Abstract
Background: A recent retrospective comparative effectiveness study found that use of the FLOSEAL Hemostatic Matrix in cardiac surgery was associated with significantly lower risks of complications, blood transfusions, surgical revisions, and shorter length of surgery than use of SURGIFLO Hemostatic Matrix. These outcome improvements in cardiac surgery procedures may translate to economic savings for hospitals and payers.
Objective: The objective of this study was to estimate the cost-consequence of two flowable hemostatic matrices (FLOSEAL or SURGIFLO) in cardiac surgeries for US hospitals.
Methods: A cost-consequence model was constructed using clinical outcomes from a previously published retrospective comparative effectiveness study of FLOSEAL vs SURGIFLO in adult cardiac surgeries. The model accounted for the reported differences between these products in length of surgery, rates of major and minor complications, surgical revisions, and blood product transfusions. Costs were derived from Healthcare Cost and Utilization Project’s National Inpatient Sample (NIS) 2012 database and converted to 2015 US dollars. Savings were modeled for a hospital performing 245 cardiac surgeries annually, as identified as the average for hospitals in the NIS dataset. One-way sensitivity analysis and probabilistic sensitivity analysis were performed to test model robustness.
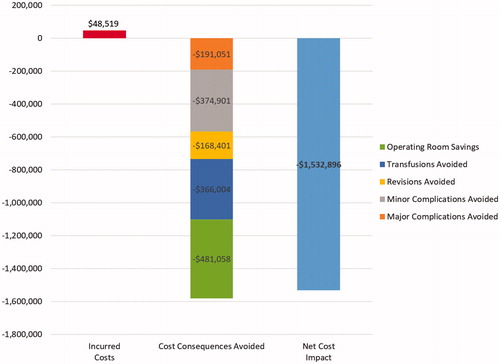
Results: The results suggest that if FLOSEAL is utilized in a hospital that performs 245 mixed cardiac surgery procedures annually, 11 major complications, 31 minor complications, nine surgical revisions, 79 blood product transfusions, and 260.3 h of cumulative operating time could be avoided. These improved outcomes correspond to a net annualized saving of $1,532,896. Cost savings remained consistent between $1.3m and $1.8m and between $911k and $2.4m, even after accounting for the uncertainty around clinical and cost inputs, in a one-way and probabilistic sensitivity analysis, respectively.
Conclusions: Outcome differences associated with FLOSEAL vs SURGIFLO that were previously reported in a comparative effectiveness study may result in substantial cost savings for US hospitals.
Introduction
Achieving hemostasis is critical with any surgical procedure. Inadequate hemostasis during surgery may lead to a number of major and minor complications that can result in increased morbidity and mortalityCitation1–3. Approximately 4% of patients may require surgical revision due to excessive blood loss during and after cardiac surgeryCitation4–6. Patients requiring surgical revision following cardiac surgery have been associated with a greater risk of renal failure, prolonged mechanical ventilation, acute respiratory distress syndrome, sepsis, atrial arrhythmias, and operative mortality in a study of over 6,000 patientsCitation4 and increased hospital length of stay (LOS) and mortality in a study of 8,586 patients undergoing a coronary artery pass graft (CABG)Citation5. Patients requiring resternotomy because of excessive bleeding following cardiac surgery were associated with a prolonged Intensive Care Unit (ICU) stay, the need for intra-aortic balloon counterpulsation, and deathCitation6.
In a study of over 1 million surgeries (including cardiac, vascular, non-cardiac thoracic, solid organ, general, knee/hip replacement, reproductive organ, and spinal surgery), patients who experience bleeding-related complications and/or blood product transfusions during surgical hospitalization have been found to have longer overall hospital LOS (10.4 vs. 4.4 days) and ICU LOS (3.3 vs. 0.5 days), which increases the economic burden relative to patients with adequate hemostasisCitation7. In all surgical sub-populations, the bleeding-related complication and/or blood product transfusion groups had higher total hospital costsCitation7. The ratio of average total costs estimated for patients with bleeding complications vs those without complications ranged from 1.31 for cardiac surgery to 1.93 for vascular surgeryCitation7.
Conventional methods of controlling intra-operative bleeding, such as suture, cautery, or ligature, may be ineffective or impractical in certain surgery settingsCitation8. Consequently, several topical adjunctive hemostatic products have been developed to decrease perioperative bleeding. Hemostatic agents such as glues, adhesives, and sealants help to stop bleeding when applied directly to the site of a bleed. However, some bleeding sites are difficult to reach, and their effectiveness can be limited in patients who receive heparinCitation8,Citation9.
In the US, two gelatin-based hemostatic matrices often used are FLOSEAL Hemostatic Matrix (Baxter Healthcare Corporation, Deerfield, IL)Citation10 and SURGIFLO Hemostatic Matrix (Ethicon Incorporated, Somerville, NJ)Citation11. These flowable agents both contain gelatin and thrombin in a single application product. However, the old preparation of SURGIFLO did not include thrombin; therefore, studies from the past compared outcomes of the use of the two products with the adjunct of thrombin to the original SURGIFLO preparation (FLOSEAL vs SURGIFLO + Thrombin). The role of gelatin is to induce hemostasis via platelet activation, as well as mechanical compression. The role of thrombin is to cleave the fibrinogen to form fibrin. Thus, these agents have a dual mechanism of action due to their ability to act at the beginning and end of the coagulation cascade to promote physical contact activation of platelets and facilitate fibrin formation, respectively. Additionally, these agents also hold the thrombin on the bleeding site, preventing the dispersion of thrombin in its natural liquid form. Randomized clinical trials have demonstrated that these flowable hemostatic agents have increased efficacy relative to conventional methods in a variety of surgical specialties due to their ability to effectively conform to wound crevices, fill in deep lesions, and ease irrigationCitation12,Citation13.
However, despite their similar mechanisms of action, FLOSEAL and SURGIFLO have demonstrated hemostatic performance differences due to the composition of their gelatin granulesCitation14,Citation15. These hemostats differ in the following gelatin granule characteristics: species type (bovine vs porcine), shape, size, smoothness, and surface areaCitation14. FLOSEAL is comprised of a self-expandable gelatin matrix component and lyophilized bovine thrombinCitation10. In contrast, SURGIFLO is comprised of a partially reconstituted porcine gelatin and is prepared with EVITHROM (Topical Human Thrombin)Citation11. Furthermore, the gelatin within FLOSEAL is arranged with smooth distinct round particles, while the gelatin within SURGIFLO is arranged with stellate coalescing ribbon-like particlesCitation14.
Both in vitro and in vivo animal studies have proven that FLOSEAL is superior to SURGIFLO in achieving effective hemostasis due to its smooth gelatin matrixCitation14,Citation15. In vitro results indicate that smooth gelatin retains significantly more thrombin relative to stellate gelatin before being mixed with blood (6.81 vs 10.89 IU/mL, p = .0013) and after a clot formation (0.613 vs 1.289 IU/ml, p = .0003)Citation14. In vivo animal studies found similar results indicating that smooth gelatin is able to provide superior hemostatic efficiency as compared to stellate gelatin at 5 min and 10 min after applicationCitation14.
There are no randomized controlled trials comparing FLOSEAL and SURGIFLO in cardiac surgery or other types of surgery. However, three retrospective comparative effectiveness analyses have been conducted in spine and cardiac surgeries using the Premier hospital databaseCitation2,Citation3,Citation16. The Premier database captures approximately one of every four hospital discharges in the US with date-stamped log including procedures, medications, laboratory, and diagnostic services encountered by a patient during a given hospital stayCitation17.
The Price et al.Citation3 observational database analysis using the Premier’s Hospital Database concluded that, for major spine surgery cases, SURGIFLO was associated with increased risk of blood product transfusion (Odds Ratio, OR = 2.56, p < .001), longer surgery time (+8.84 min, p < .0001), and increased product usage (+3.34 mL, p < .001), compared to FLOSEAL. Similarly, for severe spine surgery cases, use of SURGIFLO was associated with longer surgical time (+26.9 min, p < .001) and increased product usage (+1.52 mL, p < .01) compared to FLOSEALCitation3.
David et al.Citation16 also evaluated SURGIFLO vs FLOSEAL in spinal surgeries using the Premier Hospital Database. The authors concluded that the choice of hemostatic agent had no effect on clinical outcomes and that SURGIFLO could lead to hospital cost savings of $65 per surgery and an additional $21 savings per additional hour of surgery. However, contrary to Price et al.Citation3, David et al.Citation16 did not include procedures such as corpectomies and tumor removal that have a high risk of bleeding challenges and allowed for multiple hemostatic agents to be used, hence confounding the results.
The Tackett et al.Citation2 analysis of cardiac surgical cases captured in the Premier Hospital Database from 2006–2012 assessed real-world outcomes of FLOSEAL and SURGIFLO in cardiac patients. Cohort A included 4,480 FLOSEAL and 326 SURGIFLO cases, and results indicated that SURGIFLO cases were associated with significantly higher risk of multiple adverse outcomes, including major (OR = 2.12, p = .001) and minor complications (OR = 1.84, p < .001); surgical revisions (OR = 2.01, p = .042); transfusions for any blood products (OR = 4.90, p < .001), and longer surgery times (adjusted mean difference = 64 min, p < .001) than the FLOSEAL group. There were no significant differences in mortality and hospital LOS between the comparator groupsCitation2.
The objective of this analysis was to understand the cost-consequence (i.e. direct cost savings) and the economic implications associated with the usage of two absorbable flowable hemostatic agents (FLOSEAL vs SURGIFLO) when used for controlling intra-operative bleeding during cardiac surgery procedures.
Methods
Overview
A cost-consequence model was developed in Microsoft Excel (Redmond, WA) to estimate the annualized clinical and economic impact of FLOSEAL vs SURGIFLO. The model was based on the average number of cardiac surgeries performed in US hospitals, accounting for the difference in clinical outcomes between the two products, and the corresponding costs. The cardiac procedures included coronary artery bypass grafting (CABG), valve surgery, and thoracic aortic surgery in an adult population. The model uses the clinical data as published by Tackett et al.Citation2 and supplemented by additional outputs (e.g. adjusted rates of complications, revisions, and transfusion) derived from the same analysis using the Premier’s US Perspective Hospital DatabaseCitation17. An analysis of 2012 Healthcare Cost and Utilization Project’s (HCUP’s) National Inpatient Sample (NIS)Citation18 data was also performed to provide inputs for the costs of complications. All inputs and their sources are described below.
Clinical inputs
All clinical inputs from Cohort A (i.e. FLOSEAL only vs SURGIFLO only) in Tackett et al.Citation2 shown to be statistically different were included in the model (). These include: (1) average surgery time per procedure; (2) rate of overall major post-operative complications; (3) rate of overall minor post-operative complications; (4) rate of cases requiring surgical revision; and (5) rate of cases requiring blood product transfusions (any blood product transfusion, i.e. packed red blood cell and plasma product). It is noted that surgical revisions were identified if the patient was returned to the operating room to address surgical bleeding by identification of the ICD-9 code for a rethoracotomy to an existing surgical site within the same hospitalization. Reoperations were excluded because of the unpredictable impact on intra-operative bleeding which would have had significant effects on the data analysis.
Table 1. Clinical inputs.
It is also noted that, while the rates of major and minor complications overall were significantly different between FLOSEAL and SURGIFLO, the distribution of individual major (stroke, shock, sepsis, myocardial infarction, or any combination of two or more major complications) and minor (renal failure, respiratory insufficiency, inotropic support lasting more than 24 h, or any combination of two or more minor complications) complications was assumed to be the same whether one uses FLOSEAL or SURGIFLO ().
Additional analyses were conducted on the same dataset to identify adjusted values for certain parameters that had not been published. This was the case for the adjusted rates of revisions, transfusion, major complications, and minor complications. Additionally, the estimated average volume used per case were 9.0 mL and 9.7 mL for FLOSEAL and SURGIFLO, respectively.
All clinical inputs used in the model are summarized in .
Cost inputs
US cost inputs were broken down into product costs and surgery-related costs ().
Table 2. Cost inputs—surgery-related costs.
Product costs
The cost of product was obtained by multiplying the average product volume (i.e. FLOSEAL: 9.0 mL; SURGIFLO: 9.7 mL) with publicly available product prices (Baxter Wholesale Acquisition Cost (WAC) for FLOSEAL and Average Sales Price (ASP) for SURGIFLO from the IMS Health Hospital Supply Index). This provides estimated total product cost figures at the population level, which is relevant from the hospital perspective.
Operating room costs
Using estimates from Chatterjee et al.Citation19 and updating to 2015 US dollars using the Medical Care Consumer Price Index (CPI), the operating room costs used in the model were estimated to be $462 for each 15-min interval. This estimate was calculated from an average per minute cost for operating room time across five different high-volume surgical proceduresCitation19.
Surgery and complication costs
The 2012 NISCitation18 dataset was used to estimate surgery and complication costs. The NIS is a database of inpatient stays from billing data submitted by hospitals to statewide data organizations across the US. The NIS is used to make national estimates of healthcare utilization, access, charges, quality, and outcomes. The NIS covers all patients, including individuals covered by Medicare, Medicaid, or private insurance, as well as those who are uninsured. The NIS dataset contains data from ∼8 million hospital stays from over 1,000 hospitals sampled to approximate a 20% stratified sample of US community hospitals. For each hospital stay, the NIS database records ICD-9 diagnosis and procedure codes. Weights are provided to calculate national estimates. We used full year 2012 NIS data as it was the latest year of data available.
Appropriate ICD-9 procedure codes () were used to identify cardiac surgeries (CABG, valve surgery, thoracic aortic surgery), as well as the associated major and minor complications included in the model. HCUP hospital-level Cost-to-Charge Ratios were applied to calculate the mean total hospital cost. These costs were then converted to 2015 US dollars using the Medical Care CPI.
Surgery-related costs () included (1) Average base cost of a cardiac surgery procedure without complications, revision, or blood product transfusionCitation18, (2) Average cost of cardiac surgery procedure with blood product transfusion, but without complications or revision, (3) Average cost of cardiac surgery procedure with revision, but without complications or blood product transfusion, (4) Average costs of the surgical procedure with one major (stroke, shock, sepsis, or myocardial infarction) or minor (renal failure, respiratory insufficiency, or inotropic support lasting more than 24 h) complication, but without revision or blood product transfusionCitation18, and (5) Average costs of the surgical procedure with a combination of two or more major or minor events, but without revision or blood product transfusion.
Incremental costs for each complication were calculated as the difference between the total cost for surgery with the complication and the base cost of the surgical procedure without complication ($38,486).
Model analyses
To make our cost modeling relevant to the reader, cost savings were estimated for a hospital performing the average number of mixed cardiac surgeries per year in the US. The NIS 2012 data was again used to identify the number of mixed cardiac procedures for the average hospital in the US. ICD-9 procedure codes identified each CABG, Valve Surgery, and Thoracic Aortic surgery at the hospital level. On average, 245 mixed cardiac surgeries were performed in 2012 per hospital from the 1,178 hospitals included in the NIS datasetCitation18. Therefore, the model assumes a population size of 245 mixed cardiac surgery patients.
The outputs of the cost-consequence model were calculated in terms of the annualized comparative clinical outcomes avoided (major complication, minor complications, surgical revisions, transfusions) and the corresponding cost savings of using FLOSEAL relative to SURGIFLO. By doing so, the net cost impact of using FLOSEAL vs SURGIFLO was estimated.
Sensitivity analyses
One-way and probabilistic sensitivity analyses were performed to identify the major cost drivers accounting for uncertainty around the estimates.
One-way sensitivity analyses were conducted by varying all clinical and economic inputs one at a time using the lower and upper boundaries of the 95% confidence intervals or by ±20% when the 95% confidence interval was not available.
Probabilistic sensitivity analysis (PSA) was conducted using the Monte Carlo simulation method. All key clinical and economic inputs were varied at the same time by randomly selecting a value for each parameter within the 95% confidence interval of that parameter according to a distribution probability. All parameters, their 95% confidence interval and the distribution probabilities can be found in Appendix .
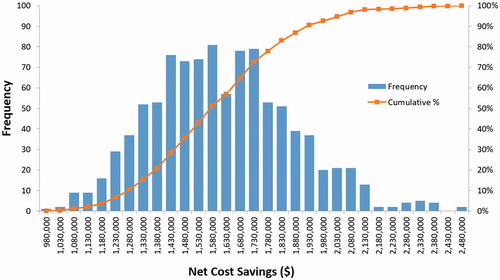
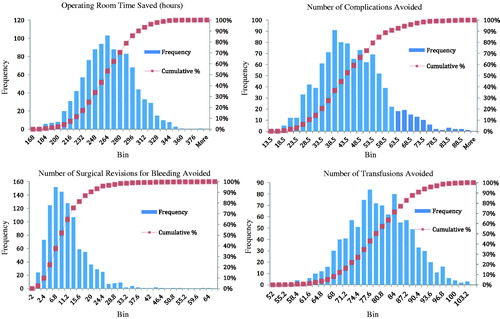
Distributions of economic and clinical outcomes based on 1000 simulations were created. Histograms of hours of operating room time saved, the number of complications avoided, the number of surgical revisions avoided, as well as the number of transfusions avoided can be found in Appendix .
Results
Base model
The model estimates that, for an average hospital that performs 245 cardiac surgeries per year, using FLOSEAL rather than SURGIFLO could avoid 42 complications (11 major, 31 minor), nine surgical revisions, and 79 blood product transfusions. In addition, operating room time for these cardiac surgeries could be reduced by 260 h. shows that, even if its acquisition cost is higher ($48,519 for 245 cardiac surgeries), FLOSEAL is expected to generate substantial annual net cost savings ($1,532,896 for 245 cardiac surgeries) compared to SURGIFLO.
Sensitivity analyses
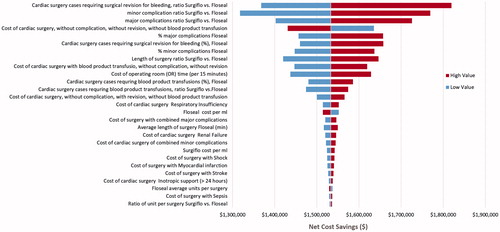
Varying each model parameter one at a time in the one-way sensitivity analyses showed that FLOSEAL’s savings for the same 245 patient cohort ranged from $1,317,675–$1,819,536. The most important cost drivers were: (1) the ratio of cardiac surgery cases requiring surgery revision for bleeding, (2) the ratio of minor complications, (3) the ratio of major complications, (4) the cost of the base cardiac surgery, (5) the percentage of major complications, (6) the percentage of cardiac surgery cases requiring surgical revision for bleeding, (7) the percentage of minor complications, (8) the surgery time ratio, (9) the cost of cardiac surgery with blood product transfusion, and (10) the cost of operating room time (). Variation in the cost of hemostatic agents had only a minor impact on the results. In all analyses, the difference was always in favor of FLOSEAL, consistently demonstrated in savings.
When varying all parameters at the same time in the PSA, the minimum and maximum savings associated with FLOSEAL ranged from $911,010–$2,435,589 (). FLOSEAL was cost saving in all 1000 iterations. Approximately 56% of simulations were above the base case and, thus, the model’s net economic savings of $1.5 million can be considered a relatively robust estimate in approximating the benefit of using FLOSEAL relative to SURGIFLO.
Discussion
We estimate that the average US hospital that utilizes FLOSEAL as an adjunct to hemostasis in cardiac surgery procedures may see a reduction of 11 major complications, 31 minor complications, nine surgical revisions, 79 blood product transfusions, and 260.3 h of operating room time compared to SURGIFLO. This can result in significant net savings with FLOSEAL when compared to SURGIFLO in cardiac surgery procedures (base case analysis) ($1.5 million per 245 cardiac surgery procedures per year). The net cost impact of using FLOSEAL vs SURGIFLO remained cost-saving after sensitivity analyses and scenario analyses. After adjusting all clinical and cost inputs, FLOSEAL’s savings still remained between $1.3–$1.8 million for a US hospital performing 245 cardiac surgery procedures per year. Furthermore, in the PSA, after 1000 iterations, FLOSEAL’s cost savings ranged between $911k and $2.4 million. Thus, both types of sensitivity analyses indicated the robustness of the cost savings associated with use of FLOSEAL vs SURGIFLO.
Our results support prior research which estimated a significant cost saving when using FLOSEAL vs non-flowable topical hemostats (SURGICEL Nu-Knit, Ethicon SARL, Neuchatel, Switzerland; GELFOAM Sterile Compressed Sponge, Pfizer, New York, NY) in cardiac surgical proceeduresCitation20. While both analyses used the same modeling framework, there are two important differences besides the comparator: (1) while the prior economic analysisCitation20 utilized results from a randomized controlled trial of 415 patients, our clinical inputs were derived from a recent real-world effectiveness analysis of 4806 patients; (2) while the prior economic analysisCitation20 used the 2010 US Hospital discharge data from six different states to obtain cost inputs, we used the 2012 NIS database to identify cost estimates and adjusted these figures to 2015 dollars. The strength of the NIS data is that it is representative of 95% of the US population and, thus, more generalizable. Therefore, using real-world data and cost estimates from a more representative dataset may help increase the generalizability of our findings.
Limitations
All economic models are a simplification of a complex healthcare situation and, hence, bear the limitations associated with a simple representation of reality. For example, our model assumed the same distributions for major and minor complications for FLOSEAL and SURGIFLO. This may not be the case in reality, but we had no solid evidence supporting a different assumption.
There is likely departmental-level variation in cost to charge ratios; however, HCUP only provides charge data per discharge. Therefore, we are unable to use departmental level CCRs with this data source.
Model results are highly dependent on the nature, amount, and quality of the data available. We used the comparative effectiveness study published by Tackett et al.Citation2 as the basis for the clinical end-points in our model. Although this study is an observational study and had significantly more use of FLOSEAL (n = 4,480) compared with SURGIFLO (n = 326), it is the only evidence comparing FLOSEAL to SURGIGLO in cardiac surgery procedures. Also, while the sample sizes of FLOSEAL and SURGIFLO use were different by over an order of magnitude, results were highly statistically significant at the p < .001 level, and this distribution reflects the market penetration of the two products in the US market during the study period. Similarly, we have used the best evidence available at this time (i.e. HCUP) for costing healthcare resources/consequences.
This study was also performed using data from 2006–2012. Since then, the introduction of new treatment guidelines for management of intra- and post-operative coagulopathy has positively impacted the rate of complications and transfusions compared to the study period. Within this context, the use of viscoelastic hemostasis assays, such as ROTEM or TEG, as tools to optimize the management of coagulopathy and reduce bleeding complications, was not evaluated in the study analysis since use of the technology was not widespread, nor standardized during the study period.
Our model was built for the US healthcare environment (i.e. using clinical evidence and costs from the US healthcare system), and, although it is very relevant for US hospitals, the generalizability of our results should be adjusted to other jurisdictions.
Conclusion
Despite the limitations described above, this cost consequence analysis suggests that cost savings at the hospital level may be generated by using FLOSEAL rather than SURGIFLO in cardiac procedures. While various sensitivity analyses demonstrated the robustness of the estimated cost savings in cardiac procedures, an additional study with the primary objective of investigating both the comparative outcomes and cost associated with different hemostatic matrices could be pursued to confirm this finding in Cardiac procedures, as well as other surgery types.
Transparency
Declaration of funding
This study was funded by Baxter Healthcare Corporation.
Declaration of financial/other relationships
DM, MR, and MRA are all paid employees and stockholders at Baxter. YX and JDE are employees at Stratevi, which was retained for this work.
Acknowledgments
The authors would like to acknowledge Samira Massachi for her contributions in the preparation of this manuscript.
References
- Nasso G, Piancone F, Bonifazi R, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg 2009;88:1520-6
- Tackett S, Calcaterra D, Magee G, et al. Real world outcomes of hemostatic matrices in cardiac surgery. J Cardiothorac Vasc Anesth 2014;28:1558-65
- Price J, Tackett S, Patel V. Observational evaluation of outcomes and resource utilization from hemostatic matrices in spine surgery. J Med Econ 2015;18:777-86
- Moulton MJ, Creswell LL, Mackey ME, et al. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J Thorac Cardiovasc Surg 1996;111:1037-46
- Dacey LJ, Munoz JJ, Baribeau YR, et al. Reexploration for hemorrhage following coronary artery bypass grafting: incidence and risk factors. Arch Surg 1998;133:442-7
- Unsworth-White MJ, Herriot A, Valencia O, et al. Resternotomy for bleeding after cardiac operation: a marker for increased morbidity and mortality. Ann Thorac Surg 1995;59:664-7
- Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res 2011;11:1-13
- Renkens KL, Payner TD, Leipzig TJ, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spine surgery. Spine 2001;26:1645-50
- Oz MC, Cosgrove DM, Badduke BR, et al. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. Ann Thorac Surg 2000;69:1376-82
- FLOSEAL VH S/D Instructions for Use. Deerfield, IL: Baxter Healthcare Corporation; 2005
- SURGIFLO Instructions for Use. Somerville, NJ: J & J Wound Management; 2008
- Mozet C, Prettin C, Dietze M, et al. Use of Floseal and effects on wound healing and pain in adults undergoing tonsillectomy: Randomised comparison versus electrocautery. Eur Arch Otorhinolaryngol 2012;269:2247-54
- Chapman WC, Singla N, Genyk Y, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surg 2007;205:256-65
- Lewis K, Atlee H, Mannone A, et al. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Invest Surg 2013;26:141-8
- Coenye KE, Bourgain C, Keibl C, et al. A qualitative morphological comparison of two haemostatic agents in a porcine liver trauma model. Surg Sci 2013;4:359-64
- David G, Lim S, Gunnarsson C, et al. Similar patient outcomes yet different hospital costs between flowable hemostatic agents. J Med Econ 2015;18:735-45
- Premier Inc. Premier PerspectiveTM Hospital Database. Charlotte, NC: Premier Inc.; 2012
- 2012 Healthcare Cost and Utilization Project’s (HCUP’s) National Inpatient Sample (NIS) Database. https://www.hcup-us.ahrq.gov/databases.jsp. Accessed February 10, 2016
- Chatterjee A, Payette MJ, Demas CP, et al. Opportunity cost: a systematic application to surgery. Surgery 2009;146:18-22
- Tackett S, Sugarman R, Kreuwel H, et al. Hospital economic impact from hemostatic matrix usage in cardiac surgery. J Med Econ 2014;17:670-6
Appendix
Table A1. Probability distributions for clinical inputs.
Table A2. Probability distributions for cost inputs.