The price of a life
In our first lesson together we used to ask to our students "what is the price of a life?". The answer was always "there is no price of a life". Although nice, it’s not true; unfortunately, the only costless man is a dead one.
In the last few years there has been a heated debate about cost and benefit in oncology, focused in particular on breast cancer screening and the availability and affordability of new drugs.
About breast-cancer screening
Breast cancer incidence has been decreasing in developed countries since the early 2000s, but only in post-menopausal women, mainly because of the decreases in endocrine treatments for menopauseCitation1, alongside usual decreases in developed-countries natural mortalityCitation2, even with disparities related to socioeconomic statusCitation1,Citation3.
A recent discussion about breast cancer screening in the USCitation4 focused on the never-ending story of screening cost-effectiveness based on different factors such as age of first screen and other technical dataCitation5,Citation6, the number of lives saved reported in the literatureCitation7–11, the rate of false positives, over-diagnosis, and consequent over-treatmentCitation12–16.
There are strong opinions in favor or against new/further researchCitation17,Citation18. In 2014, the Swiss Federal suggestionCitation11 was to stop any ongoing, and cancel any planned, screenings; however, not one Swiss canton has enforced this so far.
We believe that we do not need further research or data; rather, we need to look at how the different climates were established (e.g. Australia vs others)Citation19. Furthermore, when discussing death risk-reduction, we should take into account the role of advances in adjuvant treatmentsCitation20; it is clear that the best long-term outcomes in breast cancer so far are reported in patients with both an earlier diagnosis and the best adjuvant treatmentCitation21.
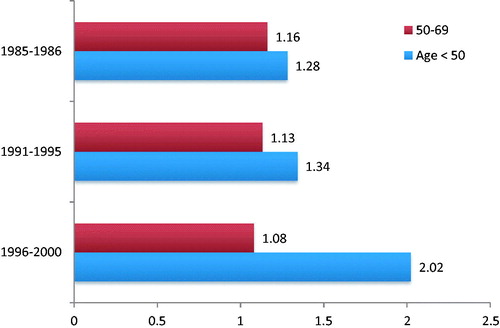
In cost-benefit evaluations we need a wider goal than just lives saved. Breast-cancer screening is very expensive, but can be used to also regulate related issues: shared protocols according to national guidelines for radiology, surgery (reconstructive surgery included), adjuvant treatments, rehabilitation, counseling. Furthermore, there are published data showing impressive improvement in 20-year breast cancer survival in screened lower socioeconomic women compared to more affluent women; the specific mortality of less affluent women, when screening for age, is doubledCitation22 ().
Figure 1. Hazard ratios (HR) of the 10-years breast cancer survival in lower socioeconomic vs affluent women stratified and adjusted for age, class, period, pT, and pN. Data from Puliti et al.Citation22.
The problem of investment in screenings is that the governments of today should pay for the benefits partially measurable by subsequent governments, which is true for breast cancer, but even more applicable for colorectal cancer screening; it can take 10–15 years for a polyp to degenerate into cancer, thus the decrease in cancer incidence and mortality rate are late resultsCitation23,Citation24.
It is unfortunate that there seems to be global agreement that screening policies are possible only in rich countriesCitation3,Citation10.
The economic situation and healthcare system of a given country play a pivotal role not only in economic decision-making, but also in overall health outcomes.
The price for drugs: to buy or not to buy? This is the question
I have had to sit down with my pharmacy and therapeutics committee and say “What am I going to not purchase in order to purchase these drugs?” (Otis Brawley MD, Emory University’s Winship Cancer Institute and Grady Hospital in Atlanta, cited by C Vanchieri)Citation25.
Many papers have been published about the cost-benefit evaluation in oncology, in particular in the adjuvant setting. The WHO defines the willingness-to-pay (WTP) threshold at 3-times the per capita gross domestic product (GDP)Citation26. Thus, the availability of life-saving therapies or drugs is different across different countries, and that is not what we would define as "socially correct"; one could imagine a direct relationship between the economic situation of a given country and drug availability. Actually, it is not always true, and the literature shows in some cases a wide gap between GDPCitation27 and the published "cost-effectiveness decision".
We can use adjuvant trastuzumab as an illuminating example. Adjuvant trastuzumab has been found cost-effective in the more affluent countries of Taiwan (GDP 2015 = $22,288)Citation28, Malaysia (GDP 2015 = $9,557)Citation29, and Singapore (GDP 2015 = $52,888)Citation30. In Colombia (GDP 2015 = $6,084) it was deemed not cost-effectiveCitation31. In Greece, currently under strong observation by the EU due to their risk of economic default, the GDP is $18,064, and both intravenous and subcutaneous formulations of trastuzumab were found to be cost-effective (s.c. more than i.v.)Citation32, the standard i.v. formulation being provided by the national health system.
Reading the above cited papers, one understands that there is a mix of data from private and public clinics, most of them based on modeled data from Phase III trials of adjuvant trastuzumab and applied to the given country; however, the Taiwan studyCitation28 was based on real world data.
The pivotal element driving effectiveness seems to be the cost payed by the patients themselves, and/or the unit cost of human resources, much lower than in western Europe or the US. Actually, 2015 GDP in Croatia and Egypt was $11,573 and $3500, respectivelyCitation27, and in both countries adjuvant trastuzumab is provided by the national healthcare system.
In conclusion, it seems the reliability of cost-benefit analyses is quite low, because of the variability of used models and in particular of the different cost for human resourcesCitation28–30. Fortunately for patients, adjuvant trastuzumab is given free of charge in many low-income countries, regardless of cost-effectiveness.
The equity matter
The US have outstanding quality of research and medical care on offer, but the healthcare system is definitely not equal. Even after "Obamacare" there are still 33 million Americans without health insurance (10.4%), 4.5 million of them childrenCitation33,Citation34. The risk of death for several cancers is significantly higher for insolvent patientsCitation35,Citation36, bankruptcy and high debt in cancer survivors are twice that of the overall populationCitation37, and there is growing concern about new-drug affordabilityCitation38. Access to care is unequal in many other countries, some in Europe, both high and low incomeCitation39. This is true not only for drugs, but also for other treatment strategies, for example, the rate of breast-conserving surgery and radiotherapy for very early breast cancer in the EU ranges from 20–64%, according to the amount of money spentCitation40.
Cost-benefit evaluation in the metastatic setting
For 1 month of palbociclib at online saleCitation41 the price ranges from US$10,309–$10,632 via Kroge Pharmacy, CVS, or Walgreens. According to the data of a Phase III trialCitation42,Citation43, the estimated cost per month-of-benefit could be ∼$15,000. The FDA granted accelerated approval, and annual sales of 3 billion dollars are estimated by 2020. Pfizer are currently running several programs for helping uninsured or low-income patients acquire the drug free of charge, or for as little $10 a monthCitation44. A cost-benefit analysis presented at ASCO 2016 showed that the sustainability for palbociclib in the Swiss healthcare system (GDP 2015 = $80,675) could be accepted only after the price of the drug was reduced by 75%Citation45. Probably the same reasoning could be applied to the similar "new entry" drug ribociclibCitation46.
Equity and solidarity (provided by the government and not by charities) should reflect a world one would offer to their family. In countries with a so far "socially equal" healthcare system, such as Canada, Italy, the UK, Switzerland, and France, the overall outcome is excellent as shown by indicators such as child mortality within the first 2-years and life expectancy of the elderly. Healthcare is expensive, but the overall population benefits.
It seems also that the best results in terms of survival rate in oncology are related to the amount of money spent on treatment, independently, by the health system, whether social insurance-based or national system-basedCitation47.
Both in high- and low-income countries health expenditure grows at a faster rate than the global economyCitation47, and there is no one country able to manage the costs without a radical revision of price definition with companies, like India did with Roche/Genentech regarding trastuzumabCitation48,Citation49. We suggest a recent editorial by SaltzCitation50 with appealing section titles such as "If You Don’t Know the Cost, You Can’t Know the Value", "The Disconnect Between Value and Cost", "Perverse Incentives", and "The Rules of the Game, and the Gaming of the Rules". These are not just titles, but insightful statements.
Conclusion
In our opinion, an affordable, ethically and politically correct budget for healthcare is government issued as part of a social healthcare system. In this context, all governments should declare how much is affordable in relationship with the economic situation of the country and decide what drugs and treatments can be provided for free (as part of a life-long system of national taxation) and which cannot. In 2005, in a health supplement of a large Italian economics newspaper, “Il Sole 24 Ore”, MustacchiCitation51 wrote
Every country has its own level of expense sustainability for a given topic. Health is probably the most important issue after freedom and hunger. Nevertheless, there is a border of sustainability, according to differing economic status (GDP). It is possible that Italy will soon be in need to define this border, which is a typical governmental duty.
Ten years later, unfortunately, there is still definitely a price for a life.
Giorgio Mustacchi and Daniele Generali University Clinical Dept of Medical, Surgical and Health Sciences, University of Trieste, Trieste, Italy [email protected]
Transparency
Declaration of funding
Nothing to declare.
Declaration of financial/other relationships
Nothing to declare.
References
- Kohler BA, Sherman RL, Howladeret N, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 2015;107:1-26
- Siegel R, DeSantis C, Virgo K, et al. Cancer Treatment and Survivorship Statistics, 2012. CA Cancer J Clin 2012;62:220-241
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-1717
- Saurabh J. The mammogram debate: how much should be spent to save one life? Medscape Oncol 2016;February 25th. Available from http://www.medscape.com/viewarticle/859234
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2016;164:279-96
- Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol 2010;7:18-27
- Tabár L, Vitak B, Chen TH, et al. Swedish Two-County Trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260:658-63
- Miller AB, Wall C, Baines CJ, et al. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ 2014;348:g366
- Prasad V, Lenzer J, Newman DH. Why cancer screening has never been shown to “save lives”—and what we can do about it. BMJ 2016;352:h6080
- Lauby‐Secretan B, Scoccianti C, Loomis D, et al, for the International Agency for Research on Cancer Handbook Working Group. Breast-cancer screening—Viewpoint of the IARC Working Group. N Engl J Med 2015;372:2353-2358
- Biller-Andorno N. A view from the Swiss medical board. N Engl J Med 2014;370:1965-7
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012;367:1998-2005
- Esserman LJ, Thompson IM, Reid B. Overdiagnosis and overtreatment in cancer. JAMA 2013;310:797-8
- Helvie MA, Chang JT, Hendrick RE, et al. Reduction in late-stage breast cancer incidence in the mammography era: implications for overdiagnosis of invasive cancer. Cancer 2014;120:2649-56
- Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014;311:1327-35
- Ong MS, Mandl KD. National expenditure for false-positive mammograms and breast cancer overdiagnoses estimated at $4 billion a year. Health Aff (Millwood) 2015;34:576-83
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012;380:1778-86
- Gøtzsche PC, Jørgensen KJ, Donzelli A, et al. Comments to: The benefits and harms of breast cancer screening Lancet 2013;381:799-800
- BreastScreen Australia Evaluation Taskforce. BreastScreen Australia Evaluation. Evaluation final report. Canberra: Australian Government Department of Health and Ageing; 2009. Report No.: Screening Monograph No.1/2009. http://www.health.gov.au/internet/screening/publishing.nsf/content/8463830B90E5BDF5CA25. Last accessed February 17, 2016
- Birnbaum J, Gadi VK, Markowitz E, et al. The effect of treatment advances on the mortality results of breast cancer screening trials: a microsimulation model. Ann Intern Med 2016;164:236-43
- Berry DA, Cronin KA, Plevritis SK, et al., for the Cancer Intervention and Surveillance Modeling Network (CISNET). Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784-92
- Puliti D, Miccinesi G, Manneschi G, et al. Does an organised screening programme reduce the inequalities in breast cancer survival? Ann Oncol 2012;23:319-23
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006;30:1491-1501
- Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging 2016;11:967-76
- Vanchieri C. When will the US flinch at cancer drug prices? J Natl Cancer Inst 2005;97:9
- Murray CJ, Evans DB, Acharya A, et al. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ 2000;9:235-51
- https://knoema.com/sijweyg/gdp-per-capita-ranking-2015-data-and-charts. Accessed July 17, 2016
- Hui-Chu L, Hsiao-Wei C, Tzeon-Jye C, et al. The real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu positive early breast cancer in Taiwan. J Med Econ19:923-927
- Wei Ching L, Mohd Roslan H, Kong Leong Y, et al. Economic analysis of intravenous vs subcutaneously administered trastuzumab for the treatment of HER2+ early breast cancer in Malaysia. Adv Breast Cancer Res 2016;5:1-13
- de Lima Lopes Jr G. Societal costs and benefits of treatment with trastuzumab in patients with early HER2neu-overexpressing breast cancer in Singapore. BMC Cancer 2011;11:178
- Buendía JA, Vallejos C, Pichón-Rivière A. An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia. Biomedica 2013;33:411-17
- Mylonas C, Kourlaba G, Fountzilas G, et al. Cost-minimization analysis of trastuzumab intravenous versus trastuzumab subcutaneous for the treatment of patients with HER2+ early breast cancer and metastatic breast cancer in Greece. Value Health 2014;17:A640-1
- Barry AM, Casselman B. 33 Million Americans still don’t have health insurance. Here’s who they are. Report on public health, food and culture for FiveThirtyEight. Sep 28, 2015. Available from https://fivethirtyeight.com/features/33-million-americans-still-dont-have-health-insurance/; accessed on Feb 17th, 2016
- de Souza JA, Hunt B, Asirwa FC, et al. Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. J Clin Oncol 2015;34:6-13
- Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer (USA). J Clin Oncol. 2016;34:980-6
- Huntington SF, Weiss BM, Vogl DT, et al. Financial toxicity in insured patients with multiple myeloma: a cross-sectional pilot study. Lancet Oncol 2015;2:e408-e416
- Banegas MP, Guy GP Jr, de Moor JS, et al. Cost of cancer care for working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Aff 2016;35:154-61
- Bach PB. New math on drug cost-effectiveness. N Engl J Med 2015;373:1797
- Cherny N, Sullivan R, Torode J et al. ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe.Ann Oncol. 2016;27:1423-43
- Allemani C, Storm H, Voogd AC, et al. Variation in ‘standard care’ for breast cancer across Europe: A EUROCARE-3 high resolution study. Eur J Cancer. 2010;46:1528-36
- GoodRX, online sale of drugs. http://www.goodrx.com/palbociclib. Accessed July 17, 2016
- Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 2015;373:209-19
- Cristofanilli M, Turne NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425-39
- IBRANCE. https://www.ibrance.com/getting-ibrance. Accessed July 17, 2016
- Matter-Walstra K, Schwenkglenks M, Brauchli P, et al. A cost-effectiveness analysis of palbociclib plus letrozole as first-line treatment for estrogen receptor-positive, HER2-negative, metastatic breast cancer. J Clin Oncol 2016;34(suppl):abstr 567
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738-48
- Gatta G, Trama A, Capocaccia R. Variations in cancer survival and patterns of care across Europe: roles of wealth and health-care organization. JNCI Monographs 2013;46:79-87
- Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol 2011;12:933-80
- Ruff P, Al-Sukhun P, Blanchard C, et al. Access to cancer therapeutics in low- and middle-income countries. ASCO Educational Book. asco.org/edbook; 2016;58:65
- Saltz LB. The value of considering cost, and the cost of not considering value. J Clin Oncol 2016;34:659-60
- Mustacchi G. L'Italia sta per affrontare il bivio costi-opportunitá e il rebus sostenibilità: la decisione sia di chi governa. Il Sole 24 Ore. Health Suppl to n49; Dec 20th 2005
