Abstract
Aims: Patients treated with anticoagulants may experience serious bleeding or require urgent surgery or intervention, and may benefit from rapid anticoagulant reversal. This exploratory analysis assessed healthcare resource utilization (HCRU) in patients treated with idarucizumab, a specific reversal agent for dabigatran etexilate.
Materials and methods: RE-VERSE AD™ (NCT02104947), a prospective, multi-center open-label study, is evaluating idarucizumab for dabigatran reversal in patients with serious bleeding (Group A) or undergoing emergency surgery/procedures (Group B). HCRU outcome measures evaluated in the first 90 patients enrolled were use of blood products and pro-hemostatic agents, length of stay (LOS) in hospital, and LOS in intensive care unit (ICU).
Results: Blood products or pro-hemostatic agents were given to 63% (32/51) of patients in Group A and 23% (9/39) of patients in Group B on the day of/day after surgery. An overnight hospital stay was reported for 82% (42/51) of patients in Group A with median LOS = 7 (range = 1–71) bed-days. For Group B, 92% (36/39) had an overnight hospital stay with a median LOS = 9 (range = 1–92) bed-days. In Group A, 17 patients were admitted to the ICU for at least 1 day with median LOS = 4 (range = 1–44) days; in Group B the number was 15 with median LOS = 2 (range = 1–92) days.
Limitations: The lack of a control group and the small patient numbers limit the strength of the conclusions.
Conclusions: The use of idarucizumab may simplify emergency management of dabigatran-treated patients with life-threatening bleeds and reduce perioperative complications in patients undergoing emergency surgery.
Introduction
The anticoagulant dabigatran is a direct thrombin inhibitor that is orally administered as the prodrug, dabigatran etexilate, and is indicated for the prevention of ischemic stroke and systemic arterial embolism in patients with non-valvular atrial fibrillationCitation1,Citation2 and for the prevention and treatment of venous thromboembolismCitation3,Citation4. For stroke prevention, dabigatran is more effective than warfarin when administered at a dose of 150 mg twice daily and equally effective as warfarin when administered at a dose of 110 mg twice dailyCitation1,Citation2. In comparison with warfarin, dabigatran 110 mg twice daily was associated with a lower rate of major bleeding, while dabigatran 150 mg twice daily was associated with a similar rate. Notably, rates of intracranial bleeding were significantly lower with dabigatran when administered at either dose level. The risk of gastrointestinal (GI) bleeding, however, was higher with dabigatran 150 mg twice daily than with warfarin and similar with dabigatran 110 mg twice dailyCitation1–4.
Despite the reassuring bleeding profile with dabigatran, as with any anticoagulant, life-threatening bleeding may still occurCitation5, and patients receiving dabigatran without bleeding may require urgent surgery or procedural interventions. In these cases, rapid reversal of the anticoagulant effect of dabigatran is desirable. Previous therapeutic approaches to treat bleeding or reverse anticoagulant effects have included the use of non-specific pro-hemostatic agents, such as prothrombin complex concentrates (PCCs), activated PCC (aPCC), or recombinant activated factor VIIa (rFVIIa), but clinical experience with these agents is limitedCitation6. Hemodialysis may be considered to accelerate plasma clearance of dabigatran, especially in patients with renal failure; however, this is a time-consuming procedure that does not completely eliminate dabigatran and is not feasible in an actively bleeding patientCitation6.
Idarucizumab, a monoclonal antibody fragment (Fab), is a specific reversal agent for dabigatran, which produced immediate, complete, and sustained reversal in phase I studies, as demonstrated by normalization of the diluted thrombin time, ecarin clotting time, and activated partial thromboplastin timeCitation7,Citation8. Idarucizumab is currently being evaluated for the emergency reversal of dabigatran, in the ongoing RE-VERSE AD™ (REVERSal Effects of Idarucizumab in Patients on Active Dabigatran; NCT02104947) phase 3 studyCitation9. An analysis of interim data from the first 90 patients enrolled in the study showed that idarucizumab completely reversed dabigatran’s anticoagulation effect within minutes, paralleled by a profound reduction in unbound dabigatran concentrationCitation10.
The ability to rapidly and completely reverse dabigatran-induced anticoagulation may reduce delays to urgent surgical or major invasive procedures, reduce the risk of perioperative bleeding complications, and facilitate management of patients with life-threatening bleeding. The impact that this will have on healthcare resource utilization (HCRU) is currently unknown; therefore, the objective of the present exploratory analysis was to provide baseline data regarding HCRU in patients treated with idarucizumab across different diagnosis-related groups (DRGs) using a post-hoc analysis of interim data from the first 90 patients enrolled in the RE-VERSE AD™ study. An economic assessment was not included in the current analysis.
Methods
Study design
The design of the RE-VERSE AD™ study has been previously describedCitation9, as have the primary and secondary outcomes of an interim analysis based on data from the first 90 patientsCitation10. In brief, the RE-VERSE AD™ study is a prospective, multi-center, open-label, single treatment arm, phase 3 study evaluating idarucizumab for the emergency reversal of dabigatran in patients with overt, uncontrolled, and/or life-threatening bleeding judged by the physician to require a reversal agent (Group A) or those with a condition requiring emergency surgery or procedure (within the following 8 h) for whom adequate hemostasis is required (Group B)Citation9.
All patients received 5 g of intravenous idarucizumab administered as two infusions no more than 15 min apart. For all patients, additional treatment with fresh frozen plasma (FFP), packed red blood cells (PRBC), platelets, PCC (three-factor or four-factor), aPCC, rFVIIa, a repeat dose of idarucizumab, or with hemodialysis was permitted before or after administration of idarucizumab.
The protocol was approved by the institutional review board or ethics committee of each of the participating centers. All patients or their authorized representative provided written informed consent. An independent Data Monitoring Committee periodically reviewed study outcomes.
Study participants
Eligibility requirements for patients in groups A and B included current treatment with dabigatran and age ≥18 years. Main exclusion criteria for patients in Group A were minor bleeds, such as epistaxis or hematuria, which could be managed with standard supportive care, and the absence of clinical signs of active bleeding. Main exclusion criteria for patients in Group B were elective surgery or procedures, which could be safely postponed for more than 8 h, and patients with a low risk of uncontrolled or unmanageable bleeding. For patients in Group A, the qualifying bleeding events were further categorized into the following protocol-specified groups: GI bleeds, intracranial hemorrhages (ICH) (and sub-groups: subdural hemorrhage, deep intracerebral and subarachnoid hemorrhage), and other bleeds (e.g. hematuria after traumatic urinary catheter, epistaxis, menstrual bleeding, unknown). Patients in Group B were retrospectively grouped to the most plausible DRG, based on available information from patient narratives, in particular qualifying diagnosis at the time of enrollment. The following DRGs were identified: musculoskeletal trauma surgery/procedure; cardiac and vascular surgery/procedure; major therapeutic open or endoscopic abdominal surgery/procedure; exploratory abdominal surgery/procedure; infections of skin and subcutaneous tissues or joint infection, and other surgery/procedures.
Clinical outcomes
In Group A, time to cessation of bleeding since the first idarucizumab infusion up to 24 h after completion of the second infusion was assessed by the investigator. Coagulation status was categorized before and at several timepoints after treatment (after the first vial, between 10–30 min after treatment, and 1, 2, 4, 6, 12, and 24 h after treatment). However, criteria for determining bleeding cessation were not specified in the protocol, which was particularly challenging in the case of ICH, where cessation of bleeding can only be evaluated by a follow-up CT scan. In Group B, the time from the decision to operate (assumed to be at the point of idarucizumab infusion), to the start of surgery was assessed, as was occurrence of major bleeding at the time points specified above for Group A, up to 24 h post-surgery. Additionally, in Group B, the treating clinician categorized hemostasis during the procedure as normal, mildly abnormal, as judged by quantity of blood loss (e.g. slight oozing), moderately abnormal (e.g. controllable bleeding), or severely abnormal (e.g. severe refractory hemorrhage).
Healthcare resource utilization measures
Measures taken to evaluate HCRU in groups A and B included utilization rate of blood products and pro-hemostatic agents (number of patients treated and number of units administered), hospital admissions, length of stay (LOS) in hospital (bed-days), intensive care unit (ICU) admission rate, and ICU LOS (days). In Group A, information on timing of blood product use was not available, but blood products were sometimes used during the follow-up period (up to 90 days). Descriptive statistics such as mean (standard deviation), median (range), or frequencies were applied as appropriate. Statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
A total of 90 patients (51 in Group A and 39 in Group B) were enrolled between June 2014 and February 2015, in 35 countries. The number of patients in each sub-group ranged from three for the sub-group undergoing cardiac or vascular surgery to 20 for the GI bleeding category. Over 95% of patients were being treated with dabigatran for the prevention of stroke on the background of atrial fibrillation. The median age was 76.5 years (range = 48–93) and median patient-reported time since the last dose of dabigatran was 15.4 h. Age and other baseline characteristics varied across sub-groups; patients in the exploratory abdominal surgery group were the oldest (median age = 87.5; range = 80–93 years) and had the lowest creatinine clearance values (median = 22.74; range = 19.49–25.99 mL/min) compared with other surgery types or DRGs. Patients in this group typically underwent exploratory surgery because of incurable disease. Additional baseline characteristics have previously been reportedCitation10 and are included in Supplementary Tables 1a and b.
Clinical outcomes
Time to bleeding cessation in Group A
As previously reportedCitation10, time to bleeding cessation could be assessed in 35 of the 51 patients in Group A. Cessation could not be ascertained for 13 patients because of prior death, missing data, or due to the time point of the report, and in an additional three patients there were no evaluable bleeding assessments at baseline. In 27 of the 35 patients (77%), bleeding cessation occurred within 24 h of idarucizumab administration (Supplementary Table 2). The median time to hemostasis in Group A was 11.4 h (range = 0–1497 h; n = 35). The prolonged times (and the presence of outliers in the data reported) were due to an inability to visualize some GI bleeds; in such cases, stoppage of bleeding was based on the disappearance of melena, which may take several days. Although the median time to cessation of bleeding was recorded as 23.1 h (range = 17–408 h; n = 8) in patients with intracerebral/subarachnoid hemorrhage and 32.7 h (range = 5–1497; n = 5) in patients with subdural hemorrhage, these are not accurate measures because bleeding could only be assessed by repeated brain imaging in these patient groups, and such imaging was delayed in several patients (Supplementary Table 2). Surgical intervention was used to control bleeding in 11 of the patients with assessable bleeding, most frequently in those with subdural hemorrhage (in all of the five patients in whom bleeding cessation could be assessed; drainage of subdural hematoma was reported in all of these patients).
Time to surgery in Group B
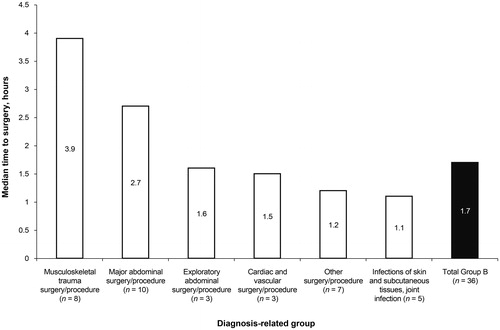
In Group B, idarucizumab averted the need for emergency dialysis in one patient who had taken an overdose of dabigatranCitation11. Two patients were too unstable for surgery, despite reversal of dabigatran with idarucizumab. The remaining 36 patients underwent urgent surgery or procedures. The median time from idarucizumab infusion to surgery was 1.7 h, with a range of –1–26 h (; Supplementary Table 3). The shortest time to surgery was –1 h, which indicates that surgery began prior to completion of idarucizumab administration.
Normal intra-operative hemostasis was reported in 33 of 36 (92%) patients. Hemostasis was mildly abnormal in two patients and moderately abnormal in one patient. No patient experienced major bleeding within 24 h post-surgery, defined according to the International Society on Thrombosis and Haemostasis (ISTH), Thrombolysis in Myocardial Infarction (TIMI), and Global Strategies for Opening Occluded Coronary Arteries (GUSTO) classifications.
Healthcare resource utilization
Utilization rate of blood products and pro-hemostatic agents
Blood products and pro-hemostatic agents, most commonly PRBC and FFP, were used in 50% (45/90) of patients. rFVIIa and three- or four-factor PCC were never used; aPCC was given to 4% (4/90) of patients. In Group A, 63% (32/51) of patients were given treatment with blood products or pro-hemostatic agents at least once. The most frequently used product was PRBC, which was administered to 57% (29/51) of patients (). Of these 29 patients, 26 were given ≥2 units and 14 were given ≥4 units. The median quantity of PRBC transfused per patient was 3 units (range = 1–20 units). FFP was administered to 27% (14/51) of patients (median = 2 units; range = 1–10 units). Six patients received platelets (median = 2 units; range = 1–4 units), three patients were given whole blood (median = 2.0 units; range = 1–4 units), and two patients were given cryoprecipitate (median = 3.5 units; one patient received 3 units, the other received 4 units). Two patients were given aPCC, which was administered before idarucizumab in both cases. Patients with intracerebral/subarachnoid hemorrhage (n = 12) did not receive any blood products or pro-hemostatic agents.
Table 1. Blood product and pro-hemostatic agent use in Group A (patients with uncontrolled bleeding).
In Group B, 23% (9/39) of patients received their first blood products or pro-hemostatic agents on the day of surgery or the day after surgery. During this time period, seven patients required transfusion of FFP (median = 2 units; range = 1–4 units) and five patients received PRBC (median = 3 units per patient; range = 1–9 units). Platelets were given to two patients; one patient received 1 unit and the other received 6 units. aPCC was given to two patients. No patients were treated with three-factor PCC, four-factor PCC, FVIIa, whole blood, or cryoprecipitate.
During the study, which included follow-up to 90 days, 33% (13/39) of patients in Group B received treatment with a blood product or pro-hemostatic agent at least once (). Use of blood products and pro-hemostatic agents in the late follow-up period is expected to be primarily attributable to late post-operative complications rather than the anticoagulant effect of dabigatran or emergency surgery/procedure; some of the patients treated during this period were receiving parenteral anticoagulants at the time when bleeding occurred. The most commonly used blood products in Group B were PRBCs and FFP, which were each given to nine patients. The median quantity of PRBCs used per patient was 4.0 units (range = 1–16 units); the median quantity of FFP was 3.0 units (range = 1–4 units). Platelets were given to two patients; one patient received 1 unit and one patient received 6 units. Treatment with aPCCs was reported in two cases (one before and one after idarucizumab infusion). None of the patients in Group B were given cryoprecipitate, whole blood, three- or four-factor PCC, or FVIIa.
Table 2. Blood product and pro-hemostatic agent use in Group B (patients undergoing emergency surgery/procedures).
Length of hospital stay and ICU admissions
Median hospital LOS for patients in groups A and B was 7 bed-days (range = 1–92 bed-days). In Group A, admission to hospital with overnight stay was reported for 82% (42/51) of patients, with a median LOS of 7.0 bed-days (range = 1–71 bed-days) (). LOS could not be evaluated for the remaining nine patients due to incomplete information. In patients with subdural hemorrhage, median LOS in hospital was 7.0 bed-days (n = 5; range = 6–71 bed-days); in patients with intracerebral and subarachnoid hemorrhage median LOS was 9.0 bed-days (n = 9; range = 4–47); in patients with “other bleeds”, median LOS was 6.5 bed-days (n = 10; range = 1–18 bed-days); in patients with GI bleeding median LOS was 6 bed-days (n = 18; range = 1–24). Of the 42 patients admitted to hospital in Group A, five died before discharge. Only one patient was re-admitted to hospital within 30 days, and this patient was from the “other bleeds” sub-group.
Table 3. Hospital admissions for patients in Group A (patients with uncontrolled bleeding).
In Group A, 17 patients had a documented ICU stay of at least 1 day, with a median LOS of 4 days (range = 1–44 days). Median LOS in the ICU was 3.0 days for patients with GI bleeding (n = 6; range = 1–11) and 3.0 days for patients with intracerebral and subarachnoid hemorrhage (n = 4; range = 1–5). Median LOS in the ICU was 4.0 days for patients with other bleeds (n = 5; range = 1–8) and 24.0 days for patients with subdural hemorrhage, however, this was only based on data from two patients (LOS was 4 days for one patient and 44 days for the other patient).
In Group B, admission to hospital with overnight stay was reported for 92% (36/39) of patients. The remaining three could not be evaluated due to incomplete information at the time of interim reporting. The median LOS was 9 bed-days (range = 1–92) (). For patients undergoing musculoskeletal trauma surgery/procedures, median LOS in hospital was 24.5 bed-days (n = 8; range = 4–92); in patients with infectious diseases requiring surgery/procedure, median LOS was 20.5 bed-days (n = 6; range = 1–61 bed-days); in patients undergoing cardiac and vascular surgery/procedure, median LOS was 12.5 bed-days (n = 2; one patient with 7 and the other with 18 bed-days of stay); for patients with major therapeutic abdominal surgery/procedure, median LOS was 9.0 bed-days (n = 10; range = 2–45); in “other surgeries/procedures”, median LOS was 3 bed-days (n = 7; range = 1–29 bed-days), and in patients requiring exploratory abdominal surgery/procedure, median LOS was 1 bed-day (n = 3; range = 1–17). Of the 36 patients admitted to hospital, seven died before discharge. Two patients were re-admitted to hospital within 30 days of discharge; one had undergone abdominal surgery and one had undergone surgery for an infection. In Group B, 15 patients had a reported stay in the ICU for at least 1 day with a median LOS of 2 days (range = 1–24 days). Median LOS in the ICU was longest in patients with infectious diseases that required surgery/procedures (4 days; n = 3; range = 3–24).
Table 4. Hospital admissions for patients in Group B (patients undergoing emergency surgery/procedures).
Discussion
The current analysis was undertaken to provide baseline data regarding HCRU in dabigatran-treated patients with uncontrolled or life-threatening bleeds (Group A), or in those requiring urgent surgery/procedures with high risk of bleeding (Group B), who receive idarucizumab. Rapid onset of action is an important characteristic of any anticoagulant reversal agent to prevent major bleeding or delays to urgent surgery. Although the median time to bleeding cessation in Group A was reported as 11.4 h, this end-point could not accurately be assessed in many patients due to difficulties in visualizing bleeds. For example, in ICH patients bleeding may have ceased prior to the recorded time of repeated imaging. In Group B, median time to surgery was 1.7 h, normal intra-operative hemostasis was reported in 92% of patients, and no patient was judged to have severely abnormal intra-operative hemostasis. The occurrence of uncontrolled intra-operative bleeding is associated with significantly higher healthcare resource use, hospital costs, and worse clinical outcomes compared with controlled bleedingCitation12. In patients who had to undergo emergency surgery in RE-LY®, the major perioperative bleeding rate was 17–23%Citation13,Citation14. By reducing procedural and perioperative bleeding, which remain common surgical complications, idarucizumab has the potential to improve outcomes and reduce the use of hospital resources. In RE-VERSE AD™, no major bleeding complication has so far been observed in the perioperative setting.
Blood products or pro-hemostatic agents were given to 63% (32/51) of patients in Group A and 23% (9/39) of patients in Group B; the use of these agents varied according to the underlying condition. The utilization rate of pro-hemostatic agents (FVIIa, PCCs, and aPCC) was low; no patient received FVIIa or three- or four-factor PCC, and four were administered aPCC. Reducing the use of blood products and pro-hemostatic agents may result in indirect cost savings associated with a reduction in downstream complications. The risks of thromboembolic complications associated with pro-hemostatic agents must be carefully considered. In a meta-analysis of patients receiving vitamin K antagonists, who were treated with three- or four-factor PCC for bleeding or before urgent surgery, the incidence of thromboembolic events was found to be 1.8% (95% CI = 1.0–3.0) with four-factor PCC and 0.7% (95% CI = 0.0–2.4) with three-factor PCC; PCCs were associated with a low but quantifiable riskCitation15.
In both Group A and Group B of RE-VERSE AD™ the median volume of transfused FFP was 2 units per patient. Although FFP transfusion is an essential aspect of managing major bleeding, its administration has been associated with a risk of transfusion-related morbidity, particularly transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload, resulting in increased duration of mechanical ventilation and ICU stay. Furthermore, TRALI is the most common cause of transfusion-related deathCitation16. It is worth examining the FFP usage in the RE-LY® study, which compared dabigatran with warfarin in patients with atrial fibrillation at risk of strokeCitation17. This RE-LY® analysis used a broader definition of major bleeding events than did RE-VERSE AD™, allowing assessment of less severe bleeding than in RE-VERSE AD™. Although the two studies are not comparable, it is interesting that the median quantity of FFP given to patients with major bleeding was 4 units (interquartile range = 2.0) in RE-LY®Citation17, but was only 2 units in RE-VERSE AD™. Reversal of dabigatran-mediated anticoagulation with idarucizumab requires administration of two 50 mL bolus infusions; these small volumes, relative to FFP, potentially reduce the burden of transfusion-related morbidity associated with delivering large volumes over a short period of time.
A major limitation of the current analysis is the lack of a control group, which was not possible for ethical reasons. As such, HCRU outcomes in patients who did or did not receive idarucizumab cannot be compared. HCRU findings from the current analysis cannot be compared with historical data due to a paucity of HCRU information in the populations studied and the heterogeneity in patient groups and conditions. However, this analysis provides the first data of its type, giving an indication of what can be expected in these patient groups in terms of resource utilization. These findings can be viewed as baseline data, which will enable local facilities to make their own comparisons based on their existing healthcare systems. For example, comparisons could be made by hospitals that do not currently stock idarucizumab, or payers could compare resource utilization for major bleeding events/surgical interventions using anticoagulants for which there are no currently available reversal agents. Furthermore, these data may be used as a reference going forward, allowing comparisons to be made with similar data for other reversal agents, once available. The current analysis is based on interim results from RE-VERSE AD™, once data from the full study population are available, a more detailed assessment of HCRU may be possible.
Another limitation of the study is the difficulty in assessing the time to cessation of bleeding; this was an investigator-determined end-point, which required visualization of the bleeding site. A further consideration is the potential influence of local healthcare regulation upon utilization of certain healthcare resources such as LOS in hospital and ICU and ICU admission rate.
Conclusions
The current analysis demonstrates significant but expected overall HCRU in the management of anticoagulated patients with uncontrolled or life-threatening bleeds, or in those requiring urgent surgery/procedures with high risk of bleeding. It also highlights that a number of resources (rather than cost alone) should be considered for patient management, which many clinicians may fail to recognize. While data are not sufficient to assess the role of idarucizumab with regard to HCRU, a baseline has now been established for further assessment and comparison. Idarucizumab rapidly reversed the anticoagulant effect of dabigatran and was associated with both a low use of factor concentrates and a low use of FFP. A reduction in pro-hemostatic agent use could positively impact HCRU and costs. In dabigatran-treated patients with life-threatening bleeds, idarucizumab may simplify emergency management; in those undergoing urgent interventions it may reduce time to surgery, complications associated with delayed surgery, and bleeding complications during surgery. However, the lack of a control group and the small sample sizes in this analysis limit interpretation of these data.
Transparency
Declaration of funding
This study was funded by Boehringer Ingelheim Pharma GmbH & Co. KG.
Declaration of financial/other interests
CVP Jr has received consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, and Janssen, and research support from AstraZeneca. RB has received travel support and fees for consulting, lectures, and serving on steering committees from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Medtronic. RD, FG, EK, PR, and AU are employees of Boehringer Ingelheim. MVH has received honoraria for presentations as well as research grants from Boehringer Ingelheim, Bayer HealthCare, Pfizer, GlaxoSmithKline, and Actelion. JHL has received consulting fees/honoraria from Boehringer Ingelheim, Janssen, The Medicines Company, and Instrumentation Labs, and has served on steering committees for CSL Behring and Grifols. FWS has received consulting fees from Boehringer Ingelheim and CSL Behring. TS has received speaker’s honoraria and consultancy fees from Boehringer Ingelheim, Bayer, Bristol-Myers Squibb/Pfizer, and Daiichi Sankyo. JIW has received consulting fees/honoraria from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Bayer, Johnson & Johnson, Daiichi Sankyo, Portola, Ionis Pharmaceuticals, and Merck. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Data have previously been presented at the American College of Cardiology Scientific Sessions 2016, Chicago, IL, USA.
Supplemental material
Download Zip (48.5 KB)Acknowledgements
Medical writing assistance, supported financially by Boehringer Ingelheim Pharma GmbH & Co. KG, was provided by PAREXEL during the preparation of this article.
References
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51
- Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363-72
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52
- Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764-72
- Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e44S-e88S
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate–a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116-27
- Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554-62
- Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet 2015;386:680-90
- Pollack CV, Jr., Reilly PA, Bernstein R, et al. Design and rationale for RE-VERSE AD: a phase 3 study of idarucizumab, a specific reversal agent for dabigatran. Thromb Haemost 2015;114:198-205
- Pollack CV, Jr., Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511-20
- Peetermans M, Pollack C, Jr., Reilly P, et al. Idarucizumab for dabigatran overdose. Clin Toxicol (Phila) 2016;54:644-6
- Corral M, Ferko N, Hollmann S, et al. Health and economic outcomes associated with uncontrolled surgical bleeding: a retrospective analysis of the Premier Perspectives Database. Clinicoecon Outcomes Res 2015;7:409-21
- Douketis JD, Healey JS, Brueckmann M, et al. Urgent surgery or procedures in patients taking dabigatran or warfarin: analysis of perioperative outcomes from the RE-LY trial. Thromb Res 2016;139:77-81
- Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012;126:343-8
- Dentali F, Marchesi C, Giorgi PM, et al. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta-analysis. Thromb Haemost 2011;106:429-38
- Marshall A, Levine M, Howell ML, et al. Dose-associated pulmonary complication rates after fresh frozen plasma administration for warfarin reversal. J Thromb Haemost 2016;14:324-30
- Majeed A, Hwang HG, Connolly SJ, et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation 2013;128:2325-32