Abstract
Objective: Two disease-modifying therapies are licensed in the EU for use in rapidly-evolving severe (RES) relapsing-remitting multiple sclerosis (RRMS), fingolimod and natalizumab. Here a discrete event simulation (DES) model to analyze the cost-effectiveness of natalizumab and fingolimod in the RES population, from the perspective of the National Health Service (NHS) in the UK, is reported.
Methods: A DES model was developed to track individual RES patients, based on Expanded Disability Status Scale scores. Individual patient characteristics were taken from the RES sub-groups of the pivotal trials for fingolimod. Utility data were in line with previous models. Published costs were inflated to NHS cost year 2015. Owing to the confidential patient access scheme (PAS) discount applied to fingolimod in the UK, a range of discount levels were applied to the fingolimod list price, to capture the likelihood of natalizumab being cost-effective in a real-world setting.
Results: At the lower National Institute of Health and Care Excellence (NICE) threshold of £20,000/quality-adjusted life year (QALY), fingolimod only required a discount greater than 0.8% of list price to be cost-effective. At the upper threshold of £30,000/QALY employed by the NICE, fingolimod was cost-effective if the confidential discount is greater than 2.5%. Sensitivity analyses conducted using fingolimod list-price showed the model to be most sensitive to changes in the cost of each drug, particularly fingolimod.
Conclusions: The DES model shows that only a modest discount to the UK fingolimod list-price is required to make fingolimod a more cost-effective option than natalizumab in RES RRMS.
Introduction
Rapidly-evolving severe (RES) relapsing-remitting multiple sclerosis (RRMS) is a severe sub-type of multiple sclerosis (MS)Citation1,Citation2, which is a chronic, progressive, autoimmune disease affecting the central nervous system. MS is estimated to affect 100,000 people in the UKCitation3, and is the leading cause of serious physical disability in the working-age populationCitation4. Symptoms include visual and sensory disturbances, loss of balance, muscle weakness, fatigue, bladder and bowel problems, and often begin to manifest in patients’ late 20sCitation5.
As in the majority of patients diagnosed with MS, RES RRMS initially follows an unpredictable relapsing-remitting pattern, with alternating periods of stability (remission) and worsening (relapses), resulting in an overall decline in physical condition with timeCitation6. In patients affected by RES RRMS, disease progression is particularly rapidCitation7: RES RRMS is defined by the presence of two or more disabling relapses in 1 year, with evidence of increasing lesions on magnetic resonance imaging (MRI) scans (one or more gadolinium-enhancing lesions or a significant increase in T2 lesion load as compared to a previous recent MRI)Citation1. This RRMS phase is usually followed by a secondary progressive phase (SPMS), during which physical function continues to decline, without remissions.
As RRMS progresses, problems with mobility, hand function and fatigue, as well as cognitive impairment, may all affect a patient’s ability to work, and unemployment rates amongst patients are high. Given that the disease predominantly strikes the working age population, RES RRMS exerts a substantial economic burden, on patients, carers, and the health serviceCitation8,Citation9. Moreover, treatment options for the RES sub-group are limited. Two DMTs are licensed in the European Union (EU) specifically for RES: natalizumabCitation2 and fingolimodCitation1. However, only natalizumab is approved for use in RES patients in England and Wales by the National Institute of Health and Care Excellence (NICE)Citation10, as fingolimod has not been appraised by NICE in RES patients to date (both natalizumab and fingolimod are recommended for use in RES disease in Scotland by the Scottish Medicines ConsortiumCitation11,Citation12 [SMC]).
Natalizumab is an intravenously administered treatment that received European Medicines Agency (EMA) approval in 2006 following submission of data from two 2-year Phase III studies (AFFIRM & SENTINEL); AFFIRM was a placebo-controlled trialCitation13, while SENTINEL studied the addition of natalizumab to beta-interferon (such combination therapy was not licensed)Citation14. Due to the risk-benefit profile of natalizumab, it was limited to the RES sub-group of RRMS, as well as the sub-group of highly active (HA) disease despite previous DMT treatmentCitation2.
Fingolimod was the first oral DMT approved in the EU (in 2011), following submission of data from two Phase III studies: one 2-year placebo-controlled (FREEDOMSCitation15) study and one 1-year beta-interferon-controlled (TRANSFORMSCitation16) study. Like natalizumab, fingolimod was only approved for use in patients with RES RRMS or with HA disease despite previous DMT treatmentCitation1. Subsequent to EMA approval, a third Phase III study, the 2-year placebo-controlled FREEDOMS II studyCitation17, was completed and published.
Alemtuzumab is a licensed therapy that has recently been approved by NICE for use in all RRMS patients, including those with RES RRMSCitation18. Alemtuzumab is an intravenous infusion taken over 5 days in the first year and over 3 days in the second year; it modulates the immune system and is associated with a strict monitoring schedule due to the risk of severe and potentially fatal adverse events. The available data do not easily allow for indirect comparisons of fingolimod, natalizumab, and alemtuzumab in the RES sub-group to inform an economic model, as it is not possible to form a network of published evidence to allow the conduct of a network meta-analysis, as the two alemtuzumab trials are Rebif-controlledCitation19,Citation20. For many RES patients, the choice remains between natalizumab and fingolimod, the analysis considered in this paper; however, work is ongoing to develop an economic model comparison to alemtuzumab, which will be published separately.
Historically, the cost-effectiveness of MS treatments has been compared using cohort Markov modelsCitation21. However, considering the long-term consequences of certain adverse events (AEs) associated with some of the more recently licensed disease modifying therapies (DMTs) for RRMS raises the issue that they cannot easily be modeled with the Markovian assumption of memoryless homogenous cohorts, as has been noted elsewhereCitation22. An alternative methodology in this situation is to use discrete event simulation (DES) modeling, which tracks individual simulated patients, their attributes, and the events that occur to them over timeCitation23. This facilitates the development of more complex model structures, with improved flexibility to model the impact of switching between therapies and a greater ability to more realistically model long-term outcomes and AEs, thereby providing greater face validity in the outputs producedCitation24.
Markov modeling has shown fingolimod to be cost-effective vs other oral DMTs in the treatment of HA RRMSCitation21, and fingolimod is the only oral DMT recommended by NICE for use in HA RRMSCitation25–27. However, the comparison of fingolimod and natalizumab in the RES population in the UK has not previously been published. Here, a new DES model to compare the cost-effectiveness of natalizumab and fingolimod in the treatment of RES RRMS in the UK is reported.
Methods
Patient populations and included studies
Individual patient baseline characteristics were taken from the pivotal phase III trials of fingolimod (TRANSFORMSCitation16, FREEDOMSCitation15, and FREEDOMS IICitation17) specifically for the sub-group of patients experiencing two or more disabling relapses in 1 year with one or more gadolinium-enhancing lesions or a significant increase in T2 lesion load compared to a previous recent MRICitation1,Citation2. The disease level characteristics of the initial population are summarized at a cohort level in Supplementary Table 1.
Table 1. Clinical parameters for natalizumab and fingolimod in RES RRMS
Structure of the model
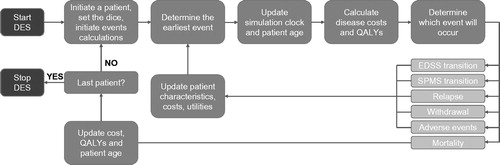
A DES model was developed using Microsoft Excel and C++ to determine the cost-utility of natalizumab vs fingolimod in the treatment of RES RRMS, from the perspective of the National Health Service (NHS) and Personal Social Services (PSS; as specified in the NICE reference case)Citation28. In the model, disease status follows the abbreviation of the Expanded Disability Status Scale (EDSS) used in previous Markov modelsCitation21.
At the beginning of the simulation, four attributes are fixed for each simulated patient: initial age, sex, initial EDSS, and the time since diagnosis of MS. Throughout the simulation, the following attributes of each simulated patient vary: current age, the time since treatment initiation on the current DMT, utility, current MS type (RRMS or SPMS), current EDSS, and logical variables identifying current relapses, washout periods, and modeled AEs.
For each therapy arm of the simulation, a set of result variables are tracked. Separate cost variables track the administration cost, disease cost, relapse cost, treatment cost, and the cost of the adverse events; a utility variable tracks the cumulative quality-adjusted life years (QALYs) experienced by the patients in that arm. No constraints on resource availability were applied in the DES.
The model simulates the events experienced based on patient-specific attributes, and calculates the associated costs and utilities for each individual patient in the cohort. Patients initiate therapy with either fingolimod (intervention therapy) or natalizumab (comparator). The specific baseline characteristics of the individual are then used to calculate the probability of each possible event, and the time to each event randomly selected from a distribution for each patient. The possible events that can occur are: a change in attributes equivalent to higher EDSS within RRMS, progression from RRMS to SPMS, a change in attributes equivalent to higher EDSS within SPMS, relapse, withdrawal, adverse event, or death.
For each simulated patient, the earliest event is determined, and this is the one that is considered to occur. Once this event has been selected, the current time is saved, and the simulation clock is updated to the time of occurrence of the event. The previous time is needed for calculations of the QALYs and continuous costs accumulated in the evaluated period. Updated patient characteristics, costs, and utilities are calculated and the process repeated until the patient either dies or discontinues (). Patients discontinue fingolimod/natalizumab treatment based on the drug withdrawal probability from the respective phase 3 trials. Following withdrawal, patients are transferred to best supportive care (BSC) until death, with mortality adjusted to incorporate age and gender weightings as well as a further risk of mortality associated with having MS. Additionally, patients who reach the EDSS threshold for discontinuing DMT (EDSS 6.5, based on Associate of British Neurologists [ABN] recommendations)Citation29, or have transitioned to SPMS, are assumed to receive BSC. Note that patients may experience changes in their attributes which relate to higher EDSS, without passing through all intermediate levels. This process is repeated for every simulated patient.
Clinical inputs
The risk of a patient’s EDSS level progressing, or of the patient transitioning from RRMS to SPMS, is calculated according to disease natural history data, modified to incorporate the published clinical efficacy of natalizumab or fingolimod. For EDSS levels 0–7, the natural history of the disease is modeled based on the RES sub-group of patients in the placebo arm of the pooled FREEDOMSCitation15 and FREEDOMS IICitation17 studies. Unfortunately, insufficient data are available in these studies to calculate risks at higher EDSS levels, and so the risk for EDSS score 8 is based on the London Ontario datasetCitation30. The natural history risks for changing from RRMS to SPMS and for progression within SPMS are also taken from this sourceCitation30. These natural history risks of disease progression are then adjusted to incorporate the relative risks for treatment vs placebo, taken from the phase III trials of the two DMTs. Note that, whilst the DES model is able to incorporate changes to lower EDSS levels, the inputs used here, based on disease natural history data, do not allow for disease improvementsCitation30.
For both natalizumab and fingolimod, clinical efficacy parameters are derived from sub-group analyses of RES RRMS patients (). There are differences in the published data available for each of the two DMTs considered in this report; for natalizumab data is only available for treatment-naïve patientsCitation31, whereas for fingolimod the data covers the whole RES sub-group.
It may be noted that, due to the limitations of the data collected in the trials, the definitions used to define the RES sub-group in both the fingolimod and the natalizumab analyses was a proxy for the RES population: two or more relapses in the year prior to baseline and one or more Gd-enhancing lesion at baseline. This proxy definition differs slightly from the licensed indication, but was used previously as a proxy definition of RES used in the fingolimod European Public Assessment Report (EPAR) published by the EMACitation32, and has been accepted in previous NICE appraisals for RESCitation10.
Additionally, disability progression was defined differently in the two studies. To overcome this and avoid bias in the comparison, the AFFIRM definitionCitation31 of disability was applied to the patient level data from the FREEDOMS trials. Clinical efficacy was then further adjusted to account for assumed waning effects in the drug’s efficacy caused by prolonged exposure. Based on the preferred assumptions used by NICE in the most recent published appraisal of a DMT in RRMS (that for dimethyl fumarate)Citation27, the efficacy of both treatments is assumed to wane to 75% at 2 years and 50% at 5 years in the base case.
Adverse events were incorporated into the model based on their incidence in the clinical trials informing the efficacy parameters; the choice of AEs modeled for each DMT was based on their respective manufacturers’ NICE submissions, with the addition to each of progressive multifocal leukoencephalopathy (PML), a serious but rare AE for which more recent data were available for both DMTs considered here. The incidence rate of each AE could vary over time in the model to a maximum of four periods specified. Similarly, the disutility and cost of adverse events could vary over time after the occurrence of the event where necessary.
Cost inputs
Costs include the costs for each EDSS level, drug acquisition costs, drug administration and monitoring costs, and costs associated with AEs and with relapses, all from an NHS and PSS perspective (, and Supplementary and ). Administration, monitoring, and adverse event costs were based on NHS Reference Costs (Supplementary )Citation34. Costs of relapse were obtained from the 2014–15 NHS National Tariff (owing to a lack of available data, relapse rate was assumed to be constant across different relapse severities)Citation35. EDSS level costs were based on the natalizumab submission to NICECitation36 and inflated to 2015 costs. For natalizumab, drug acquisition costs were based on list price, since no national Patient Access Scheme (PAS) discount is recorded on the NICE websiteCitation37; for fingolimod, where a confidential national PAS is stated to be in placeCitation37, a range of possible PAS discounts were applied, as summarised in . The incidences for AEs are provided with the AE disutilities in Supplementary Table 5.
Table 2. Annual drug acquisition costs for natalizumab and fingolimod.
Table 3. Cost-effectiveness of fingolimod vs natalizumab in RES RRMS.
Utility inputs
Utility weightings by EDSS score were based on the UK population and collected using the EuroQoL 5-Dimension (EQ-5D) SurveyCitation42, as recommended by NICE and previously employed in other MS modelsCitation21,Citation36,Citation39,Citation43,Citation44. The model applies a utility gain of 0.01 for every 5-year period with MS (0.002 per year), on the basis that, as patients come to terms with their condition, utility improvesCitation42. Males experience a further utility gain of 0.017 every year compared with femalesCitation42. An average disutility value of 0.071 was applied to all relapses, irrespective of severityCitation42. This is a simplifying assumption driven by data availability. The disutilities applied to specific AEs associated with natalizumab and fingolimod were obtained from the respective NICE submissions. When AE disutility values were not available, assumptions were made (see Supplementary Table 5 for details). A full summary of modeled utilities is given in Supplementary Tables 4 and 5.
Model outcomes
This analysis measures benefit in the form of QALYs. QALYs and costs are calculated over a lifetime horizon (maximum permissible modeled age was 100) and both costs and benefits are discounted at an annual discount rate of 3.5%, in accordance with the NICE reference caseCitation28. The cost-effectiveness is reported in terms of net monetary benefit (NMB), whereby a QALY is valued at a particular level in the calculations to obtain a single numerical result. NMB was chosen rather than the incremental cost-effectiveness ratio (ICER) to avoid the difficulties of displaying ICER values that lie in different quadrants of the cost-effectiveness plane and, therefore, have different interpretations. NMB was calculated using the £20,000 and £30,000 per QALY willingness-to-pay thresholds, as per the upper and lower threshold employed by NICE in the UK. A positive NMB can be interpreted as meaning that the intervention represents a cost-effective use of resources, at that willingness-to-pay threshold.
Sensitivity analyses
A deterministic sensitivity analysis was carried out to investigate the impact individual variables have on the model results. Upper and lower confidence limits were used as model inputs for each of the variables detailed above. Where confidence limits were not available, an assumed change of ±20% was used. The analysis was conducted under the scenario using the fingolimod list-price, as with no PAS discount applied clinical parameters will have the most influence over the model.
Additionally, probabilistic sensitivity analysis (PSA) was performed by running 1000 iterations, in which each input parameter was drawn randomly from its probability distribution (see Supplementary Table 6 for details). Scenario analyses were carried out to investigate whether the base case result remained stable under various conditions, including different waning assumptions (applied to both drugs) and treatment withdrawal probabilities.
Results
Base case and probabilistic sensitivity analysis
DES models are inherently stochastic, with the model results varying according to the seed value for the random number generator set in the inputs. To determine that the cohort iteration setting was sufficiently large to produce computationally stable outputs, the model base case was run for seeds values of 1–10, using a cohort iteration setting of 2000, and the variation in the results was examined, as presented in Supplementary Table 7. It was concluded that the variation in the net monetary benefit results using a cohort iteration setting of 2,000 was acceptable at below 1% on average; therefore, a seed value of 1 was used for all further analyses, and stochastic variation was not further assessed.
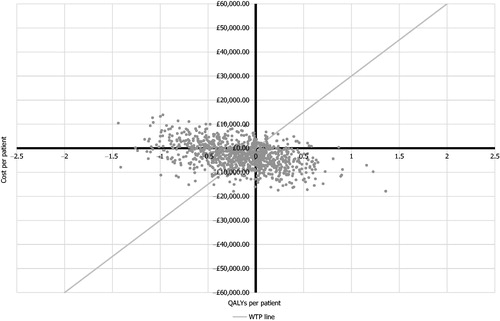
The probabilistic analysis () found that, at a willingness-to-pay threshold of £20,000, with no PAS discount applied, the probability of fingolimod being cost-effective was 50.8% (95% confidence interval = 47.7–53.9%); therefore, it was found to be marginally more likely that fingolimod is cost-effective even without the confidential discount which is known to be available to the NHS. In the deterministic results, for the lower NICE threshold of £20,000/QALY, fingolimod was not cost-effective at list price, requiring a discount greater than 0.8% to be cost-effective. In considering the effect of the confidential national PAS discount from list price for fingolimodCitation37, it was found that, at all discounts above 2.5%, fingolimod was cost-effective at the upper NICE threshold of £30,000/QALY (). It may be noted that it is known that no national PAS discount is in place for natalizumabCitation37.
Deterministic sensitivity analysis
The deterministic sensitivity analysis was undertaken on incremental net monetary benefit at the NICE £20,000/QALY threshold (incremental NMB = [incremental QALY difference × value of a QALY] – [incremental cost difference]). The deterministic sensitivity analysis results showed the model to be most sensitive to changes in the drug acquisition costs and the relative disability progression risks of the two DMTs, particularly fingolimod (). The cost of each infusion visit for natalizumab was the next most influential parameter, followed by the treatment withdrawal rate of each drug.
Figure 3. Deterministic sensitivity analysis on the net monetary benefit (NMB) at £20,000/QALY for the fingolimod vs natalizumab comparison (both drugs at list price).
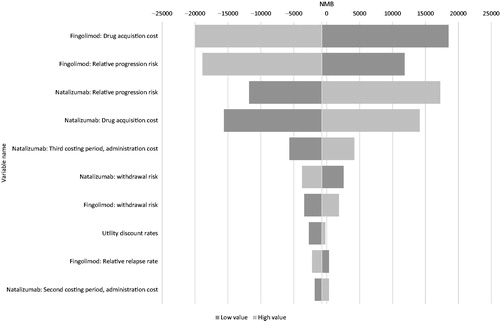
The NICE website notes the existence of a simple confidential discount PAS for fingolimod, but there is no PAS in place for natalizumabCitation37. Consequently, there is greater uncertainty around the price of fingolimod. As noted above, the effect of almost any discount will result in fingolimod being cost-effective. These considerations leave the hazard ratios for confirmed disability progression for each comparator as the parameters subject to most uncertainty; such uncertainty is to be expected as these figures are necessarily derived from post hoc sub-group analyses following the licensing process. Furthermore, such uncertainty is best taken into consideration by way of a comprehensive probabilistic sensitivity analysis, as was undertaken and presented in ; the probabilistic result marginally favored fingolimod as cost-effective even without any discount. The other parameters were considerably less influential and were in any case also considered in the comprehensive probabilistic analysis.
Scenario analyses
Scenario analyses were conducted to further examine the conditions under which natalizumab or fingolimod would be the more cost-effective treatment option (). The results of the base case analysis were found to be affected by each assumption, with the NMB for fingolimod increasing when withdrawal probabilities were set equal for both drugs and decreasing when either the waning assumptions or the modeled adverse events were removed from the model.
Table 4. Scenario analyses.
Discussion
Two DMTs are licensed in the EU for the treatment of the RES sub-group of RRMS patients; natalizumab and fingolimodCitation1,Citation2. Whilst both are recommended by the SMC for use in RES in ScotlandCitation11,Citation12, at present only natalizumab is recommended by NICE for this patient population in England and WalesCitation10. This study sought to evaluate the cost-effectiveness of natalizumab compared to fingolimod in the RES RRMS sub-group, through discrete event simulation modeling.
A comparison of natalizumab to the list-price of fingolimod is not relevant to UK clinical practice, as fingolimod is offered at a discounted price under an approved PASCitation37. Whilst the level of the discount is confidential, our results show that any plausible level of discount to the fingolimod list-price renders it likely to be the cost-effective option for RES RRMS in comparison to natalizumab.
The probabilistic sensitivity analysis showed that, at a willingness-to-pay threshold of £20,000, with no PAS discount applied, the probability of fingolimod being cost-effective was 50.8%. Furthermore, the model was found to be most sensitive to changes in the drug acquisition cost of fingolimod in the deterministic sensitivity analysis; it is, therefore, clear that any level of discount applied to fingolimod would increase the probability of being cost-effective further above 50%. Given that it is known that a confidential national PAS discount is in place for fingolimod and that it is known that a national PAS discount is not in place for natalizumabCitation37, the model results show fingolimod has greater than a 50% probability of being cost-effective vs natalizumab for the treatment of RES RRMS at the nationally available prices for both DMTs in the UK.
The main limitations of this economic evaluation primarily stem from the sub-group data from the natalizumab and fingolimod clinical trials. The phase III trials were powered to detect treatment effects in the overall enrolled RRMS population. Thus, the RES RRMS sub-group is subject to broader confidence intervals than the overall trial results. This is reflected in the deterministic sensitivity analysis, which found that the relative risk of confirmed disability progression for fingolimod and natalizumab were the second and third most influential parameters in the model, respectively. Additionally, the fingolimod sub-group reflects the overall RES population, whereas the natalizumab sub-group only includes treatment-naïve patients; it is unclear what effect, if any, this difference between the sub-populations may have on the relative results, which is further compounded by the lack of published baseline demographic data for the two sub-groups. As discussed in the methods, there is the additional complication that the definition used to define the RES sub-group in both the fingolimod and the natalizumab analyses was a proxy for the RES population, which differs slightly from the exact wording in the licensed indication. This may mean that the RES patients seen in the clinical trials are not as severe as those seen in clinical practice, but, as this proxy sub-group applies to both fingolimod and natalizumab equally, it is unlikely to have an impact on the relative cost-effectiveness results.
Other limitations include the fact that multiple parameters were derived from the overall RRMS population, who may be treated with DMTs other than natalizumab and fingolimod. The values of these inputs may, therefore, not be directly reflective of the RES RRMS population, in which both disease progression and treatment pathways may vary, especially if other DMTs are used. However, sensitivity analyses showed that these inputs did not substantially impact the model results. In addition, the model does not differentiate between relapses of different severities. The clinical data used are an average across all relapses for both therapies and, seeing as it is not expected that one therapy is likely to have a different effect on relapse severity than the other, this simplification in the model is unlikely to have a significant effect on the results. In terms of adverse events, there are several limitations in the way that PML is modeled; first, the model does not account for the early mortality impact of PML in the first year after its incidence and, second, it is assumed that the patients who are found to be JCV-positive remain on natalizumab rather than switching. The latter assumption is likely to be true for the UK settingCitation50, but may limit the applicability of the results to other countries where this is not the practice.
It should also be noted that the model did not incorporate improvements in patients’ disease. Although an alternative source for the natural history risk of EDSS progression in RRMS is available that does allow improvement in EDSS level, this source does not provide data specifically on the RES sub-groupCitation51. As the definition of RES disease is based on a high level of disease activityCitation1,Citation2, the risk of EDSS change should be derived specifically from a RES population; thus, the data from the fingolimod trials were used.
A previous study comparing a Markov model with this DES model in HA RRMS found generally high agreement in the results of the two models, with similar ICERs per QALY for the treatments examined, similar probabilities of one treatment being cost-effective over the other, and similar sensitivities to the model inputs suggesting that DES modelling is an appropriate strategy in RRMSCitation52. Although this comparison was based on an analysis of fingolimod and dimethyl fumarate, and focused on a sub-group of patients with HA RRMS, many of the inputs are aligned with those used here. The key point of difference with the present study, however, is that the comparison of fingolimod to dimethyl fumarate in HA RRMS did not incorporate any long-term consequences of adverse events and, therefore, the finding of similar results provided an indication that the DES model would produce similar outcomes to the Markov model when the DES model was limited to assumptions that were in the Markov model; in effect, this comparison was a form of model cross-validation.
Moving beyond such a like-for-like comparison, DES models can take into account chronic effects of AEs, which would extend beyond the individual cycle time of a Markov model and would, therefore, be lost, as well as rare events which are most likely to be seen over long time frames such as patient lifetimes. By incorporating infrequent but serious AEs, such as PML in the present report, DES models are able to model situations more reflective of real-world care, and, as such, the model developed here represents an important advance in the economic modeling approaches used in RRMS to date. An anticipated further key application of DES modeling to RRMS will be to consider DMTs where ongoing treatment is triggered by events occurring in the model rather than being continuous; such modeling would naturally fit with the pattern of alemtuzumab administration reported in clinical trials where initial treatment cycles are followed by as-needed re-treatments driven by the occurrence of relapses in future yearsCitation53,Citation54. Further work is ongoing and will be reported separately that considers this scenario and, indeed, the DES model approach may become the standard approach for modeling the treatment of RRMS in the future, as such models are more easily adapted to deal with the increasing complexity of the therapeutic pathwaysCitation22.
In conclusion, through the development and use of a DES model to facilitate a more complex and clinically-relevant approach to modeling in RRMS than has previously been possible, it has been shown that at all levels of confidential national PAS discount to the UK list price greater than 2.5%, fingolimod is the cost-effective option at a willingness-to-pay threshold of £30,000/QALY compared to natalizumab at its national list price (there being no PAS availableCitation37) in the treatment of RES RRMS.
Transparency
Declaration of funding
This study was funded by Novartis Pharmaceuticals UK Ltd, Camberley, UK.
Declaration of financial/other relationships
NA is a paid employee of Novartis Pharmaceuticals UK Ltd, Camberley, UK. RN is a paid employee of Imperial College Healthcare NHS Trust, London, UK, and has acted as a paid consultant in the past for Biogen, Roche, Teva, Merck, and Novartis. He has also received research funds from and worked on clinical trials run by Biogen and Novartis. RN did not receive financial compensation for authorship of this manuscript. SM and JK are, and MM and DS were at the time this work was prepared, paid employees of Costello Medical Consulting Ltd, Cambridge, UK, which was contracted by Novartis to undertake some of the work. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary tables
Download MS Word (84.9 KB)Acknowledgments
The authors take full responsibility for the content of the paper. Helen Chambers, an employee of Costello Medical Consulting Ltd, Cambridge, UK, provided medical writing and editorial assistance in the preparation of this article, funded by Novartis Pharmaceuticals UK Ltd, Camberley, UK.
References
- Summary of Product Characteristics: Gilenya 0.5 mg hard capsules. London: European Medicines Agency, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf. Accessed October 30, 2015
- Summary of Product Characteristics: Tysabri 300 mg concentrate for solution for infusion. London: European Medicines Agency, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000603/WC500044686.pdf. Accessed October 30, 2015
- Mackenzie IS, Morant SV, Bloomfield GA, et al. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry 2014;85:76-84
- Kingwell E, Marriott JJ, Jette N, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol 2013;13:128
- Miller D, Barkhof F, Montalban X, et al. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol 2005;4:281-8
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83:278-86
- Gani R, Nixon RM, Hughes S, et al. Estimating the rates of disability progression in people with active relapsing-remitting multiple sclerosis. J Med Econ 2007;10:79-89
- McCrone P, Heslin M, Knapp M, et al. Multiple sclerosis in the UK: service use, costs, quality of life and disability. Pharmacoeconomics 2008;26:847-60
- Montgomery S, Kusel J, Allen F, et al. Paucity and inconsistency: a systematic review and critique of budget impact analyses of disease-modifying therapies for multiple sclerosis in the UK and the implications for policy in the UK. Appl Health Econ Health Policy 2016;14:545-58
- National Institute for Health and Care Excellence. Natalizumab for the treatment of adults with highly active relapsing-remitting multiple sclerosis (TA127). London, UK: NICE; 2007
- Scottish Medicines Consortium. Natalizumab 300 mg concentrate for solution for infusion (Tysabri). NHS Scotland: Glasgow; 2007
- Scottish Medicines Consortium. Fingolimod (Gilenya). NHS Scotland: Glasgow; 2014
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899-910
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006;354:911-23
- Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387-401
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402-15
- Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:545-56
- National Institute for Health and Care Excellence. Alemtuzumab for treating relapsing-remitting multiple sclerosis (TA312). London, UK: NICE; 2014
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819-28
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829-39
- Maruszczak MJ, Montgomery SM, Griffiths MJ, et al. Cost-utility of fingolimod compared with dimethyl fumarate in highly active relapsing-remitting multiple sclerosis (RRMS) in England. J Med Econ 2015;18:874-85
- Allen F, Montgomery S, Maruszczak M, et al. Convergence yet continued complexity: a systematic review and critique of health economic models of relapsing-remitting multiple sclerosis in the United Kingdom. Value Health 2015;18:925-38
- Caro JJ, Moller J. Advantages and disadvantages of discrete-event simulation for health economic analyses. Expert Rev Pharmacoecon Outcomes Res 2016;16:327-9
- Karnon J, Haji Ali Afzali H. When to use discrete event simulation (DES) for the economic evaluation of health technologies? A review and critique of the costs and benefits of DES. Pharmacoeconomics 2014;32:547-58
- National Institute for Health and Care Excellence. Fingolimod for the treatment of highly active relapsing-remitting multiple sclerosis (TA254). London, UK: NICE; 2012
- National Institute for Health and Care Excellence. Teriflunomide for treating relapsing-remitting multiple sclerosis (TA303). London, UK: NICE; 2014
- National Institute for Health and Care Excellence. Dimethyl fumarate for treating relapsing-remitting multiple sclerosis (TA320). London, UK: NICE; 2014
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. London: National Institute for Health and Care Excellence, 2015; http://www.nice.org.uk/article/pmg9/. Accessed October 30, 2015
- Association of British Neurologists. Guidelines for prescribing in multiple sclerosis. London, UK: Association of British Neurologists; 2009
- Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1989;112(Pt1):133-46
- Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol 2009;256:405-15
- European Medicines Agency. Gilenya: EPAR - Assessment Report; 2011. London, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002202/WC500104529.pdf Accessed October 30, 2015
- Novartis. Data on file: HR vs placebo for 3-month confirmed disability progression (with the definition of progression adjusted to match that from the AFFIRM trial), pooled FREEDOMS + FREEDOMS II RES sub-group, Camberley, Surrey 2016
- NHS. National Schedule of Reference Costs 2014–15 for NHS trusts and NHS foundation trusts. London, UK: NHS; 2015
- NHS. NHS Tariff 2014–15. London, UK: NHS; 2015
- Biogen Idec Ltd. Natalizumab (Tysabri(r)) for the treatment of adults with highly active relapsing remitting multiple sclerosis. Manufacturer submission of evidence to NICE. London, UK: National Institute for Health and Clinical Excellence: TA127; 2007
- National Institute for Health and Care Excellence. List of technologies with approved Patient Access Schemes. London: National Institute for Health and Care Excellence, https://www.nice.org.uk/about/what-we-do/patient-access-schemes-liaison-unit/list-of-technologies-with-approved-patient-access-schemes. Accessed November 30, 2016
- Curtis L, Burns A. Unit costs of health and social care 2015. Canterbury,Kent: University of Kent, 2015
- Genzyme. Alemtuzumab for the treatment of relapsing remitting multiple sclerosis in adults. Manufacturer submission of evidence. London, UK: National Institute for Health and Care Excellence: TA312; 2013
- Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol 2015;21:637-44
- McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 2016;87:117-25
- Orme M, Kerrigan J, Tyas D, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health 2007;10:54-60
- Genzyme. Teriflunomide for the treatment of relapsing-remitting multiple sclerosis in adults. Manufacturer submission of evidence. London, UK: National Institute for Health and Care Excellence: TA303; 2013
- Novartis Pharmaceuticals UK Ltd. Fingolimod for the treatment of relapsing remitting multiple sclerosis in adults. Manufacturer submission of evidence. London, UK: National Institute for Health and Clinical Excellence: TA254; 2011
- Espallargues M, Czoski-Murray CJ, Bansback NJ, et al. The impact of age-related macular degeneration on health status utility values. Invest Ophthalmol Vis Sci 2005;46:4016-23
- Petrou S, Hockley C. An investigation into the empirical validity of the EQ-5D and SF-6D based on hypothetical preferences in a general population. Health Econ 2005;14:1169-89
- Currie CJ, McEwan P, Peters JR, et al. The routine collation of health outcomes data from hospital treated subjects in the Health Outcomes Data Repository (HODaR): descriptive analysis from the first 20,000 subjects. Value Health 2005;8:581-90
- Putzki N. Characteristics of PML cases in multiple sclerosis patients switching to fingolimod from natalizumab. Presented at the 6th Triennial Congress of the European and Americas Committees for Treatment and Research in Multiple Sclerosis, September 10–13, 2014, Boston, MA
- Biogen Idec. TYSABRI (natalizumab) Benefit/risk update & PML risk stratification. Reactive presentation for healthcare professionals (approval number: TY-PAN-0683(2) Date: September 2015). Cambridge: Massachusetts http://www.slideshare.net/gavingiovannoni/tysabri-benefit-risk-update-q3-2015/. Accessed May 20, 2016
- Raffel J, Gafson AR, Malik O, et al. Anti-JC virus antibody titres increase over time with natalizumab treatment. Mult Scler 2015;21:1833-8
- Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open 2014;4:e004073
- Kusel J, Maruszczak M, Adlard N. Cost-utility of fingolimod compared with dimethyl fumarate (Dmf) in highly active relapsing remitting multiple sclerosis (Rrms) in England: comparison of a Markov and discrete event simulation model. Value Health 2015;18:A759
- Coles AJ, Arnold DL, Cohen JA, et al. Patients with active RRMS and an inadequate response to prior therapy demonstrate durable improvements in relapse and disability following treatment with alemtuzumab: 5-year follow-up of the CARE-MS II study. Presented at the 68th Annual Meeting of the American Academy of Neurology, April 15–21, 2016, Vancouver, BC, Canada
- Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 2015;86:208-15