Abstract
Background: A Phase-3 study of defibrotide compared with historical controls demonstrated a 23% improvement in 100-day survival post-hematopoietic stem cell transplantation (HSCT) among patients with veno-occlusive disease with multi-organ dysfunction (VOD with MOD).
Aim: To estimate the budget impact and cost-effectiveness of introducing defibrotide to a transplant center.
Methods: The authors developed a budget impact model from the perspective of a bone-marrow transplant center. It was estimated that 2.3% of adults and 4.2% of children would develop VOD with MOD following HSCT based on a retrospective hospital database analysis and the effect that treating patients with defibrotide would have on costs for adult and pediatric centers was estimated. A cost-utility analysis (CUA) was also developed to capture the long-term cost-effectiveness of defibrotide. Projected life expectancies in the two groups were estimated based on trial data, transplant registry data, studies of long-term survival among HSCT patients, and US population life-tables.
Results: There was an estimated 3% increase ($330,706) per year in total adult transplantation center costs associated with adopting defibrotide, and a <1% increase ($106,385) for pediatric transplant centers, assuming 100 transplants per year. In the CUA, the lifetime increase in cost per patient was $106,928, life expectancy increased by 3.74 years, and quality-adjusted life-years (QALYs) increased by 2.24. The incremental cost-effectiveness ratio (ICER) was $47,736 per QALY gained; 88% probability defibrotide was cost-effective at a $100,000/QALY threshold.
Conclusion: The budget impact of defibrotide for a transplant center is relatively modest compared to the overall cost of transplantation. Defibrotide provides an important survival advantage for VOD with MOD patients, and the life years gained lead to defibrotide being highly cost-effective.
Introduction
Hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome, with multi-organ dysfunction (MOD), is a significant complication of hematopoietic stem cell transplantation (HSCT) and is likely associated with substantial economic burden due to the direct medical costs of treatment and indirect costs associated with high mortality rates (>80% by 100 days post HSCT)Citation1–4. The incidence of VOD with MOD is related to the volume of HSCTs, which has steadily increased over the past 2 decades in the US; ∼11,000 autologous transplants and 8,000 allogeneic transplants in 2013Citation5. While incidence estimates for VOD are wide-ranging, a 2010 review reported 13.7% for VOD of any severityCitation2, and a recent study found an incidence of 8.8% among a cohort of HSCT patients who had received reduced-intensity conditioningCitation6. A retrospective analysis of a hospital database found a 5.4% incidence of VOD and a 2.5% incidence of VOD with MODCitation7.
Prior to approval of defibrotide, standard care for VOD was supportive care, comprised mainly of adequate fluid and sodium balance, and avoidance of hepato- and nephrotoxic drugsCitation8,Citation9. Diuretics can be used if weight gain progresses despite the basic measures above, and treatments such as analgesia, paracentesis, thoracentesis, and oxygen therapy are used to alleviate symptoms produced by ascites and pleural effusion.
Defibrotide (Defitelio®) is the first and only FDA-approved pharmacological agent for the treatment of VOD with renal or pulmonary dysfunction post-HSCT (subsequently also referred to as MOD throughout the paper) in the US. It significantly increases survival 100 days post-HSCT (Day +100) and Day +100 complete response rates (CR) in terms of improvements in total bilirubin and resolution of multi-organ failure measured by renal and/or pulmonary dysfunction, with a manageable safety profile in VOD with MOD patients compared to historical controls (HC)Citation10,Citation11. The phase 3 study included 102 patients treated with defibrotide and 32 historical controls (HC), and examined the efficacy of defibrotide 25 mg/kg/day in VOD patients with MOD compared to HC patients. Patients in the defibrotide group were treated for a median of 22 days (range = 1–60), and had 23% improvement in survival (p = 0.0109) at Day +100 compared to HC using a propensity adjusted analysis (observed survival rates: defibrotide = 38.2% vs HC = 25.0%)Citation12.
Despite the promising clinical benefit demonstrated by the clinical trial, the economic impact and value of defibrotide in this patient population has not been studied. The objective of this analysis was to estimate, from a transplant center perspective, the likely budget impact and long-term economic value of defibrotide for the indication of VOD with MOD. Below, we describe two evaluations: a budget impact model (BIM) and a cost-utility analysis (CUA) of defibrotide vs standard care in HSCT patients who develop VOD with MOD.
Methods
Budget Impact Model (BIM)
Approach and model structure
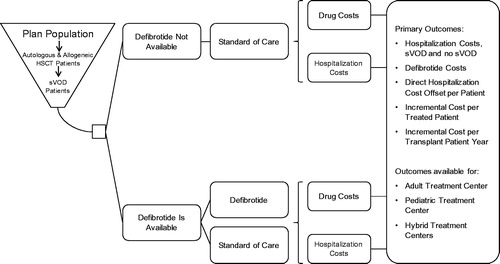
A budget impact model was undertaken to anticipate the economic impact of introducing defibrotide to the US market. The perspective was that of a bone marrow transplant center. The model estimates the net cumulative cost of treatment with defibrotide vs standard care for two scenarios: (1) an adult transplant center with an average of 100 HSCTs per year; and (2) a pediatric center with 100 transplants per year.
We estimated that 2.3% of adults and 4.2% of children would develop VOD with MOD post-HSCT, based on a retrospective analysis of the Premier Healthcare DatabaseCitation7. The analysis accounted for the cost of treating VOD with MOD with defibrotide, as well as associated hospitalization costs. An overview of the model structure is displayed in . The same model structure was used for adult and pediatric transplant center scenarios, and the results were based on costs for the items depicted in .
Assumptions and model inputs
Costs were estimated separately for adult and pediatric patients, and key costs were hospitalization cost (for patients with and without VOD with MOD) and defibrotide cost. An overview of base-case model assumptions is presented in .
Table 1. Base-case model assumptions and inputs for budget impact model.
Data specific to the Phase III trial for defibrotideCitation12 were used where available. The propensity-adjusted outcome analysis from the trial (i.e. 23% improvement in survival 100 days post-HSCT) was used for Day +100 survival. Day +100 survival without defibrotide treatment was assumed to be the same as in the historical control (HC) group (25%), and a 23% improvement was applied to obtain a Day +100 survival estimate of 48% in the defibrotide group. This survival estimate for the defibrotide group is supported by a single-arm study (Defitelio expanded access program) where the interim analysis (n = 642) showed a Day +100 survival estimate of 45%Citation13. It was assumed that the trial population was representative of VOD with MOD patients with regard to factors such as demographics, transplantation, and standard care.
Characteristics of the trial population that are relevant to the model are also listed in . A defibrotide dose per day of 6.25 mg/kg (1,848 mg for adults with a weight of 73.9 kg, and 650 mg for pediatrics with a weight of 26.0 kg) was used to estimate the number of vials per day (9.24 adults and 3.25 pediatrics). In the base-case analysis, it was assumed there was no vial sharing, so the vials per day were rounded up to 10 and 4, respectively.
Because there currently is no evidence to support differences in length of stay between those treated and not treated with defibrotide, we assumed no difference. However, the effect of defibrotide treatment on the intensity of care (inpatient costs) was estimated based on (1) findings from our analysis of a previously published observational study data setCitation3; and (2) differences in Day +100 mortality from the defibrotide trial (see )Citation12. The published observational study used the Premier Healthcare DatabaseCitation14 to estimate the cost of hospitalization for HSCT patients experiencing VOD with and without MODCitation3. We stratified the results by survival/mortality status, which indicated that patients who die of VOD with MOD have higher hospitalization costs than those who do not die (). The Premier cohort (n = 5,418) included patients with an inpatient hospitalization with a bone marrow transplant procedure as identified by ICD-9 codes (41.00, 41.01, 41.01, 41.03, 41.04, 41.05, 41.06, 41.07, 41.08, and 41.09) and CPT code (38241) and discharged between January 1, 2009 and May 31, 2014. VOD was defined as at least one ICD-9 code indicating liver injury (453.xx, 570.xx, 573.8, 573.9, 459.89, and 277.4) and at least two codes reflecting the clinical criteria used to diagnose VOD: hyperbilirubinemia (782.4), hepatomegaly (789.1), abnormal weight gain (783.1), or ascites (789.5)Citation3. Patients with graft vs host disease (GVHD) or total parental nutrition (TPN) cholestasis were excluded. To qualify as VOD with MOD (n = 134), patients had to have one or more ICD-9 diagnosis or procedure codes indicating MOD: acute/chronic respiratory failure (518.8x), other respiratory abnormalities (786.09), hypoxemia (799.02), acute respiratory failure (518.81), other dependence on machines (V46.2), other continuous invasive mechanical ventilation (96.7x), non-invasive mechanical ventilation (93.90, 93.91, 93.93, 93.99), acute renal failure (584.x), renal failure unspecified (586.x), renal failure (593.9), or dialysis (39.27, 39.42, 39.95, 54.98).
Table 2. Other model inputs for budget impact model.
The cost of defibrotide represents wholesale acquisition cost (WAC)Citation15. A list of other model inputs and their sources are presented in .
Sensitivity Analyses
Clinical parameter ranges were obtained from data-derived confidence intervals (CIs) or ±20%. Single-variable (one-way) sensitivity analyses were performed with the value of each input individually varied over ranges shown in ; one-way sensitivity analysis results are presented as tornado diagrams.
Table 3. Parameters varied in budget impact model one-way sensitivity analysis of incremental total costs.
Cost Utility Analysis (CUA)
Approach and model structure
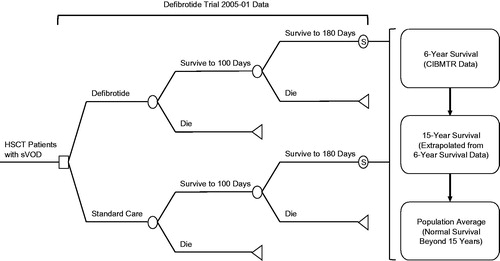
A cost utility analysis was conducted to capture the long-term cost-effectiveness of defibrotide for the treatment of VOD with MOD. A decision model was developed for defibrotide vs standard care using many of the assumptions and inputs from the BIM. Projected life expectancy in the two groups were estimated based on trial data, Center for International Blood and Marrow Transplant Research (CIBMTR) registry data, studies of long-term survival in HSCT patient, and US population life-tables. The patient population was assumed to be similar to the defibrotide trial population. The model estimates the incremental total direct costs, increase in life expectancy, incremental quality-adjusted life years (QALYs), and incremental cost-effectiveness ratio of treatment with defibrotide vs standard care. An overview of the model structure is displayed in and includes the following general approach: a third-party payer perspective, 30-year time horizon, and costs and outcomes discounted at 3% per yearCitation16.
Assumptions and model inputs
Assumptions and inputs for the CUA that are the same as the BIM include the following (see and ): difference in Day +100 survival, defibrotide dose per day, defibrotide cost, mean hospitalization cost among VOD with MOD patients who were alive at Day +100, and mean hospitalization cost among VOD with MOD patients who died by Day +100. Other inputs required for the CUA and from the trial are listed in . Patient characteristics were assumed to be similar to the trial population.
Table 4. Additional inputs for the cost utility analysis from the trial.
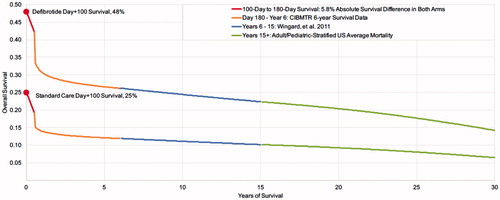
To account for the likelihood that VOD with MOD Day +100 survivors had higher mortality than average HSCT patients, we applied the additional mortality observed in the trial from Day 101 to Day 180 for the defibrotide group (5.8%) to both arms. Survival beyond Day +180 was assumed to be the same in the two groups and estimated using CIBMTR registry data (1999–2012)Citation5, and US Life TablesCitation17. Mean survival estimates from CIBMTR by age (≤16 and >16) and type of transplant (allogeneic and autologous) were applied to the overall trial population characteristics, resulting in an estimated mean survival of 44.8 months, and ∼62% alive at 6 years. Applied to the 42% of defibrotide patients alive at 180 days and the 19% standard care patients alive at 180 days, 6-year survival was 26% in the defibrotide group and 12% in standard care group. Survival from year 7 to year 15 was estimated based on long-term survival outcomes for HSCT patients in whom 84% of patients alive at 5 years were alive at 15 yearsCitation18. Survival beyond 15 years was assumed to be similar to the US population averageCitation17. depicts the estimated survival from the defibrotide pivotal trial and these additional three sources.
Figure 3. Estimated mean long-term survival of patients who survive to 180 days (based on Phase III trial data)Citation11 undergoing HSCT and experiencing VOD with MOD.
Quality-of-life among patients who survived VOD with MOD (and HSCT) was assumed to be 0.85 for both groups. Because of the uncertainty in this estimate, it was varied from 0.69–0.95 in sensitivity analyses.
Outcomes
The model calculated 30-year direct healthcare costs, life-years (LYs), quality-adjusted life years (QALYs), and incremental differences in these outcomes between the defibrotide and no defibrotide arms. The incremental cost-effectiveness ratio (ICER) was calculated as the difference in costs divided by the difference in QALYs.
Sensitivity analyses
We performed sensitivity analyses to examine the influence of uncertainties in the model inputs and to judge the robustness of the findingsCitation19. Clinical parameter ranges were obtained from reported literature (when available), data-derived CIs, or ±20%. Single-variable (one-way) sensitivity analyses were performed with the value of each input individually varied over ranges shown in ; one-way sensitivity analysis results are presented as tornado diagrams. We also performed probabilistic sensitivity analysis (PSA)Citation20, wherein all model parameters were jointly varied over 3000 simulations using pre-specified statistical distributions, enabling the calculation of 95% credible ranges (CR) for model outcomesCitation21,Citation22. Results of the PSA were plotted on an incremental cost-effectiveness planeCitation23,Citation24. In addition to standard sensitivity analyses, we estimated the ICER with the unadjusted Day +100 survival in the defibrotide arm (38.2% vs 48%).
Table 5. Range of model inputs for cost utility sensitivity analyses.
Results
Budget Impact Model (BIM)
Base-case analysis
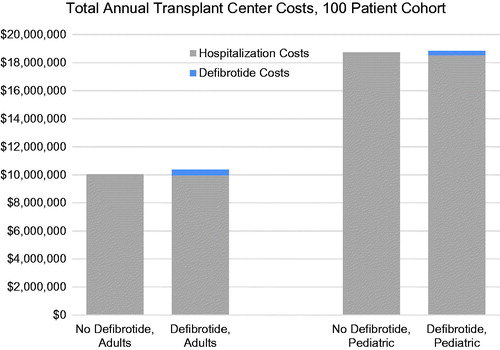
Results of the base-case analysis show separate costs for a cohort of 100 transplanted adults or 100 transplanted pediatric patients by VOD with MOD treatment status (defibrotide vs standard care) ( and ). The additional cost of adopting defibrotide was ∼$330,706 per year for adult transplant centers and $106,385 for pediatric transplant centers, which represents a 3% and <1% increase over the total annual transplantation costs in those centers, respectively. The additional cost of treating all expected cases of VOD with MOD per HSCT patient at the transplant center was $3,307 and $1,064 for adult and pediatric patients, respectively.
Table 6. Results of budget impact model for 100 patients per year: adult transplant center.
Table 7. Results of budget impact model for 100 patients per year: pediatric transplant center.
When only hospital costs for VOD with MOD were considered for the two groups (i.e. drug costs not included), the costs for the defibrotide-treated patients was lower on average than the standard care group, with a cost offset of $29,465 per treated patient and $43,970 per treated patient ( and ). The total incremental cost per defibrotide patient is also presented below, calculated as the cost of defibrotide minus the offset in hospitalization costs. Graphical depictions of the total costs are displayed in for adults and pediatrics.
Sensitivity analyses
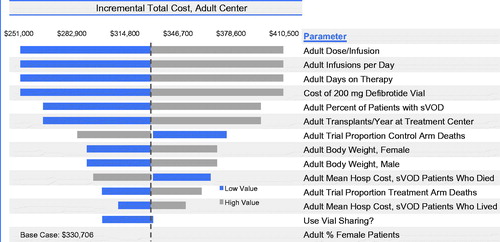
Results of sensitivity analyses indicate that, for adults, the incremental cost of introducing defibrotide is influenced most by defibrotide costs, dose per infusion, number of infusions per day, and days on therapy (). Vial sharing reduced the incremental cost. Varying the cost of hospitalization among adult VOD with MOD patients who both were and were not alive at Day +100 had a small effect on the results. As expected, incremental costs varied according to the number of transplants per year and the proportion of transplant patients who experienced VOD with MOD. Overall, the cost difference was robust to varying model assumptions, with the range of total annual incremental costs to an adult transplant center associated with the introduction of defibrotide between $251,011 and $410,401. In similar sensitivity analyses, the incremental cost per HSCT patient varied from $2,510–$4,104.
Figure 5. Budget impact model one-way sensitivity analyses for total annual incremental costs for an adult transplant center with the introduction of defibrotide for VOD with MOD. The widths of the horizontal bars represent the change in results when each parameter was varied over the ranges specified in .
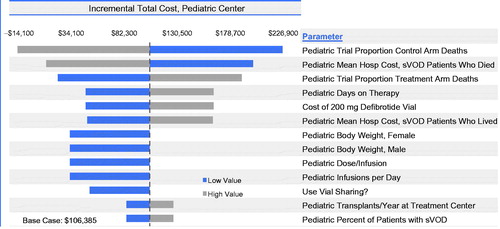
In contrast to adults, incremental costs for the pediatric population were influenced by Day +100 mortality rates and cost of hospitalization among those who died (). Vial sharing influenced results, as did the cost of defibrotide (although to a lesser extent than in adults because of the lower weight-based dosing resulting in lower drug costs in pediatric patients), the number of days on therapy, dose (as determined by weight), and number of infusions per day. The total annual costs to a pediatric transplant center associated with the introduction of defibrotide varied from a saving of $14,055 to an incremental increase of $226,825. The incremental cost per HSCT patient varied from −$141 to $2,268 when inputs were varied, as specified in .
Figure 6. Budget impact model one-way sensitivity analyses for total annual incremental costs for a pediatric transplant center with the introduction of defibrotide for VOD with MOD. The widths of the horizontal bars represent the change in results when each parameter was varied over the ranges specified in .
Cost Utility Analysis (CUA)
Base case analysis
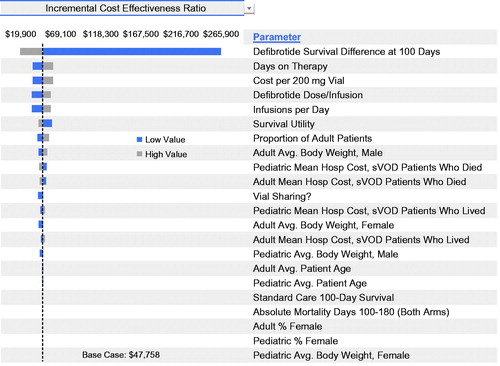
The costs, LYs, QALYs, and lifetime cost-effectiveness of defibrotide vs standard care in VOD with MOD patients is summarized in . The total increase in cost per patient with VOD with MOD treated was $106,929, the increase in life expectancy was 3.74 years, and the increase in QALYs was 2.24. The incremental cost-effectiveness ratio (ICER) was $47,758 per QALY gained.
Table 8. Cost utility analysis results: incremental cost effectiveness of defibrotide versus standard care in the treatment of VOD with MOD among patients undergoing HSCT.
One-way sensitivity analysis
Defibrotide remained generally cost-effective when parameters were varied, as specified in . The ICER ranged from $19,966–$265,822 when model inputs were individually varied (). Results were most sensitive to the survival difference between defibrotide and standard care at Day +100. Other inputs such as the mean hospitalization costs for adult VOD with MOD patients who died by Day +100, defibrotide cost, days on therapy, number of doses infused per day, survival utility, and Day +100 survival in the standard care arm had a relatively smaller effect.
Probabilistic Sensitivity Analyses (PSA)
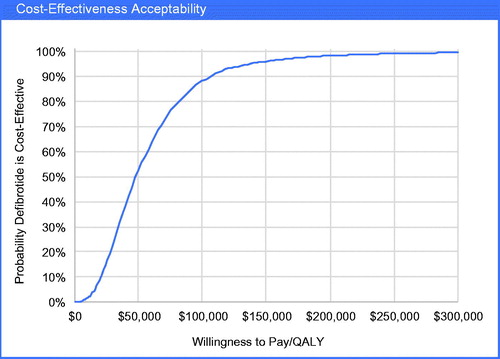
Results of the probabilistic sensitivity analysis showed that defibrotide treatment increased both life-years (95% credible range (CR) = 1.30–7.01) and QALYs (95% CR = 0.77–4.29) per patient (). The 95% CR for incremental cost per patient spanned from a cost savings of $40K to an additional cost of $176K for defibrotide-treated patients (95% CR = −$40,551–$176,776). The ICER ranged from $12,374–$178,523 per QALY gained. The cost-effectiveness acceptability curve () indicated the probability that defibrotide was cost-effective at a $100,000 per QALY threshold was 88%.
Sensitivity analysis with unadjusted Day +100 survival
Results of this sensitivity analysis yielded a doubling of the ICER ($95,054; 95% CR = $12,060–$165,918), but still just under the $100,000 threshold. The total increase in cost per patient with VOD with MOD treated was $122,144, the increase in life expectancy was 2.14 years, and the increase in QALYs was 1.28.
Discussion
This is the first study to report on the budget impact and cost-utility of defibrotide in the treatment of VOD with MOD. The following limitations of the models should be considered when interpreting results. First, the generalizability of the trial population and results to all transplant patients should be considered. The most common underlying disease in both treatment groups was AML (defibrotide: 28.4%; HC: 25.0%). Other common (>5%) underlying diseases in the defibrotide group included acute lymphoid leukemia (defibrotide: 16.7%; HC: 21.9%), myelodysplastic syndrome (defibrotide: 6.9%; HC: 9.4%), and neuroblastoma (defibrotide: 5.9; HC: 0). The pediatric population was less represented than adults in both arms of the trial, and, therefore, the 100 day and 100–180 day mortality rates from the trial may be a slight under-estimate for the pediatric population. The proportion of adult vs pediatrics was accounted for when modeling long-term survival. Although there were some differences between the defibrotide and control groups, the adjusted analysis of Day +100 mortality utilized a standard approach, propensity score stratification, to control for differences. The Day +100 mortality rate in the pivotal trial HC group (75%) was lower but somewhat similar to the Coppell meta-analysis of 19 studies reporting mortality rates in VOD with MOD patients (84.3%; 95% CI = 79.6%–88.9%)Citation2. Day +100 mortality for defibrotide could also differ by factors not evaluated in the models such as delay of defibrotide use, type of transplant (allogeneic vs autologous), type of donor (sibling, relative, unrelated), prior transplants, remission status, and extent of organ failure with VOD. The relatively small sample size of the HC group led to some uncertainty in the mortality difference, although it was statistically significant, and this range was explored in the sensitivity analysis.
Second, cost data were generated on a broad group of patients based on an algorithm to identify likely HSCT patients and cases of VOD. HSCT, VOD, and VOD with MOD were identified in the Premier Healthcare Database using ICD-9 diagnosis and procedural codesCitation3. Such a method is subject to potential misclassification secondary to the lack of specific ICD-9 code for VOD. Medical chart review to validate the algorithm was not possible. While Premier data contains 535 million patient encounters, only 134 cases of VOD with MOD were identified during the study period. Patients identified as having VOD with MOD differed from the trial population with respect to measured characteristics such as age (mean of 49 years) and type of transplantation (48.5% allogeneic). Inpatient mortality for VOD with MOD patients in the Premier data was only ∼40%, so could represent a less severe disease population and, thus, more conservative cost estimates. Hospital costs for HSCT and VOD with MOD were overall, and did not consider factors that may influence the length and type of hospital stay (e.g. ICU) such as type of transplant, prior transplants, remission status, and extent of organ failure. Long-term survival was projected based on estimates from several data sources. While there is uncertainty in these estimates, the model was conservative in assuming that there was additional mortality (for both groups) from Days 101–180, and the survival benefit in the defibrotide group was only realized in the first 100 days. These data represent best available evidence, and estimates were varied in sensitivity analyses. Results are presented as 100 patients per year, but transplant programs vary, and pediatric programs are likely smaller.
Limitations withstanding, this study provides important information from the perspective of transplant centers and providers practicing within such settings. In March 2016, the US Food and Drug Administration approved Defitelio® (defibrotide) to treat adult and pediatric patients who develop VOD with additional kidney or lung abnormalities after they receive HSCT. This is the first FDA-approved therapy for treatment of severe hepatic VOD. While the disease is rare, the uptake of defibrotide expected post-approval will provide additional opportunities to study the health economic and clinical implications of defibrotide use.
Conclusion
In conclusion, the economic analyses indicate that the budget impact of defibrotide for a transplant center is relatively modest compared to the overall cost of transplantation, and there are important hospital cost offsets ($43,970 pediatrics; $29,465 adults), particularly for pediatric patients. Furthermore, defibrotide provides an important survival advantage for VOD with MOD patients, and the quality adjusted life years gained lead to defibrotide being highly cost-effective. The ICER was well below the commonly cited threshold for cost-effectiveness in the US of $100,000 per QALY.
Transparency
Declaration of funding
This analysis was funded by Jazz Pharmaceuticals, Inc.
Declaration of financial/other interests
DMB, GG, and DLV served as consultants to Jazz Pharmaceuticals, Inc. KFV is a full-time employee of Jazz Pharmaceuticals, Inc., who in the course of this employment has received stock options exercisable for, and other stock awards of ordinary shares of Jazz Pharmaceuticals, Inc. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Poster at the Academy of Managed Care Pharmacy, San Francisco, CA, April 2016.
References
- Carreras E, Diaz-Beya M, Rosinol L, et al. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl 2011;17:1713-20
- Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl 2010;16:157-68
- Dvorak CC, Nejadnik B, Cao Z, et al. Hospital cost associated with veno-occlusive disease (VOD) in patients with hematopoietic stem cell transplant (HSCT). Biol Blood Marrow Transpl 2016;22:S275
- Quock T, Zhou Z, Dai B. et al. A model estimating indirect costs of premature death associated with severe hepatic veno-occlusive disease (sVOD) among hematopoietic stem cell tranplant (HSCT) patients in the United States (US). Blood 2015;126:3273
- Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. Center for International Blood & Marrow Transplant Research Conference; 2014; http://www.cibmtr.org
- Tsirigotis PD, Resnick IB, Avni B, et al. Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transpl 2014;49:1389-92
- Dvorak C, Nejadnik B, Cao Z, et al. Incidence of hepatic veno-occlusive disease (VOD) in premier healthcare database. Blood 2015;126:3280
- Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol 2015;168:481-91
- Richardson PG, Ho VT, Cutler C, et al. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: novel insights to pathogenesis, current status of treatment, and future directions. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl 2013;19:S88-S90
- Richardson PG, Ho VT, Cutler C, Glotzbecker B, Antin JH, Soiffer R. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: novel insights to pathogenesis, current status of treatment, and future directions. Biol Blood Marrow Transplant 2013;19:S88-90
- Richardson PG, Soiffer RJ, Antin JH, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl 2010;16:1005-17
- Richardson PG, Riches ML, Kernan NA, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood 2016;127:1656-65
- Richardson P, Smith A, Triplett B, et al. Pediatric and adult subgroup results from an ongoing defibrotide expanded access program in the US for patients with hepatic veno-occlusive disease. 20th Congress of Asia-Pacific Blood and Marrow Transplantation Group (APBMT); 2015; Okinawa, Japan
- Premier Research Services™ creates insights through data. Premierinc.com: Premier Inc.; 2016. https://www.premierinc.com/transforming-healthcare/healthcare-performance-improvement/premier-research-services/. Accessed November 30, 2016
- AnalySource. Analy$ource – Suite of Drug Pricing Services; 2016. https://www.analysource.com/. Accessed November 30, 2015
- Manning W, Fryback DG, Weinstein M. Reflecting uncertainty in cost-effectiveness analysis. In: Gold MR, Russell LB, Weinstein M, editors. Cost effectiveness in health and medicine. New York: Oxford University Press; 1996. p. 247-75
- Arias E. United States Life Tables, 2009; Natl Vital Stat Rep. 2014;62:1-63
- Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 2011;29:2230-9
- Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making 2012;32:722-32
- Critchfield GC, Willard KE, Connelly DP. Probabilistic sensitivity analysis methods for general decision models. Comput Biomed Res 1986;19:254-65
- Briggs AH, Gray AM. Power and sample size calculations for stochastic cost-effectiveness analysis. Med Decis Making 1998;18:S81-S92
- Doubilet P, Begg CB, Weinstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making 1985;5:157-77
- Briggs A, Fenn P. Confidence intervals or surfaces? Uncertainty on the cost-effectiveness plane. Health Econ 1998;7:723-40
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401
- Richardson P, Tomblyn M, Kernan N, et al. Defibrotide (DF) in the treatment of severe hepatic veno-occlusive disease (VOD) with multi-organ failure (MOF) following stem cell transplantation (SCT): results of a phase 3 study utilizing a historical control. Blood 2009;114:654
- Keating GM. Defibrotide: a review of its use in severe hepatic veno-occlusive disease following haematopoietic stem cell transplantation. Clin Drug Investig 2014;34:895-904