Abstract
Objective: To quantify healthcare utilization and costs in patients with tuberous sclerosis complex (TSC) and renal angiomyolipoma (AML) in a matched cohort of patients without TSC or AML.
Methods: Administrative data from the MarketScan Research Databases were used to select patients with TSC and renal AML during January 1, 2000–March 31, 2013 from the Commercial database and January 1, 2000–June 30, 2012 from the Medicaid database. Patients were required to have at least 30 days of follow-up from initiation into the study, and were followed until inpatient death, end of insurance coverage, or the end of study. Age, calendar year, and payer-matched controls that had no TSC and no AML were selected. All-cause annualized healthcare utilization and costs were calculated by service category.
Results: A total of 218 patients under 18 years and 377 patients 18 years and older with TSC-renal AML were selected from the Commercial database, and matched to 654 and 1,131 controls, respectively. Thirty-eight patients under 18 years and 110 patients 18 years or older with TSC-renal AML were selected from the Medicaid database, and matched to 54 and 212 controls, respectively. Within the Commercial cohort, and across both age groups, TSC-renal AML patients utilized more healthcare services than their matched controls. Within the Medicaid cohort, in both age groups, utilization was higher in TSC-renal AML patients vs control patients for inpatient admissions, emergency room visits, physician office visits, and hospital-based outpatient visits. Across age groups and in both the Commercial and Medicaid cohorts, the annual average total costs were significantly higher in TSC-renal AML patients compared to control patients (p < 0.05 for all). Healthcare costs ranged from $29,240–$48,499 for TSC-renal AML patients and from $2,082–$10,864 for control patients.
Conclusions: Compared to controls, TSC-renal AML patients incurred substantially higher annual healthcare utilization and costs.
Introduction
Tuberous sclerosis complex (TSC) is a rare hereditary disease affecting 25,000–40,000 persons in the US and ∼1–2 million persons worldwide, regardless of age, gender, race, and ethnicityCitation1,Citation2. TSC is defined by mutations of the TSC1 and TSC2 genes located at the 9q34 and 16p13.3 chromosomes, respectivelyCitation1,Citation3,Citation4. The TSC1 and TSC2 genes produce hamartin and tuberin, and act in concert to inhibit the mammalian target of rapamycin (mTOR) protein complex responsible for sensing nutrients and controlling protein synthesisCitation1,Citation3,Citation5. The loss of regulation as a result of these mutations leads to over-activation of mTOR, followed by abnormal cell differentiation and development, and the tuberous lesions characteristic of the diseaseCitation1,Citation3,Citation6.
While the tuberous lesions from TSC are benign, they can result in considerable morbidity by impairing normal functioning of affected organsCitation1–5,Citation7–10. While skin lesions are the most common manifestation of TSC, occurring in more than 90% of individuals with TSCCitation3,Citation5, neurological complications are a key driver of morbidityCitation4,Citation8. Central nervous system involvement is found in ∼90% of casesCitation3, often including seizures and behavioral disordersCitation4,Citation8. Renal complications are the second leading cause of morbidity and the primary cause of death in TSC patients over 30 years of ageCitation8, occur in 70–90% of persons with TSCCitation3, and typically appear as renal cysts or angiomyolipomas (AMLs)Citation2,Citation7,Citation9,Citation10. AML diagnoses carry significant risks, including impaired renal function and spontaneous hemorrhage from the formation of aneurysmsCitation2,Citation3,Citation10. The disease is chronic and lifelong—children with TSC are born with normally functioning kidneys and develop complications as they ageCitation2. The progression from cystic disease or angiomyolipoma (AML) to chronic kidney disease (CKD) is well established, with renal failure, by one historical study, being the leading cause of death among TSC patients based on death certificate dataCitation11.
Despite the potential for a substantial economic burden from TSC-renal angiomyolipoma, the healthcare utilization and costs of these patients have not been studied. The objective of the current study was to quantify the healthcare utilization and costs of patients with TSC-renal AML compared to those of patients without the disease using administrative claims from a large, geographically diverse US population including both Commercial and Medicaid payers.
Methods
Study design
A retrospective, observational study was conducted using US administrative claims data. Patients diagnosed with TSC-renal AML were selected and followed until the earliest of the following events: inpatient death; end of enrollment from a health plan in the database; or the end of the study period. Healthcare utilization and costs were quantified in patients with TSC-renal AML and in a matched cohort of patients without TSC or renal AML.
Data source
Patients were selected using data from the Truven Health MarketScan Commercial Claims and Encounters (Commercial) and Multi-State Medicaid (Medicaid) databases (Truven Health Analytics, Ann Arbor, MI, US). These databases include medical claims for healthcare services performed in the inpatient and outpatient settings, outpatient prescription drug information, and enrollment data including demographic information and eligibility data. The medical claims files include service dates, provider reimbursement amounts, and patient co-payment and deductible amounts. Additional information about the MarketScan databases can be found in a Truven Health White PaperCitation12.
The Commercial database provides detailed utilization and cost data for ∼35 million enrollees annually covered under a variety of health plans with fee-for-service and capitated payment arrangements, including preferred provider organizations, point of service plans, indemnity plans, and health maintenance organizations. The Medicaid database contains the pooled healthcare experience of ∼10 million Medicaid enrollees each year from 15 states of varying sizes and industrial compositions across the US. Patients selected from the Medicaid database were further screened to include only those covered under non-capitated plans, as payment information for capitated plans is not available.
Study variables were defined using enrollment data and International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, Current Procedural Terminology 4th edition (CPT-4) codes, Healthcare Common Procedure Coding System (HCPCS) codes, and hospital revenue codes, as appropriate.
Because the databases are de-identified in compliance with the Health Insurance Portability and Accountability Act (HIPAA) regulations, Institutional Review Board approval was not required.
TSC-renal AML patient selection
Patients were selected from two distinct databases: the Commercial database from January 1, 2000 through March 31, 2013, and the Medicaid database from January 1, 2000 through June 30, 2012. TSC-renal AML patients were required to have at least one medical claim with a diagnosis of TSC (ICD-9-CM 759.5) and evidence of renal AML. Renal AML was determined using the following ICD-9-CM diagnosis code algorithm applied to medical claims: [223.0] or [593.81] or [599.71 and (593.9, 789.35, or 739.36) within 30 days] or [789.09 and 593.9 within 30 days] or [789.09 and (789.35 or 789.36) within 30 days] and not [441.3, 441.4, 577.8, 759.0, 789.1, or 789.2 at any time during follow-up].
The date of the first TSC or renal AML diagnosis in the selection period was assigned as the index date. Duration of follow-up was variable, with patients followed from the index date until the earliest of inpatient death, the end of continuous health plan enrollment, or the end of the study period. Patients were required to have a minimum of 30 days of follow-up after the index date.
Control patient selection
A pool of control patients was identified by age and payer type for matching. Two hundred potential controls were identified for each TSC-renal AML patient (case), randomly selected within the case’s age range on their index date (age ranges <18, 18–34, 35–44, 45–54, 55–64, ≥65 years), and with the same payer (Commercial or Medicaid). The potential controls were excluded if they had a medical claim with a diagnosis of TSC or renal AML based on the above definitions. Of the remaining potential controls, up to three patients were randomly selected for each case and assigned the index date of that case.
Patient characteristics and outcome measures
Demographic variables included mean age on the index date, gender, and mean months of follow-up subsequent to the index date. All-cause healthcare utilization and cost measures were determined for place of service (e.g., inpatient admissions, emergency room (ER) visits, physician office visits, hospital-based outpatient visits, or ambulatory surgical center visits) and unique therapeutic classes of medications. The average number of services per category and mean costs per category were calculated during the variable duration of follow-up and were annualized and reported as per patient per year (PPPY). All costs were reported as total reimbursed amount in 2013 dollars.
Analyses
Descriptive analyses were performed to assess study variables, stratified by age on the index date (<18 years and ≥18 years). Categorical variables were summarized by proportions, while continuous variables were reported by mean and standard deviation. Differences between the TSC-renal AML patients and the matched controls were tested for statistical significance using Chi-square tests for categorical variables and t-tests for continuous variables.
Results
Sample selection
A total of 595 patients with TSC-renal AML and 1,785 age and index year-matched patients without TSC-renal AML met the study selection criteria in the Commercial database. Within the Commercial cohort, 36.6% of the case and control patients were under 18 years (TSC-renal AML, n = 218; control, n = 654) and 63.4% were 18 years or older (TSC-renal AML, n = 377; control, n = 1,131).
Within the Commercial cohort, TSC-renal AML patients were demographically similar to their matched controls, with few exceptions (). Among patients under 18 years, control patients were older than the TSC-renal AML patients (11.2 vs 9.7 years; p < .05). Although this age difference was statistically significant, the absolute difference between the age groups was small (1.5 years). For both age groups, the proportion of females in the TSC-renal AML cases was higher compared to that in the controls, although the difference was significant only for patients aged ≥18 years (age <18 years 54% vs 47%, p = .07; age ≥18 years 73% vs 56%; p < .05). Among patients under 18 years, the average duration of follow-up did not differ significantly between cases and controls; mean follow-up duration was 47.9 months in the TSC-renal AML patients and 50.1 months in the control patients (p = .22). Among patients 18 years or older, TSC-renal AML cases had significantly less follow-up than controls (37.0 vs 40.8 months; p < .05).
Table 1. Characteristics of TSC-renal angiomyolipoma patients and matched controls.
A total of 148 patients with TSC-renal AML were matched to 266 controls in the Medicaid database. As three qualified controls for each Medicaid Case were not available, a 3:1 matching process within the Medicaid cohort was not performed. Of the matched patients, 25.7% of TSC-renal AML patients (n = 38) and 20.3% of the controls (n = 54) were under the age of 18, while 74.3% of TSC-renal AML patients (n = 110) and 79.7% of the controls (n = 212) were 18 years or older.
Within the Medicaid cohort, among patients ≥18 years, there was a significantly higher percentage of females in the controls compared to the TSC-renal AML patients (67% vs 58%; p < .05) (). The TSC-renal AML patients had approximately twice the duration of follow-up as their controls (87.2 vs 34.4 months for patients under 18 years; 75.9 vs 36.0 months for patients 18 years or older; p < .05 for both). A potential explanation for this difference is that TSC-renal AML patients were more likely to meet Medicaid eligibility for a longer time than their controls, due to disability benefits that cover neurological, mental disorders, skin disorders, and other comorbidities that are associated with TSC-renal AML.
Healthcare utilization
summarizes the annualized healthcare utilization of TSC-renal AML patients and matched controls. Within the Commercial cohort and across both age groups, TSC-renal AML patients utilized more healthcare services than their matched controls: among patients under 18, TSC-renal AML patients had a significantly higher annual mean number of inpatient admissions (0.44 vs 0.02), ER visits (0.67 vs 0.27), physician office visits (7.49 vs 3.07), hospital-based outpatient visits (7.48 vs 0.68), ambulatory surgical center visits (0.23 vs 0.02), and unique therapeutic classes of medications (3.16 vs 1.91) (all p < .05). Among those 18 and over, statistically significant differences in all categories except for use of ambulatory services (inpatient admissions 0.54 vs 0.04; ER visits 0.88 vs 0.21; physician office visits 8.46 vs 3.42; hospital-based outpatient visits 6.29 vs 1.34; ambulatory surgical center visits 0.07 vs 0.05; and unique therapeutic classes of medications 4.99 vs 3.37) (all p < .05 except ambulatory services) were observed.
Table 2. Annualized healthcare utilization in TSC-renal angiomyolipoma patients and matched controls.
Among Medicaid patients under 18 years, PPPY utilization of each of the healthcare services was higher in TSC-renal AML patients than in the control patients for inpatient admissions (0.56 vs 0.06; p < .05), ER visits (1.22 vs 0.52; p < .05), physician office visits (4.85 vs 2.55; p < .05), and hospital-based outpatient visits (6.52 vs 1.19; p < .05). Similar findings were observed in patients 18 years or older, where TSC-renal AML patients compared to control patients had higher PPPY inpatient admissions (0.47 vs 0.28), ER visits (1.94 vs 0.65), physician office visits (5.00 vs 2.11), and hospital-based outpatient visits (4.35 vs 1.93) (all p < .05, except for inpatient admissions). In patients under 18 years, the PPPY number of ambulatory surgical center visits was lower in the TSC-renal AML patients than in the control patients (0.01 vs 0.04; p = .53); however, in patients 18 years or older, TSC-renal AML patients had a higher annual mean number of ambulatory surgical center visits than control patients (0.05 vs 0.01; p < .05). In both age groups, the annual mean number of unique therapeutic classes of medications was higher in the control patients than in the TSC-renal AML patients, although differences were not significant (age <18 years; 3.52 vs 3.10; age ≥18 years; 6.33 vs 3.98).
Regardless of insurance type, TSC-renal AML patients under 18 years had fewer annual healthcare service visits than TSC-renal AML patients of 18 years and over, with three exceptions: older patients with commercial insurance had fewer PPPY ambulatory surgical center visits; older patients with Medicaid insurance had a smaller average number of inpatient admissions; and, for both payers, the average number of hospital-based outpatient visits was lower in the older age group compared to the younger age group. Among TSC-renal AML patients across both age groups, Medicaid patients had lower utilization than Commercial patients, except a higher number of ER visits (under 18 years: 1.22 vs 0.67; 18 years or older: 1.94 vs 0.88) and a higher number of inpatient admissions (for under 18 years only: 0.56 vs 0.44).
Healthcare costs
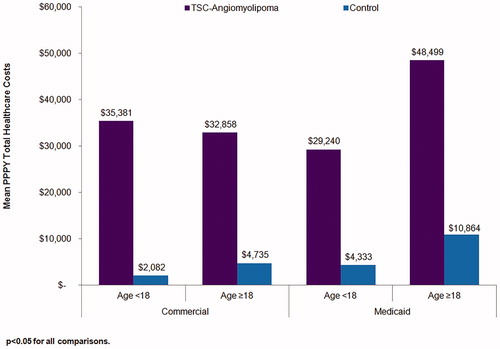
Annualized total healthcare costs of TSC-renal AML patients and their matched controls are shown in . Across age groups and insurance types, the annual average total healthcare costs were significantly higher in TSC-renal AML patients compared to control patients (p < .05 for all). The mean PPPY total healthcare costs ranged from $29,240–$48,499 for TSC-renal AML patients and from $2,082–$10,864 for control patients.
Figure 1. Annualized total healthcare costs in TSC-renal angiomyolipoma patients and matched controls.
Annualized total healthcare costs stratified by service type are presented in for the Commercial cohort and in for the Medicaid cohort. In both payers and across both age groups, TSC-renal AML patients had significantly higher costs than their controls in all service types (p < .01 in all cases). For Commercial TSC-renal AML patients under 18 years, inpatient cost was $10,113 higher, outpatient pharmacy cost $15,749 higher, and outpatient pharmacy $7,050 higher than their controls; for Commercial TSC-renal AML patients 18 years or older, the difference was $14,527 higher in inpatient, $11,041 higher in outpatient, and $2007 higher in outpatient pharmacy departments ().
Figure 2. Annualized total healthcare costs by service type in TSC-renal angiomyolipoma patients and matched controls in the commercial cohort.
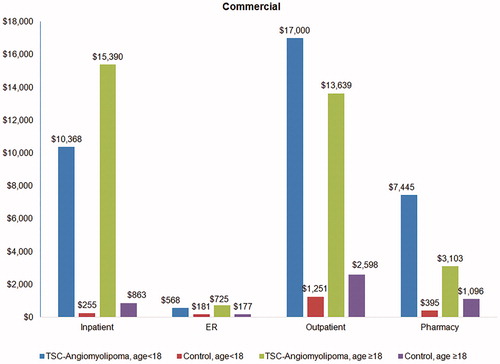
Figure 3. Annualized total healthcare costs by service type in TSC-renal angiomyolipoma patients and matched controls in the Medicaid cohort.
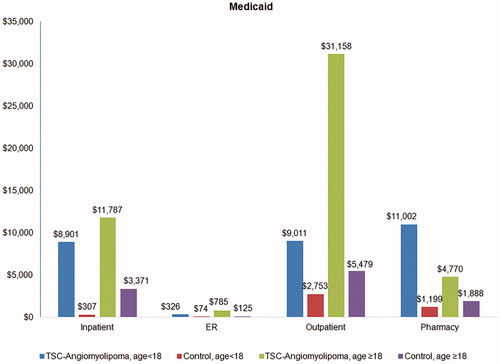
Within the Medicaid cohort under age 18, the largest cost difference between TSC-renal AML patients and controls came from outpatient pharmacy ($9,804), followed by inpatient ($8,594), and outpatient ($6,258). For the Medicaid cohort 18 years or older, the largest difference was outpatient cost ($25,678), followed by inpatient cost ($8,415) and outpatient pharmacy ($2,882) (). The difference in ER costs was the smallest in both payers and across both age cohorts ($251 and $660 higher for TSC-renal AML patients than for controls).
Discussion
Findings from the current study demonstrated that, compared with age-matched controls, TSC-renal AML patients incurred substantially higher annual healthcare utilization and costs. Within the Commercial cohort, PPPY inpatient admissions for TSC-renal AML patients were between 13.5 to 22 times higher than non-TSC-renal AML patients across both age groups; within the Medicaid cohort, admissions were 1.7 and 9.3 times higher among TSC-renal AML patients. Inpatient costs accounted for the largest proportion of costs in both the Commercial and Medicaid populations, 29–47% and 24–30% of costs, respectively. Patterns of increased utilization and costs in TSC-renal AML patients relative to controls were observed in almost every healthcare setting.
To the best of our knowledge, there are no published studies reporting on healthcare service utilization and their corresponding costs in the TSC-renal AML population in the US. Two recent studies based on small sample sizes were conducted outside of the USCitation13,Citation14. One study, conducted in the Netherlands, found an annual cost of €1,275–€31,916 (in 2012€) per patient using 376 TSC patients with CKDCitation14. Another study of 79 patients with TSC-renal AML in the UK estimated that TSC patients had mean costs of £15,162 (in 2014 costs) during a 3-year window, almost 3-times that of a comparison cohort (£5,672)Citation13.
Results from the current study provide some insight into the cost burden from this disease, with PPPY total healthcare costs for TSC-renal AML patients ranging from $29,240–$48,499. To place these costs in perspective, PPPY costs were $13,700 (in 2012 dollars) for patients with diabetesCitation15 and $7,850 (in 2009 dollars) for patients with asthmaCitation16. Given the chronic nature of TSC-renal AML, the costs may have under-estimated the long-term burden of the disease. CKD may result from the renal cysts and AML found in TSC patientsCitation2, and the costs associated with CKD are well documentedCitation17. Estimates of savings to Medicare for each patient not progressing to dialysis are $250,000 per patientCitation17.
Treatment options for TSC-renal AML have focused on interventional procedures such as renal artery embolization and nephrectomyCitation3,Citation5. As with overall costs of TSC-renal AML, costs in patient sub-groups based on treatment have not been well studied, nor have newer treatment options, such as mTOR inhibitors to reduce AML sizeCitation6,Citation18. Further research is needed to evaluate outcomes based on choice of treatment and to quantify the impact of specific treatments on both short-term and long-term costs.
Several limitations should be noted. First, administrative claims were used to identify patients with TSC and renal AML; as with all claims-based studies, any miscoding of claims may have resulted in misclassification of our patients. Second, the rarity of patients with TSC-renal AML means that outlier measures could have had a large impact on our findings. Third, to keep all TSC-renal AML patients in the sample, controls were matched based on age and payer only. Patients’ other characteristics may differ between TSC-renal AML patients and controls, which could contribute to the healthcare utilization and cost differences observed in this study. Fourth, TSC-renal AML patients can be treated for any TSC manifestation, including seizures, and the costs do not limit focus to the AML-related disease activities. In addition, the study period of January 2000–March 2013 occurred largely prior to the US Food and Drug Administration’s approval of everolimus for the treatment of renal manifestations of TSC (April 2012), thus the costs reported in this study were mostly pre-everolimus costs. Finally, this study was limited to those individuals with Commercial or Medicaid health coverage, and thus results may not be generalizable to uninsured patients or to TSC-renal AML patients covered under other payer types.
Conclusions
Compared to matched controls, TSC-renal AML patients incurred substantially higher annual healthcare utilization and costs, across both commercially and Medicaid insured patients. Further research is needed to better understand the costs of this disease and the potential impact of treatment on these costs.
Transparency
Declaration of funding
The study was funded by Novartis Pharmaceutical Corporation.
Declaration of financial/other relationship
ZL, QS, and JP are employees of Novartis. HC, JH, and JB are paid consultants to Novartis. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Michelle Shaw for providing medical writing assistance.
References
- National Institute of Neurological Disorders and Stroke. Tuberous Sclerosis Fact Sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Tuberous-Sclerosis-Fact-Sheet Bethesda, MD. Accesssed Jan. 5, 2017
- Dixon BP, Hulbert JC, Bissler JJ. Tuberous sclerosis complex renal disease. Nephron Exp Nephrol 2011;118:e15-20
- Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet 2008;372:657-68
- Rosser T, Panigrahy A, McClintock W. The diverse clinical manifestations of tuberous sclerosis complex: a review. Semin Pediatr Neurol 2006;13:27-36
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345-56
- Moavero R, Coniglio A, Garaci F, et al. Is mTOR inhibition a systemic treatment for tuberous sclerosis? Ital J Pediatr 2013;39:57
- Cook JA, Oliver K, Mueller RF, et al. A cross sectional study of renal involvement in tuberous sclerosis. J Med Genet 1996;33:480-4
- Martignoni G, Pea M, Rocca PC, et al. Renal pathology in the tuberous sclerosis complex. Pathology 2003;35:505-12
- O’Callaghan FJ, Noakes MJ, Martyn CN, et al. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int 2004;94:853-7
- Rakowski SK, Winterkorn EB, Paul E, et al. Renal manifestations of tuberous sclerosis complex: Incidence, prognosis, and predictive factors. Kidney Int 2006;70:1777-82
- Shepherd CW, Gomez MR, Lie JT, et al. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 1991;66:792-6
- Hansen LG, Chang S, Danielson E. Health Research Data for the Real World: The MarketScan Databases (White Paper). Ann Arbor, MI: Truven Health Analytics; 2013
- Kingswood JC, Nasuti P, Patel K, et al. The economic burden of tuberous sclerosis complex in UK patients with renal manifestations: a retrospective cohort study in the clinical practice research datalink (CPRD). J Med Econ 2016;19:1116-26
- Vekeman F, Magestro M, Karner P, et al. Kidney involvement in tuberous sclerosis complex: the impact on healthcare resource use and costs. J Med Econ 2015;18:1060-70
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033-46
- Carrier E, Cunningham P. Medical cost burdens among nonelderly adults with asthma. Am J Manag Care 2014;20:925-32
- National Institutes of Health. Chronic Kidney Disease and Kidney Failure Fact Sheet. https://report.nih.gov/nihfactsheets/Pdfs/ChronicKidneyDiseaseAndKidneyFailure(NIDDK).pdf Bethesda, MD. 2010. Accessed Jan. 5, 2017
- Pirson Y. Tuberous sclerosis complex-associated kidney angiomyolipoma: from contemplation to action. Nephrol Dial Transplant 2013;28:1680-5
