic myeloid leukemia. Chin J Hematol 2014;35:126-8
- Zou WY, Xu DR, Su C, et al. Therapeutic effects of imatinib on chronic myeloid leukemia in different phases and the factors affecting the effects. J Southern Med Uni 2008;28:1660-2