Abstract
Aims: To compare patient characteristics, rates, and costs of medically attended falls among patients with Parkinson’s disease (PD) and probable PD plus neurogenic orthostatic hypotension (PD + nOH).
Materials and methods: MarketScan Commercial and Medicare Supplemental databases (January 1, 2009–December 31, 2013) were used to identify PD and probable PD + nOH patients. The first medical or prescription claim suggesting these diagnoses served as the index date. Baseline characteristics and post-index all-cause and fall-related healthcare utilization and costs were compared between patient groups.
Results: A total of 17,421 PD and 281 PD + nOH patients were identified. Compared with PD patients, PD + nOH patients were older (77 vs 74 years; p < .0001) and had more comorbidities. Pre- and post-index date, more PD + nOH patients had a medically attended fall than PD patients (25% vs 20% [p = .0159] and 30% vs 21% [p = 0.0002], respectively). Fallers in both groups had similar numbers of medically attended falls 12-months pre-index (mean =1.9), but PD + nOH fallers had more falls post-index (2.5 vs 2.0; p = .0176). Compared with PD patients, more PD + nOH patients (all p < .01) had fall-related emergency department (ED) visits (18% vs 10%), hospitalizations (7% vs 3%), and non-office visit outpatient services (15% vs 10%). Adjusted total post-index medical costs for falls ($2,260 vs $1,049; p = .0002) and total all-cause costs ($31,260 vs $20,910; p < .0001) were higher for PD + nOH vs PD patients.
Limitations: This study had some limitations. There is no ICD-9-CM diagnosis code for nOH, so a combination of PD and OH diagnoses (with confounding conditions excluded) served as a proxy for an nOH diagnosis. Also, the rate of falls and associated costs in these cohorts might be under-reported because only medically attended falls were evaluated.
Conclusions: PD + nOH patients had a higher prevalence of pre-existing comorbidities and a higher rate of medically attended falls than those with PD alone, leading to increased costs of care.
Introduction
Neurogenic orthostatic hypertension (nOH) is a sub-category of orthostatic hypertension (OH) whereby an orthostatic drop in blood pressure results from an underlying neurologic disorder, most commonly Parkinson’s disease (PD), pure autonomic failure, and multiple system atrophyCitation1–4. In PD, nOH is a non-motor symptom that results from sympathetic nervous system dysfunction and subsequent deficits in norepinephrine release and may precede neurologic diagnosisCitation2,Citation5. The prevalence of symptomatic nOH in PD (PD + nOH) is estimated at 18%Citation6.
nOH increases the risk of hip fractures and head trauma secondary to falls, with a resultant disability that can affect one’s daily activities and is associated with depression/anxietyCitation2,Citation7. Fear of falling may also limit physical activity, with exercise avoidance leading to deconditioning and additional psychosocial impact such as social isolation and poor quality-of-lifeCitation7,Citation8. One US study reported that OH was the cause of 36 hospitalizations per 100,000 US adults, and the number of OH-related hospitalizations increased steadily with age; those 75 years or older had an annual hospitalization rate of 233 per 100,000Citation9. PD is also a costly condition resulting from drug costs, hospitalizations, and productivity loss—with direct and indirect costs increasing with progressionCitation10. In PD, falls rank among the most common reasons for hospital admissionsCitation11–14. Additionally, falls in PD patients have been associated with increased caregiver burdenCitation15. The increase in falls in the PD population due to nOH has not been adequately explored, although the available evidence suggests a greater fall rate with PD + nOH vs PD aloneCitation16. To date, only one published study, from France, has described higher direct medical costs over a 6-month period in Parkinsonian syndromes with vs without nOHCitation17.
Given the limited information describing characteristics, healthcare utilization, and cost outcomes in patients with PD + nOH compared to those with PD alone, these outstanding research questions were explored in the current study.
Methods
Study design
This retrospective cohort study used the Truven Health MarketScan Commercial Claims and Encounters (Commercial) and Medicare Supplemental Databases (January 2009–December 2013). The patient identification period spanned from January 1, 2010–December 31, 2012, and patients diagnosed with OH or PD during this period were eligible for study inclusion. The index date was the first claim date qualifying a patient as having OH or PD during the enrollment period. The 12-month period before the index date served as the pre-index period, and the 12-month period following the index date served as the post-index period.
Patient selection
All patients were required to have ≥1 non-diagnostic medical claim with a diagnosis code for PD (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] 332.0) in any position, or ≥1 prescription claim for a PD medication (dopamine agonists [amantadine, apomorphine, bromocriptine, cabergoline, carbidopa-levodopa, levodopa, pergolide, pramipexole, ropinirole, rotigotine], monoamine oxidase inhibitors [rasagiline, selegiline], catechol-O-methyltransferase inhibitors [entacapone, tolcapone], and anti-parkinson anti-cholinergics [benztropine, biperiden, procyclidine, trihexyphenidyl]) during the identification period. Also required was the alternative criteria (≥1 prescription claim for a PD medication or ≥1 non-diagnostic medical claim with a diagnosis code for PD in any position) anytime during the study period. Additional criteria included age ≥18 years on the index date and no diagnosis for autonomic failure/neuropathy or multiple system atrophy during the study period.
Eligibility for the OH population required ≥1 non-diagnostic medical claim with a diagnosis code for OH (ICD-9-CM diagnosis code 458.0, 458.1, 458.9) in any position or ≥1 prescription for fludrocortisone or midodrine during the enrollment period. Also required was ≥1 non-diagnostic medical claim with a diagnosis code for OH in any position (if first claim was a prescription) or ≥1 prescription for fludrocortisone or midodrine (if the first was a medical claim) anytime during the study period. Additional criteria included age ≥50 on the index date, and no diagnosis for end-stage renal disease, conditions associated with volume depletion, or autonomic failure/neuropathy or multiple system atrophy during the study period. Both patient populations must have had continuous enrollment in medical and pharmacy benefits for 12 months prior to and after their respective index dates.
No specific ICD-9-CM diagnosis code exists for nOH; we therefore used the intersection of PD and OH populations. Accordingly, the PD + nOH population included patients with an initial diagnosis of OH or PD and a subsequent diagnosis of PD or OH, respectively, within 12-months pre-index and 90 days post-index of the initial conditions’ index date. Patients with a diagnosis of OH alone were excluded from study.
Baseline characteristics
Baseline patient characteristics were measured during the pre-index period and included demographics (age, gender, geographic region, plan type, payer type, and index year) and clinical characteristics (Charlson comorbidity index [CCI] score, specific comorbidities, concomitant medications, and medically attended falls).
Outcomes
The study outcomes—medically attended falls, healthcare utilization, and healthcare costs—were evaluated during the 12-month post-index period. Based on a previously published study, medically attended falls were identified using medical claims with ICD-9-CM codes in the primary position for fractures, dislocations, sprains, strains, intracranial injuries, or contusions with intact skin surfacesCitation18.
Healthcare utilization was categorized as fall-related (as defined above) and all-cause (any diagnosis). Medical care was captured by care setting: hospitalizations, emergency department (ED) visits, physician office visits, and other outpatient visits. In addition, pharmacy claims were captured as part of all-cause healthcare utilization.
Healthcare costs were similarly captured for both medically attended falls and all-cause. Costs reflected all payments made to providers of care from both insurers (plan and co-ordination of benefits) and patients (co-payment, co-insurance, and deductible). All costs were adjusted to 2013 US dollars using the Bureau of Labor Statistics’ medical care component of the Consumer Price Index (CPI). Patients with capitated claims were excluded from cost analyses only.
Statistical analysis
Descriptive analyses
Frequencies and proportions were used to summarize categorical measures, and means and standard deviations (SDs) were used for continuous measures. Statistically significant differences between the disease cohorts (PD vs PD + nOH) were evaluated using Chi-square tests for categorical measures and t-tests for continuous measures.
Multivariable analyses
The proportion of patients with a medically attended fall, the number of medically attended falls, costs associated with medically attended falls, and all-cause costs were further evaluated in multivariable analyses controlling for patients’ baseline demographics and clinical characteristics.
The likelihood of having a medically attended fall was evaluated using multivariable logistic regression, with results presented as adjusted proportion of fallers and 95% confidence intervals (CIs). The number of medically attended falls was evaluated using a 2-part model. The first part was a logit model estimating the probability of having ≥1 fall, and the second part was a negative binomial model estimating the number of falls among patients with ≥1 fall. Results were presented as the adjusted number of fall events and 95% CIs.
Medical costs associated with medically attended falls were also evaluated using a 2-part model. A logit model estimated the probability of having non-zero costs, and a general linear model (GLM) with a log-link function and gamma distribution estimated the costs among those with non-zero costs. All-cause total and medical costs were evaluated using a GLM with log-link function and gamma distribution, and the recycled prediction method was used to obtain predicted fall-related and all-cause cost values and 95% CIs.
All statistical analyses were conducted in SAS version 9.3, and 2-sided tests with p-values <0.05 were considered statistically significant.
Results
The attrition for the PD and PD + nOH cohorts are presented in , respectively. The initial PD cohort included 199,951 patients who had at least one PD diagnosis and/or PD medication; and, of these, a total of 60,350 met the initial PD criteria. Following the application of additional inclusion/exclusion criteria, the final PD population consisted of 17,421 patients.
Figure 1. Patient attrition for the (a) PD cohort, and (b) PD + nOH cohort. *Non-mutually exclusive criteria. †Required for a secondary analysis (results not presented). OH: orthostatic hypotension; PD: Parkinson’s disease; PD + nOH: Parkinson’s disease with neurogenic orthostatic hypotension. Note: Because the data extract included patients with a diagnosis for OH or PD, patients with only a prescription for OH medication without an OH diagnosis or a prescription for PD medication without a PD diagnosis during the study period represent only a sub-set of all such patients.
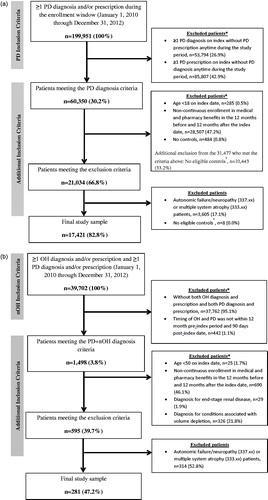
Of the initial 39,702 patients who had at least one OH diagnosis and/or OH medication and at least one PD diagnosis and/or PD medication, a total of 1,498 patients met the initial PD + nOH criteria. After applying additional inclusion/exclusion criteria, 595 patients remained. An additional 314 patients with other causes of nOH (autonomic failure/neuropathy or multiple system atrophy) were excluded, resulting in a final PD + nOH population of 281 patients.
Baseline characteristics
Baseline demographic and clinical characteristics are summarized in and , respectively. For the PD + nOH cohort, mean age was higher than the PD cohort (77 vs 74 years; p < .0001), and these patients were more likely to be male (68% vs 59%; p = .0048). A higher proportion of the PD + nOH cohort had ≥1 medically attended fall during the baseline period compared to the PD cohort (25% vs 20%; p = .0159), but the average number of falls was similar (1.9 falls; p = .7287). While the rate of hypertension was similar between the cohorts (47% PD vs 42% PD + nOH; p = .0959), significant differences were observed in the use of some anti-hypertensive medications, with proportion of users being higher in the PD cohort.
Table 1. Demographic characteristics of PD and PD + nOH patients.
Table 2. Clinical characteristics of PD and PD + nOH patients.
Medically attended fall-related events (post-index)
In the unadjusted analysis, during the post-index period, the PD + nOH cohort was more likely to have ≥1 medically attended fall compared to the PD cohort (30% vs 21%; p = .0002). The mean (SD) number of falls among the patients who fell was also higher in the PD + nOH cohort compared to the PD cohort (2.5 [2.2] vs 2.0 [1.5]; p = .0176).
After controlling for baseline characteristics, no significant difference was observed in the probability of having a post-index medically attended fall between the cohorts (25.6% vs 21.2%; difference = 4.5%; 95% CI = −0.2–9.7%). Consistent with the unadjusted analyses when assessing the number of post-index medically attended falls, PD + nOH patients had significantly more falls than PD patients (0.68 vs 0.42; mean difference = 0.26; 95% CI = 0.17–0.38; ).
Table 3. Adjusted percentage of patients with at least one medically attended fall and number of medically attended falls over the 12-month post-index period.
Medically attended fall-related and all-cause utilization (post-index)
Significantly higher proportions of the PD + nOH patients were hospitalized (7% vs 3%; p = .0004), visited the ED (18% vs 10%; p < .0001), and had other outpatient services (15% vs 10%; p = .0028) due to post-index falls compared to PD patients (). However, the average number of fall-related visits in these treatment settings did not differ between the cohorts.
Table 4. Medically attended fall-related and all-cause healthcare utilization in the 12-month post-index period.
During the post-index period, the PD + nOH cohort had a higher proportion of patients with all-cause hospitalizations (51% vs 28%; p < .0001), ED visits (58% vs 37%; p < .0001), and other outpatient visits (97% vs 94%; p = .0422) compared to the PD cohort (). Among those requiring services, the average numbers of hospitalizations (2.2 vs 1.9; p = .0485), ED visits (2.4 vs 1.9; p = .0002), other outpatient visits (21.2 vs 17.3; p = .0007), and physician office visits (11.7 vs 10.6; p = .0124) were significantly higher in the PD + nOH compared to the PD cohort. Lastly, PD + nOH patients had more post-index prescription claims than PD patients (56.5 vs 45.0; p < .0001).
Medically attended fall-related and all-cause costs(post-index)
During the 12-months post-index, PD + nOH patients incurred on average $1,211 (unadjusted p = .0002) more in fall-related medical costs than PD patients (). After accounting for baseline differences in the cohorts, fall-related costs remained significantly higher for PD + nOH patients compared to PD patients: on average $1,471 more ($2,519 vs $1,047; 95% CI = $715–$2,553; ) in fall-related medical costs.
Table 5. Medically attended fall-related and all-cause healthcare costs in the 12-month post-index period.
Unadjusted all-cause total costs were $31,260 for PD + nOH patients compared to $20,910 for PD patients (p < .0001; ). Medical costs accounted for ∼72% of total healthcare costs, with inpatient and other outpatient service costs accounting for the majority of medical costs. After multivariable adjustment, all-cause total costs for the PD + nOH cohort remained significantly higher than those for the PD cohort ($30,428 vs $20,950; mean difference = $9,478; 95% CI = $6,336–$12,982; ); all-cause medical costs were also significantly higher ($22,896 vs $15,117; mean difference = $7,779; 95% CI = $4,679–$11,366; ).
Discussion
This study showed differences in the patient characteristics, fall-related and overall disease burden, and associated healthcare costs of patients with PD + nOH relative to PD alone. The results suggest that nOH has a significant incremental negative impact on patients diagnosed with PD. The PD + nOH cohort had more pre-existing comorbidities and were more likely to have at least one medically attended fall during the baseline period, and, while the adjusted rate of post-index medically attended falls did not differ significantly between the cohorts, PD + nOH patients had more falls and correspondingly higher fall-related medical utilization and costs. In addition, the PD + nOH cohort had significantly higher medical utilization and costs overall.
Falls are commonCitation19,Citation20 and rank among the most frequent reasons for hospital admissions in patients with PDCitation11–14, yet the contribution of OH to PD-related fall risk and the resulting costs of care in this population remain uncertain given the lack of published PD + nOH vs PD comparisons. To our knowledge, only one study (n = 971) has provided insight into the cost differences between Parkinsonian syndromes and PD + nOHCitation17. This French study that found nOH significantly increased direct costs (€4,426 vs €3,074; p < .05), and this finding was mostly attributable to increased costs of hospitalization, ancillary care, physician fees, and drug therapyCitation17. However, interpretation of these results is confounded by its inclusion of patients with various Parkinsonian syndromes and limited by the 6-month study time frame. Furthermore, important differences between healthcare systems make it difficult to extrapolate the results from this French study to the US nOH population, particularly in terms of costsCitation17.
Although limited, other data are available regarding the contribution of OH to fall risk, providing some insight into its underlying pathophysiology. Recently, falls were evaluated in ambulatory non-demented patients with PD, and OH was identified as one of the contributing factorsCitation21. In a Finnish study, PD + nOH patients had a numerically greater rate of falls relative to PD patients (39.5% vs 28.1%; p = .614), accompanied by a significant increase in postural sway without altered mobility or walking speed (suggesting an impact of nOH specifically on balance)Citation16.
Our findings not only support that PD + nOH patients experience a greater number of falls and cost more than PD patients, but they also reveal possible unmet needs in PD + nOH patients for treatments that will mitigate the impact of nOH in PD. In the aforementioned French study, drug costs were a major contributor to the increased direct medical costs of nOH in Parkinsonian syndromes, with patients having concomitant nOH using more controlled-release levodopa formulations and dopamine agonistsCitation17. Of note, a recent economic analysis (Markov modeling) of a phase 3 clinical trial in PD + nOH found that droxidopa was associated with over 100,000 fewer falls per 10,000 patients (258,776 vs 365,419 falls with standard of care, for which placebo was the proxy) and is a cost-effective therapy in the USCitation22. While the drug costs for droxidopa were $30,112, fall avoidance yielded an estimated 12-month healthcare cost savings of $14,574. No such information is available for other nOH treatments. Further investigations are warranted to determine the optimal strategy for fall avoidance in the PD + nOH population and to more precisely define fall risk.
Limitations
We also acknowledge several limitations to this retrospective database analysis. Administrative database analyses may be subject to selection bias and reliance on accurate and complete coding in billing data. Although this study was not designed to study the prevalence of nOH, it is striking that the number of PD + nOH patients identified is much smaller than PD-alone patients. While the point prevalence of OH in PD has been estimated at 30.1% (95% CI =22.9–38.4%)Citation23, the relative proportion of the PD + nOH vs PD alone in our study is <2%, which may reflect that nOH remains under-diagnosed and under-treated in routine practice. In addition, patients may be misclassified as having PD alone, leading to an under-estimation of the PD + nOH population. Medical claims data contain only ICD-9-CM diagnosis codes with no other clinical information, and there are no ICD-9-CM codes specific to nOH; therefore, an algorithm was developed with clinical input (IB and CS) combining the presence of diagnosis codes with disease-specific prescription treatment to identify both PD and OH patients. Patients had to have both diagnosis codes and prescription fills related to both conditions within 12 months to meet the definition of PD + nOH. Different age criteria were applied in the selection of the PD + nOH and PD cohorts; however, this likely did not affect the study findings, as the mean age between the cohorts differed by only 3 years after all the selection criteria were applied. In the PD + nOH cohort, patients who were over 50 years old were excluded (n = 25); however, nearly all these patients (n = 24) also met other exclusion criteria and would have been excluded for other reasons. While this criterion was not applied in the PD cohort, only ∼2% of the patients were less than 50 years old. This study focused on medically attended falls, as those not requiring medical attention cannot be identified in administrative claims data. Therefore, the rate of falls overall and their associated costs are under-estimated; it is estimated that <20% of falls are medically attendedCitation24. Lastly, this analysis focused on burden specific to medical resource use and provides no insight into the relative impact of PD + nOH vs PD alone with respect to important outcomes such as patient quality-of-life, activities of daily living, and caregiver burden.
Conclusion
In conclusion, patients with PD + nOH have a higher prevalence of pre-existing comorbidities, and a higher rate of medically attended falls than those with PD alone, leading to increased direct fall-related and all-cause healthcare costs. Future studies may determine whether these patterns specific to medically attended falls persist after the introduction of additional or alternative OH treatments.
Transparency
Declaration of funding
This study was funded by Lundbeck, LLC. Xcenda, LLC received funding from Lundbeck to conduct this study and develop the manuscript.
Declaration of financial/other relationships
CF is an employee of Lundbeck, LLC, and AZ was an employee of Lundbeck, LLC, at the time of completion of the analyses and preparation of the manuscript. IB and CS are consultants for Lundbeck, LLC. AO, HCS, EF, and AD are employees of Xcenda, LLC.
Previous presentations
Presented at the International Parkinson and Movement Disorder Society Congress 2016 (June 2016) and World Parkinson Congress 2016 (September 2016).
Acknowledgments
Medical writing support was provided by Laurie Orloski, PharmD (independent medical writer) and funded by Xcenda.
References
- Arnold AC, Shibao C. Current concepts in orthostatic hypotension management. Curr Hypertens Rep 2013;15:304-12
- Goldstein DS, Sharabi Y. Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation 2009;119:139-46
- Maule S, Papotti G, Naso D, et al. Orthostatic hypotension: evaluation and treatment. Cardiovasc Hematol Disord Drug Targets 2007;7:63-70
- Metzler M, Duerr S, Granata R, et al. Neurogenic orthostatic hypotension: pathophysiology, evaluation, and management. J Neurol 2013;260:2212-19
- Goldstein DS. Orthostatic hypotension as an early finding in Parkinson’s disease. Clin Auton Res 2006;16:46-54
- Ha AD, Brown CH, York MK, et al. The prevalence of symptomatic orthostatic hypotension in patients with Parkinson’s disease and atypical parkinsonism. Parkinsonism Relat Disord 2011;17:625-8
- Perlmuter LC, Sarda G, Casavant V, et al. A review of orthostatic blood pressure regulation and its association with mood and cognition. Clin Auton Res 2012;22:99-107
- Vellas BJ, Wayne SJ, Romero LJ, et al. Fear of falling and restriction of mobility in elderly fallers. Age Ageing 1997;26:189-93
- Shibao C, Grijalva CG, Raj SR, et al. Orthostatic hypotension-related hospitalizations in the United States. Am J Med 2007;120:975-80
- Rodríguez-Blázquez C, Forjaz MJ, Lizán L, et al. Estimating the direct and indirect costs associated with Parkinson’s disease. Expert Rev Pharmacoecon Outcomes Res 2015;15:889-911
- Woodford H, Walker R. Emergency hospital admissions in idiopathic Parkinson’s disease. Mov Disord 2005;20:1104-8
- Stolze H, Klebe S, Zechlin C, et al. Falls in frequent neurological diseases—prevalence, risk factors and aetiology. J Neurol 2004;251:79-84
- Temlett JA, Thompson PD. Reasons for admission to hospital for Parkinson’s disease. Intern Med J 2006;36:524-6
- Low V, Ben-Shlomo Y, Coward E, et al. Measuring the burden and mortality of hospitalisation in Parkinson’s disease: a cross-sectional analysis of the English Hospital Episodes Statistics Database 2009–2013. Parkinsonism Relat Disord 2015;21:449-54
- Schrag A, Hovris A, Morley D, et al. Caregiver-burden in Parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord 2006;12:35-41
- Matinolli M, Korpelainen JT, Korpelainen R, et al. Orthostatic hypotension, balance and falls in Parkinson’s disease. Mov Disord 2009;24:745-51
- Desboeuf K, Grau M, Riche F, et al. Prevalence and costs of parkinsonian syndromes associated with orthostatic hypotension. Therapie 2006;61:93-9
- Bohl AA, Fishman PA, Ciol MA, et al. A longitudinal analysis of total 3-year healthcare costs for older adults who experience a fall requiring medical care. J Am Geriatr Soc 2010;58:853-60
- Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson’s disease: a systematic review. Parkinson’s Dis 2013;2013:906274
- Pickering RM, Grimbergen YA, Rigney U, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord 2007;22:1892-1900
- Rascol O, Perez-Lloret S, Damier P, et al. Falls in ambulatory non-demented patients with Parkinson’s disease. J Neural Transm (Vienna) 2015;122:1447-55
- François C, Hauser RA, Aballéa S, et al. Cost-effectiveness of droxidopa in patients with neurogenic orthostatic hypotension: post-hoc economic analysis of phase 3 clinical trial data. J Med Econ 2016;19:515-25
- Velseboer DC, de Haan RJ, Wieling W, et al. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2011;17:724-9
- Haines TP, Nitz J, Grieve J, et al. Cost per fall: a potentially misleading indicator of burden of disease in health and residential care settings. J Eval Clin Pract 2013;19:153-61
