Abstract
Objective: To evaluate the quality of Chinese pharmacoeconomic-evaluation literature published between 2012–2014 retrieved from the Chinese National Knowledge Infrastructure (CNKI) in order to assess their adherence to recommendations of the Chinese Pharmacoeconomic Guidelines.
Methods: Identified literature was screened according to pre-specified criteria to access legibility for inclusion. Each included piece of literature was systematically compared against the recommendations proposed by the relevant Chinese guidelines.
Results: After culling, 259 studies were included in the comparative analysis. When compared to a previous study evaluating the quality of similar literature published between 1997–2007, the results showed improvements in certain technical aspects over the years. Particularly, an improvement was observed in more diverse evaluation methods being used, increased use of cost-utility analysis (2.43% in 2012–2014 vs 0.26% in 1997–2007) and use of discounting (45% in 2012–2014 vs 4.35% in 1997– 2007). In addition, a small number of studies were starting to apply modeling.
Conclusion: The quality of economic evaluation literature has improved in recent years, with more researchers realizing the importance and necessity of using discounting, sensitivity analysis, and modeling when conducting economic evaluation. This study also highlights certain important areas needing further attention when conducting economic evaluations in China. These include the ICER threshold of economic analysis, more detailed guidance in performing sensitivity analysis and modeling, as well as transferability of cost data across different regions. Overall, the results would support the positive contribution of the Chinese Economic Guideline in promoting economic evaluations in China.
Introduction
In recent years, pharmacoeconomic evaluation has been attracting increasing attention in China due to many factors affecting resource allocation in the healthcare system, including strained medical resources, and less than comprehensive health insurance coverage. As drug treatment plays an irreplaceable role in maintaining public health, it is not surprising that designing an effective drug policy has become an important focus in the recent Chinese healthcare reforms, starting in 2009. Hence, national authorities are particularly interested and highly value the important role of pharmacoeconomic evaluation in essential medicines inclusion, drug pricing, and medical insurance. This is evident as, since 2009, a number of national policies and opinions, such as the “CPC Central and State Council’s opinion on deepening reform of the healthcare system” were released to confirm the significance and importance of pharmacoeconomicsCitation1. Congruent with the increased emphasis on pharmacoeconomics, the quantity of published pharmacoeconomic evaluation literature in China also increased year by year. However, pharmacoeconomic research in China started relatively late, and, compared with those published abroad, the quality of these Chinese studies would not be considered high, and most still suffered from some methodological issues.
In order to standardize and facilitate pharmacoeconomic research, the “Pharmacoeconomic Evaluation Guidelines of China” was published jointly in 2011 by the Chinese Pharmaceutical Association, China Association for Science and Technology, and the Chinese Medical Doctor AssociationCitation2. This guideline is a methodology guideline aiming to provide some recommended standard advice for performing economic evaluation. The guideline provides recommendations in 10 categories, including how to conduct pharmacoeconomic evaluation or study, covering topics such as research questions, study design, cost estimation, health outcomes, evaluation techniques, modeling analysis, variability, uncertainty, generalizability, and budget impact analysis. Since there is no mandatory requirement to use the Chinese Pharmacoeconomic Evaluation Guidelines, in order to evaluate its impact, it would be informative to assess the adherence to the recommendations in pharmacoeconomic studies performed in China since its publication. This would provide valuable insights for further improvement of pharmacoeconomic research in China, and facilitate its further potential mandatory use in assisting health resource allocation.
Methods
To assess the quality of published pharmacoeconomic studies against the published Chinese Pharmacoeconomic Guidelines (2011 edition), seven Level 1 items, and 14 Level 2 items () from the Guidelines were selected as the criteria for assessment of adherence. At the same instant, we also attempted to identify the funding sources and affiliations of the researchers.
Table 1. Assessment criteria of pharmacoeconomic evaluation literature.
Database searching
Full-text database Chinese National Knowledge Infrastructure (CNKI) was searched from January 2012 to September 2014, using the keywords “pharmacoeconomics”, “cost-effectiveness analysis”, “cost-benefit analysis”, “cost-utility analysis”, “cost minimization analysis”, and “cost analysis”.
The title and abstract of the identified literature were scanned for relevance and suitability. A parallel approach was used when screening the potential literature for inclusion as well in the final analysis of the included literature. Any disagreement of relevance and suitability was resolved by discussion among the researchers. Full texts of literature deemed relevant were retrieved, and the reference sections of these manuscripts were further scanned manually for any missing literature. In our current study, we only included experimental research literature; conference reports, reviews, and non-experimental studies were excluded.
Results
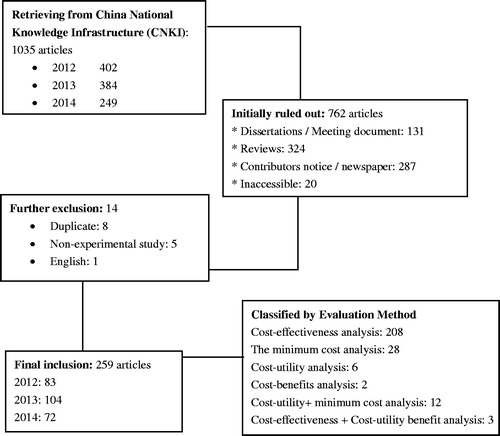
In total, the number of identified papers from the database for the period 2012–2014 amounted to 1,035. By systematically removing conference reports, literature reviews, duplicate studies, and non-experimental studiesCitation3–6, 259 studies were finally included for quality assessment. The culling process is shown in .
First, the authorship and the institute affliction of the studies were examined. The first authors of most of the published literature were clinicians and pharmacists, with relatively few (12.4%) from academic institutions. Among the retrieved studies, 35.9% originated from hospitals for the evaluation of several drugs or therapies, with most evaluation focused on respiratory diseases and tumors. A summary of the relevant information of these retrieved manuscripts is presented in .
Table 2. Summary of retrieved studies (2012–2014) and degree of adherence to recommendations in Guideline.
Assessment of quality (as adherence to guideline recommendations)
Research questions
All of the studies stated the objectives clearly, these included rational drug use, comparison between imported and domestic drugs, economic analysis of several pharmacotherapeutic treatments for the same disease, and comparison of different treatment options.
Costs and outcomes of most studies were obtained based on the perspective of the patient or the hospital. The few exceptions used the societal perspectiveCitation7, the perspective of health service providersCitation8, and fund payer perspectiveCitation9.
Almost all studies contained the selecting description of the target population. However, this was not specified in 21 studies, and 45 studies included control groups.
Study design
Among the identified articles, there were 120 (46.8%) randomized controlled trials, and 137 (53.5%) retrospective studies using data from hospital electronic patient records. The time horizon used in all studies was generally short (between 1–3 months), with more than 1 year in only 18 articles (7.03%)Citation10–27.
Research methods
In terms of evaluation methods, most retrieved studies used cost-effectiveness analysis (CEA). However, in a number of studies, CEA was still performed even when the results showed no statistically significant difference in treatment effectCitation28–35. In contrast, cost-utility analysis (CUA) was rarely used and was employed in only four studies (1.56%)Citation20,Citation36–38. Results of CEA were mostly expressed by cost-effectiveness ratio (C/E) and the incremental cost-effectiveness ratio (ICER), but most of the CEA did not present ICERCitation39–53. In addition, many ICEA generally compared C/E of the group with the least effective comparator; in other words, the ICEA produced the cost-effectiveness ratio compared with the least effective group. Other studies selected the minimum cost scheme as the standard to interpret the increment ratios obtainedCitation54–59. In contrast, several studies compared all programs when performing ICEACitation60,Citation61, but, as there is still no accepted or recommended ICER threshold value in ChinaCitation2, the results were interpreted based on the subjective judgments of the evaluatorsCitation62–69.
Cost estimation
Most studies had clear definitions and classification of costs, with the cost divided into direct costs, indirect costs, and intangible costs. However, most would claim difficulties in estimating indirect costs and intangible costs, and proceeded to estimate direct cost only in their studies. To be precise, direct and indirect costs were included in 10 studiesCitation5,Citation23,Citation50,Citation54,Citation66,Citation70–74, while direct costs, indirect costs and intangible costs were presented in only one studyCitation8. For studies comparing several drugs or treatment programs in hospitals, besides drug cost, most would assume other direct costs to be identical or negligible. Only a few studies included different medical equipment, different length of hospital stay, and diagnostic costs into direct cost estimationCitation75–83.
For discounting, most studies did not use the National Treasury rates or discounted rates recommended by the guidelines to adjust the costs, but simply used the year’s pricesCitation84–91 or retail price of hospital insteadCitation5,Citation9,Citation10,Citation29,Citation92–103. Even for studies with a duration of more than 1 year, many researchers did not apply discounting at all. This would hinder making meaningful comparison with these studies in future.
Output indicators
For outcomes used in the economic analysis, most research reported the treatment results with the intervention in patients in the natural clinical situation, while only 11 studies reported the efficacy of the intervention based on randomized controlled conditionsCitation31,Citation32,Citation51,Citation62,Citation63,Citation71,Citation90,Citation104–107. Actually this would be desirable as the effect of the intervention was estimated taking into consideration real-life factors such as doctor’s diagnostic error, complexity of the disease, complications of the situation, patient’s compliance, etc.
Use of modeling
Few studies used appropriate modeling in their evaluation, and most of these originated from academic or research institutions. Among these few studies, Markov modeling was applied in three studiesCitation14,Citation20,Citation23, and decision tree modeling in five studiesCitation9,Citation52,Citation108–110.
Handling of uncertainties
Sensitivity analysis was performed to handle uncertainties in 108 studies (41.7%). These sensitivity analyses were mainly used to evaluate the change of estimates in assumptions and certain key variables, such as the price of drugs, hospital days, the cure rate, and the discount rate. Most studies (almost 88%) applied single factor sensitivity analysis by decreasing drug prices by 10%, but only 12% of studies applied multivariate analyses using different probability of the variablesCitation20,Citation50,Citation68,Citation71,Citation90,Citation101,Citation108,Citation111–114. However, without clear guidance or standard recommendations from the guidelines, the range used for the different variables was subjectively decided by the authors.
Discussion and recommendations
The main purpose of the Chinese Pharmacoeconomic Evaluation Guidelines is to provide standardized recommendations about various technical aspects in conducting pharmacoeconomic research in China. Therefore, it would be informative to assess its level of impact on studies performed since its publication. For this reason, our current study evaluated recently published literature in China against the existing Chinese pharmacoeconomic guidelines. A better understanding of the discrepancies between current pharmacoeconomic research and criteria as recommended in the Chinese guidelines would facilitate wider application of guidelines and improve the quality of evaluation studies.
Before the Chinese guideline was published, Li and Ma et al.Citation115 had evaluated 201 pharmacoeconomic studies from 1997–2007, studying the perspective, evaluation method, discounting, cost, modeling, and sensitive analysis of each literature. A comparison of the results of the study by Li and Ma et al. and our current study is presented in . Based on the comparison, we could observe that there were improvements in certain areas over the years. Particularly, the improvement was seen in more diverse evaluation methods being used, increased use of cost-utility analysis (2.43% in 2012–2014 vs 0.26% in 1997–2007) and use of discounting (45% in 2012–2014 vs 4.35% in 1997–2007). Furthermore, a small number of studies (n = 8) were using modeling such as Markov and decision tree models. Overall, this comparison shows the quality of economic evaluation literature in China had improved in recent years.
Table 3. Analysis results of the literatures in 1997–2007 and 2012–2014.
However, despite the observed positive trends observed, based on our results, there are at least five areas that would need further improvement. These are discussed briefly in the following paragraphs.
Our study found that most current published studies did not explicitly state its research perspective and needed to be inferred, thus greatly reducing the usefulness to other users and decision-makers. In this respect, the Chinese Guideline recommends that there should be a clear perspective such as social/medical/patient, when conducting any pharmacoeconomic research.
When considering the types of economic analysis performed, CMA was rarely performed. There were a significant proportion of CEA studies being performed after no significant difference in efficacy between treatments was shown. Researchers should be advised that CEA/CUA is appropriate only when a statistical and clinical difference between treatments is established. Furthermore, in most ICEA, the result was compared with C/Emin (i.e. the ratio from the least effective comparator), or interpreted based on the evaluators’ subjective judgments only. The lack of an accepted or recommended ICER threshold value in China is noteworthy, it is recommended that a threshold value or range should be developed with collaboration of government and other stakeholders based on China’s economic situation and other relevant national conditions.
Furthermore, in estimation of cost, many studies unrealistically assumed that, besides drug costs, all other direct costs were identical. This ignores the efficacy of newer drugs which can significantly reduce the suffering of patients, loss of working time, the length of hospital stay, etc. Such assumptions would not capture comprehensively the financial benefits of the new treatment, and would potentially misguide and misinform the policy-makers. A more comprehensive and accurate evaluation of costs involved would greatly improve the applicability and generalizability of the evaluation.
Most studies used drug prices to perform univariate sensitivity analysis, but the existence of quite a huge inter-regional drug price difference in China would greatly affect the external validity of the results. In fact, the range of fluctuation in drug prices in China (and, for that matter, many other countries) is affected by many factors, such as patient demand, currency exchange rate fluctuation, inflation, bidding, etc., so how to best handle this uncertainty in drug price remains to be resolved. At this juncture, it is recommended to study the interaction between at least some of the more important parameters in multi-factor sensitivity analysis to better inform decision-making.
Probably due to a lack of expertise, modeling was seldom attempted in most of our included studies. For those few studies where modeling was attempted, the exercises were not properly conducted. Hence, learning about modeling techniques in foreign literature and guidelines and conducting training by Chinese academic institutes and experts in the field would gradually alleviate and improve this technical insufficiency.
Certainly, there are limitations in our present study. First, the guideline was released in 2011, and usually there would be a time lag before a wider adaptation by the scientific and clinical communities. Hence, we could not rule out the fact that some of the retrieved studies were conducted before the release of the guideline. In addition, despite our best effort, there existed the probability that we did not capture fully all the relevant literature of CNKI. However, we were reasonably satisfied that the captured articles would be a good representation of the status of pharmacoeconomic studies in China. Finally, the improvement observed over the years may not be totally attributable to the Guidelines, as experience gained over time by researchers would definitely be another contributing factor.
Conclusions
By comparing our results with a previous review, we conclude that the overall quality of economic evaluation literature published in China has improved in recent years after the publication of the Chinese Guidelines. However, there is still a significant deficiency in several areas that needs to be rectified for the quality of economic evaluation to be comparable with international standard. A better adherence to the recommendations from the Chinese Guidelines would contribute to achieving this.
Transparency
Declaration of funding
The authors declared that no sponsorship/funding was received for the project.
Declaration of financial/other relationships
None of the authors has any conflict of interest to declare. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgement
The authors would like to express their gratitude to the staff at the study site for their assistance during the literature collection process.
References
- CPC Central Committee and State Council on Deepening the Health Care Reform [ED/OL]. Xinhua News Agency. Retrieved April 8 2009. 2009. http://www.gov.cn/test/2009-04/08/content_1280069.htm
- Chinese Pharmacoeconomics Evaluation Guide Research Group, Liu GE, Hu SH, Wu JH. Chinese pharmacoeconomics evaluation guide (2011 edition). Chin Pharmacoeconom 2011;3:1-23
- Chang YP, Xie YM. Overview of pharmacoeconomic research on traditional Chinese and Western treatment of stroke. J Chin Med Mater 2012;37:3509-12
- Chen X, Zhai SD. Clinical efficacy and pharmacoeconomics of human insulin: weight at both ends of the balance. Drug Evaluat 2012;9:6-9
- Zhu HF, Long JL, Sun L, et al. Pharmacoeconomic evaluation of different nifedipine formulations for treating hypertension. Chin Pharmacoeconom 2013;3:33-5
- Jiang Y, Liu Li, Zhang BH. Analysing clinical usage of rheumatoid arthritis drug. Modern Prevent Med 2013;40:3891-3
- He JJ, Li YM, Zhuang Y, et al. Pharmacoeconomic evaluation of somatostatin analogues before acromegaly surgery. Chin Health Ins 2014;2:52-6
- Xu F, Liu GE, Tang Y, et al. Pharmacoeconomic evaluation of Qizheng plaster in treatment on acute lumbar sprain. Chin Pharmacoeconom 2014;8:9-13
- Zhu L, Liu GE, Li DM, et al. Cost-effectiveness analysis of pediatric seven-valent pneumococcal conjugate vaccine. Chin Health Econ 2013;32:71-5
- Zhou HY. Cost-effectiveness analysis of 3 regimens in treatment of patients with decompensated hepatitis B. Chin J New Drugs 2012;21:654-7
- Zhou L, Huang LH. Cost-effectiveness analysis of 4 regimens in patients with decompensated hepatitis B. J Chin Hosp Pharm 2012;32:1306-9
- Lao GQ, Wang JL, Wu Y. Cost-effectiveness analysis of 4 nucleoside analogs in treatment of chronic hepatitis B. Herald Med 2012;31:435-7
- Ma YH, Xu YF, Niu HL. Pharmacoeconomic evaluation of 4 antibiotics in treatment of chronic obstructive pulmonary disease complicated with pulmonary infection. Gansu Med J 2012;31:333-6
- Zhang XH, Zhao P, Zhang XL, et al. Pharmacoeconomic evaluation of domestic and imported cefuroxime sodium in the treatment of bacterial infections. J Chin Antibiot 2012;37:145-8, 162
- Chen JQ, Yin YZ, Yang R, et al. Pharmacoeconomic analysis of two chemotherapeutic regimens in treatment of advanced gastric cancer. Mod Hosp 2012;12:52-3
- Chen XJ, Xia ZH, Zhang MZ, et al. Cost-effectiveness analysis of four chemotherapy intravesical instillation in prevention of superficial bladder cancer through the urethra resection of bladder tumor recurrence. Chin Gen Prac 2012;15:2188-90
- Lin GX, Lin HL, Lu XH. Cost-effectiveness analysis of 3 dosing regimens in treating early Parkinson’s disease. Pharm Today 2013;3:142-4
- Li YQ. Cost-effectiveness analysis of 3 drugs in treating osteoporosis. J Pharm Prac 2013;2:143-4, 150
- Wang JC, Yang DK, Zheng JH, et al. Economic evaluation of 4 anti-virus programs in treating chronic hepatitis B. Chin Pharm 2013;18:1633-5
- Kang Q, Yu Z. Pharmacoeconomic evaluation of EGFR-TKI in patients with advanced non-small cell lung cancer. Chin Drug Eval 2013;5:305-8
- Deng CY, Wang YZ, Liu X, et al. Cost-effectiveness analysis of 4 interventions in treating hypertension in the workplace. Chin J Hypertens 2013;2:148-53
- Chen XY, Wang XR, Dong GL, et al. Clinical evaluation and pharmacoeconomic analysis of two regimens in treating metastatic breast cancer. Chin J Clin Oncol 2013;19:1160-4
- Fu YN, Liu GE, Zhu L, et al. Cost-effectiveness analysis of heptad pneumococcal conjugate vaccine against pneumococcal disease. Chin Health Econ 2013;1:49-52
- Zhuang QZ. Pharmacoeconomic evaluation of entecavir and recombinant human interferon a-2b in treating chronic hepatitis. Herald Med 2014;4:456-8
- Chen GW. Efficiency and safety analysis of common PPI drugs in treating reflux esophagitis. Chin Pharm Guide 2014;17:223-4
- Min TS, Sui WP, Liu J. Cost- effectiveness analysis of entecavir and adefovir in treating chronic hepatitis B. Chin J Hosp Pharm 2014;34:1499-501
- Wang GL, He Y, Wang X, et al. Cost-effectiveness analysis of Interferon α-2b and pegylated interferon α-2a in treating chronic hepatitis C. Modern West Med 2014;23:919-20, 944
- Chen LH, Huang WZ. Cost-effectiveness analysis of two PPIs in treating Helicobacter pylori positive duodenal ulcer. Chin Pharm Guide 2013;11:429-30
- Wu JW, Zhang YF. Cost-effectiveness analysis of two antibacterial drugs in treating urinary tract infections. Chongqing Med J 2013;42:1395-7
- Zhao LR. Cost-effectiveness analysis of three clinical regimens for community acquired pneumonia in elderly patients with chronic obstructive pulmonary disease. Chin Med 2013;10:122-4
- Tan H, Yuan HY, Ji P. Economic evaluation of calcium citrate drug to treat osteoporosis. Modern Med Health 2014;30:2266-7
- Zhang JH. Cost-effectiveness evaluation of losartan and benazepril in treatment of mild to moderate hypertension. Chin Pharmacoeconom 2014;3:9-11
- Wang L, Zhou LM. Minimum cost analysis of two different dosage form of acarbose in treatment with type 2 diabetes. Drug Eval 2014;1:32-5
- Xiao J. Economic evaluation of two proton pump inhibitor in treatment with helicobacter pylori infection gastric ulcer with activity. Hebei Med J 2014;20:413-15
- Fu YZ, Xu WH, Wei SH. Pharmacoeconomic study of Yinxingdamo and Salvia ligustrazine injection in adjuvant treatment of elderly patients with acute cerebral infarction. Chin Pharm Clin 2014;14:77-9
- Liu M, Zhang SC, Min J, et al. Pharmacoeconomic analysis of hospitals and community health service centers combined intervention model in patients with coronary heart disease after revascularization. Chin J Hosp Pharm 2013;33:1634-8
- Zhu JJ, Chen W. Pharmacoeconomics of vildagliptin in combination with metformin in treatment of diabetes. Chin Pharmacoeconom 2013;4:11-16
- Wu JY, Zhang FZ, Zhang FR, et al. The efficacy and cost study of Black Lands Yellow Pills in treatment of chronic renal failure. Shandong Chin Med 2014;1:23-5
- Yu ZH, Wang XR, Wang Y. Cost-effectiveness analysis of amoxicillin and moxifloxacin in treatment of senile bronchitis. Jilin Med J 2012;33:3455-6
- Zhang H. Pharmacoeconomic evaluation of two schemes of Azithromycin in treatment of lower respiratory infections. West Med 2012;24:772-3
- Pang XM, Song YH, Xu XB. Pharmacoeconomic analysis of two hypertension treatment regimens. Chin Pharm Guide 2012;10:163-4
- Yang QW, Li GD. Clinical efficacy and pharmacoeconomic evaluation of 2 inhaled therapies in treatment of patients with severe COPD. Taishan Med Coll 2012;33:363-5
- Wang Z, Jin XY. Effective analysis of different treatment schemes of severe COPD with pseudomonas aeruginosa infection. J Clin Pulmon Med 2013;18:2017-19
- Wang NQ, Wang SQ, Diao Y. Pharmacoeconomic analysis of Danshen tablets and compound Danshen dripping pills in treatment of coronary heart disease. Strait Pharm J 2013;25:212-14
- Wang WQ. Pharmacoeconomic evaluation of domestic azithromycin in treatment of pediatric infectious diseases. Chin Modern Doctor 2013;51:27-8, 31
- Li XF, Chen Y. The analysis of import and domestic pemetrexed disodium in treatment of non-small cell lung cancer. J Clin Pulmon Med 2013;18:1098-100
- Shang JH. Clinical application experience and analysis of anti-hypertensive drug in 320 cases of patients. Chin J Prac Med 2013;8:194-5
- Zheng B. Economic assessment of risperidone and clozapine in treatment of schizophrenia. Strait Pharm J 2014,26:135-6
- Shi YF, Yan H, Sun SG, et al. Systematic evaluation and pharmacoeconomic analysis of two Salvia injections in treating Coronary Angina Pectoris. Chin J Evidence-Based Med 2014;14:287-91
- Wang YH, Yu CY, Zhang J, et al. Pharmacoeconomic evaluation of 2 schemes in treatment of acute ischemic cerebrovascular disease. Chin J Biochem Pharm 2014;1:135-7
- Li L. Clinical efficacy and pharmacoeconomics of Qingzhi Powder in the treatment of hyperlipidemia. Chin J Prac Med 2014;14:179-80
- Luo XZ, Mo XX. Cost-effectiveness analysis of cefprozil and cefaclor in treatment of children upper respiratory tract infections. Chin Pharmacoeconom 2014;7:9-10
- Huo Y. Efficacy and economics between imipenem cilastatin sodium and moxifloxacin in treatment of severe community-acquired pneumonia. Chin J Prac Med 2014;19:152-3
- Zhang HJ, Zhang FP. Economic analysis of 4 calcium channel blockers commonly used in treating of hypertension. Chin J Integrat Med Cardio-/Cerebrovasc Dis 2014;12:695-6
- Liu YQ, Peng B, Li QF, et al. Cost-effectiveness analysis of three regimens in treatment of type 2 diabetes. Chongqing Med J 2014;43:2470-3
- Yan TS, Sun WP, Liu J. Cost-effectiveness analysis of entecavir and adefovir in treatment of chronic hepatitis B. Chin J Hosp Pharm 2014;34:1499-501
- Zhang YM. Cost-effectiveness analysis of Pule'an and finasteride in treatment of benign prostatic hyperplasia. Gansu J Trad Chin Med 2013;32:850-1
- Wang YY, Dou LP, Zhong Y. Cost-effectiveness analysis of Chinese medicine combined with chemotherapy in the treatment of elderly patients with advanced non-small cell lung cancer. Shandong J Trad Chin Med 2013;53:18-21
- Song JW, Li CB, Song YX, et al. Economic evaluation and the clinical effect of traditional Chinese medicine injections assisted PD in treatment of advanced ovarian cancer. Mod Oncol 2013;21:2796-8
- Shi F, Xie BQ, Shu Q. Cost-effectiveness analysis of five antibiotics for patients with urinary tract infections. Anti-infect Pharm 2014;11:231-3, 243
- Xu YY, Cai XJ, Zhang XC, et al. Pharmacoeconomics of 3 anti-coronary drugs in the treatment of angina pectoris. Strait Pharm J 2013;25:197-8
- Qiu DQ. Cost-effectiveness analysis of 4 treatment regimens of community II diabetes. Chin J Public Health Manage 2013;29:238-9
- Jiang YM, Yang XY. Pharmacoeconomic analysis of four topical treatments of oral ulcers. J Med Theory Prac 2013;26:820-1
- Wu YH, Huang JP. Cost-effectiveness analysis of 2 controlled intravenous analgesia regimens in geriatric patients. Pharm Today 2014;6:406-8
- Ding GH, Zhang Q, Feng B, et al. Cost-effectiveness analysis of 3 drugs for treating cerebrovascular disease brain dysfunction. Aerospace Med 2014;25:1001-2
- Wang YH, Yu CY, Zhang J, et al. Clinical observation of Ozagrel and Naodesheng in sequential treatment of acute cerebral infarction. Chin Pharm 2014;25:699-701
- Xue X, Gao JR, Xia LZ, et al. Cost-effectiveness analysis of glimepiride in combination with different drugs in treatment of type 2 diabetes. Anhui Med J 2014;18:441-3
- Chen SY, Cen YH. Economic evaluation of our hospital’s 2 treatment regimens of acute ankle sprain. Neimenggu Trad Chin Med 2014;33:154-5
- Wang HX. Cost-effectiveness analysis of 5 commonly used drugs in treatment of acute cerebral infarction. Chin Modern Drug Applic 2014;8:147-8
- Pan MY. Efficacy and economic analysis opioid receptor antagonist in treatment of severe traumatic brain injury. Strait Pharm J 2013;25:272-4
- Wang QH, He Y, Wang YH. Economic evaluation of randomized controlled trial of moxifloxacin and levofloxacin injection in treatment of community acquired pneumonia. Chin Pharmacoeconom 2013;3:36-9
- Yan XY, Li Z, Yu Q, et al. Cost-effectiveness analysis of 3 drug treatments of senile chronic functional constipation. Chin J New Drugs Clin Remedies 2013;32:154-7
- Lin LQ, Yang YY, Lin Y. Pharmacoeconomics of two treatments of acute obstructive pulmonary disease. Strait Pharm J 2014;26:179-82
- Xu CJ, Jiang TF, Sun Yi. Cost-minimization analysis of two chemotherapy regimens in treatment of non-small cell lung cancer. Chin J Clin Pharm 2014;23:35-8
- Tan B, Wu F, Wang Y. The effectiveness and economic evaluation of different injections of traditional Chinese medicine in auxiliary treatment of liver cancer. Evaluat Anal Drug-use Hosp China 2013;13:494-6
- Li YJ. Cost minimization analysis of two meropenem infusion regimens in treatment of ventilator-associated pneumonia. Chin J Modern Drug Applic 2013;14:121-2
- Qu LY, Zhang W, Cai S, et al. Cost-effectiveness analysis of Rina thiazole amine with vancomycin in the treatment of methicillin-resistant staphylococcal pulmonary infection. Chin J New Drugs Clin Remed 2013;32:578-81
- Liu M, Zhang SC, Xu YN, et al. Pharmacoeconomic analysis of combined management by hospital and community health service center of patients post coronary revascularization. Chin J Hosp Pharm 2013;12:1634-8
- Zhang BS, Shen WX, Wang H, et al. Cost-effectiveness analysis of 4 types of treatments in patients with type 2 diabetes in community. J Ning Xia Med Uni 2013;35:906-9
- Zhou FJ, Kuang XH. Comparison of the cost-effectiveness in two preventive treatments of postoperative infection of acute appendicitis. Chin Modern Med 2013;30:134-5
- Xu CJ, Jiang YF, Sun Y. Cost minimization analysis of two regimens in treatment of non-small cell lung cancer. Chin J Clin Pharm 2014;23:35-8
- Hou XY, Tao X, Zhang C, et al. Cost-effectiveness analysis of two metformin hydrochloride formulations in the treatment of type 2 diabetes. Chin Pharm 2014;25:1844-7
- Wu X, Chi C, Kou JG, et al. Cost-effectiveness analysis of rabeprazole and esomeprazole in treatment of reflux esophagitis. J Modern Med 2010;30:143-4
- Yang Z, Liu XH, Liu SK, et al. Cost-effectiveness analysis of 2 schemes in treating bacterial infections in acute exacerbation of severe COPD. Chin Pharm 2012;23:356-8
- Du H, Yang Z, Ge LH, et al. Cost-effectiveness analysis of 3 different regimens drug-induced liver injury in patients infected with HIV/TB using antituberculosis drugs. Chin Pharm 2012;23:3803-6
- Fang H. Pharmacoeconomic analysis of 3 antihypertensive drugs in treatment of hypertension. Strait Pharm J 2012;24:267-9
- Zhou YY. Cost-minimization analysis of 3 selective 5-HT reuptake inhibitors in treatment of somatoform disorders. Chin J Clin Pharm 2012;21:318-20
- Dong JY, Cui CL, Tao ZM, et al. Cost-minimization analysis of moxifloxacin sequential therapy of community-acquired pneumonia. Chin Pharm 2012;21:36-7
- Zhao K, Yang HT, Chang C. Cost-effectiveness analysis of 3 proton pump inhibitors combined with flupentixol melitracen in treatment of functional dyspepsia. Chin Pharmacoeconom 2013;6:9-10, 18
- Li YJ. The minimum cost analysis of two different meropenem infusion schemes in treating ventilator-associated pneumonia. Chin J Mod Drug Applic 2013;7:121-2
- Bi YZ, Zeng DX, Sheng GF, et al. Cost-effectiveness analysis of 3 antiemetic programs in treating of cisplatin-induced vomiting. J Basic Clin Oncol 2012;25:414-7
- Yu F, Pu R, Cui YM, et al. Cost-effectiveness analysis of pirarubicin and epirubicin in neoadjuvant chemotherapy of breast cancer. Chin Pharm 2012;23:347-50
- Xu XG, Zou LX. Pharmacoeconomic analysis of domestic and imported entecavir treatment of viral hepatitis B. Strait Pharm J 2012;24:247-8
- Liang XL, Yan XQi. Pharmacoeconomics of gemcitabine combined with different platinum in treatment of advanced non-small cell lung cancer. Med Innovat Chin 2012;9:155-6
- Zheng YG, An AJ, Cao JJ, et al. Cost-effectiveness analysis of 3 second-line schemes in treating of non-small cell lung cancer. Med Innovat Chin 2012;9:149-50
- Zhao F. The effect of patients with breast hyperplasia selecting different treatment programs in the guidance of pharmacoeconomics. Chin Health Ind 2012;9:101
- Xue L, Yang W. Pharmacoeconomic evaluation of different formulations of metformin in treatment of type 2 diabetes complicated with lung cancer. Pharm Care Res 2012;12:438-41
- Ma CL. Cost-effectiveness analysis and adverse reactions of Azithromycin and clarithromycin in treatment of Helicobacter pylori-induced peptic ulcer. Qinghai Med J 2013;6:71-2
- Jiang LH, Chen CY, Huo CF. Pharmacoeconomic analysis of six oral hypoglycemicregimens in National essential drug lists. Chin J Drug Applic Monit 2013;10:131-4
- Li ZQ, He X. Cost-effectiveness analysis of 3 prevention infection programs after cesarean section in a certain hospital. Drug Evaluat 2013;10:23-6
- Wang CX, Wang HY. Cost-effectiveness analysis of 3 treatment programs of advanced non-small cell lung cancer. Chin Health Ind 2013;10:152-3
- Zhong SY. Cost-effectiveness analysis of sitagliptin and pioglitazone associated with metformin respectively in type 2 diabetes. Chin Pharmacoeconom 2013;4:19-20
- Yu L, Feng SL, Jia XF. Cost-effectiveness analysis of sodium hyaluronate in treatment of knee osteoarthritis. Chin Pharm 2013;24:2265-7
- Hong HJ. Pharmacoeconomic analysis of fleroxacin sequential therapy of urinary tract infections. Chin Pharmacoeconom 2013;3:21-2
- Xu HY, Han XM, Huang JP. Cost-effectiveness analysis of Chinese and Western medicine in treatment of respiratory syncytial viral pneumonia in children. Liaoning J Tradit Med 2013;40:1188-9
- Wang ZL, Li X. Cost-effectiveness analysis of 3 sulfonylurea drugs for initial treatment of type 2 diabetes. Chin Pharm 2014;25:2019-21
- Zhao P, Zhang XH, Meng L. Cost-effectiveness analysis of 2 traditional Chinese medicine injections in treatment of diabetic peripheral neuropathy. Pharm Clin Res 2014;22:368-70
- Chen X, Xu YJ, Li Y, et al. Pharmacoeconomic evaluation of dosing regimens of imipenem anti postoperative infection combining Monte Carlo simulation. Chin J New Drugs 2013;22:691-4, 708
- Cai L. Pharmacoeconomic analysis of 4 treatments in elderly hypertensive. Chin Pract Med 2014;10:32-3
- Ding XY, Zhang GY, Li CL, et al. Pharmacoeconomics analysis of antimicrobial treatment of children with bronchial pneumonia. J Pediat Pharm 2014;20:36-40
- Zhang LF, Xu L. Cost-effectiveness analysis of 2 community-acquired pneumonia treatments. J Pharm Prac 2014;32:315-17
- Zhang BS, Shen WX, Wang H, et al. Cost-effectiveness analysis of 4 treatment regimens of Type 2 diabetes in the community. J Ningxia Med Uni 2013;35:906-9
- Chen SF. Cost-effectiveness analysis of import and domestic teicoplanin in treatment of lower respiratory tract infections. Strait Pharm J 2013;25:199-201
- Sun YF. Pharmacoeconomic analysis of different methods of anesthesia in children with colic. J Med Res 2013;42:143-6
- Li MH, Ma AX. Current status, problems and suggestions of drug economic evaluation in China. Chin Pharm 2008;11:801-5