Abstract
Objective: To assess the cost-effectiveness of panitumumab in combination with mFOLFOX6 (oxaliplatin, 5-fluorouracil, and leucovorin) vs bevacizumab in combination with mFOLFOX6 as first-line treatment of patients with wild-type RAS metastatic colorectal cancer (mCRC) in Spain.
Methods: A semi-Markov model was developed including the following health states: Progression free; Progressive disease: Treat with best supportive care; Progressive disease: Treat with subsequent active therapy; Attempted resection of metastases; Disease free after metastases resection; Progressive disease: after resection and relapse; and Death. Parametric survival analyses of patient-level progression free survival and overall survival data from the PEAK Phase II clinical trial were used to estimate health state transitions. Additional data from the PEAK trial were considered for the dose and duration of therapy, the use of subsequent therapy, the occurrence of adverse events, and the incidence and probability of time to metastasis resection. Utility weightings were calculated from patient-level data from panitumumab trials evaluating first-, second-, and third-line treatments. The study was performed from the Spanish National Health System (NHS) perspective including only direct costs. A life-time horizon was applied. Probabilistic sensitivity analyses and scenario sensitivity analyses were performed to assess the robustness of the model.
Results: Based on the PEAK trial, which demonstrated greater efficacy of panitumumab vs bevacizumab, both in combination with mFOLFOX6 first-line in wild-type RAS mCRC patients, the estimated incremental cost per life-year gained was €16,567 and the estimated incremental cost per quality-adjusted life year gained was €22,794. The sensitivity analyses showed the model was robust to alternative parameters and assumptions.
Limitations: The analysis was based on a simulation model and, therefore, the results should be interpreted cautiously.
Conclusions: Based on the PEAK Phase II clinical trial and taking into account Spanish costs, the results of the analysis showed that first-line treatment of mCRC with panitumumab + mFOLFOX6 could be considered a cost-effective option compared with bevacizumab + mFOLFOX6 for the Spanish NHS.
Introduction
Worldwide, colorectal cancer (CRC) is the third most common diagnosed cancer in men and the second in womenCitation1. In 2012, 1.4 million cases of CRC were estimated, where Australia/New Zealand, Europe, and North America had the highest regional incidenceCitation1. In Spain, CRC is the cancer with the highest incidence (15% of the total incidence of cancer), presenting 32,240 new cases annuallyCitation2,Citation3. In line with the global trend, Spanish men have a higher incidence than women, with an age-standardized rate of 43.9 and 24.2 cases per 100,000 persons, respectivelyCitation2,Citation3.
In Spain, CRC causes 14,700 deaths each year and is the second most common cause of cancer deaths, with an age-standardized mortality rate of 17.1 and 8.4 cases per 100,000 persons in men and women, respectivelyCitation2,Citation3. The high mortality of CRC is related to the large number of patients with metastatic CRC (mCRC) (25% at diagnosis)Citation4. It is calculated that 50% of CRC patients at earlier stages will develop metastasesCitation4. Furthermore, it is estimated that patients with mCRC have a total unadjusted prevalence of RAS mutation of 55.9%Citation5.
In 2015, Corral et al.Citation6 published a study carried out in Catalonia (Spain) where hospital costs associated with CRC were assessed. In this study, a cohort of 699 patients diagnosed with CRC were retrospectively analysed, identifying mean costs per patient by stage at diagnosis according to the American Joint Committee on Cancer classification (in situ, I, II, III, IV, or unknown), cost type (hospitalization, outpatient, chemotherapy, etc.), and disease phase (initial, monitoring, or advanced). The mean direct cost per patient with CRC increased from €6,573 in patients with cancer in situ to €36,894 in patients with disease in stage III (€2005)Citation6.
The clinical implementation of the most appropriate treatment for mCRC is complex due to the heterogeneity of the disease, since it is caused by the interaction of genetic and environmental factors and because a major part of the genes involved in CRC are perceived in a small proportion of casesCitation7. When choosing treatment, the characteristics of the patient, the tumor, and the treatment to be administered must be consideredCitation8. Molecular biology has allowed targeting the treatment of mCRC and has increased survival in these patientsCitation7. The determination of the molecular biomarkers predictive of response to treatment enables the choice of the therapy that may be more effective. Thus, less suitable therapies that are associated with undesirable costs and effects can be avoided. In the management of mCRC, anti-epidermal growth factor receptor (EGFR) monoclonal antibodies are only indicated in patients with wild-type RASCitation8,Citation9.
Panitumumab and bevacizumab are monoclonal antibodies. Panitumumab acts by binding to the external domain of EGFR and competing with its natural ligandsCitation10 and bevacizumab is an inhibitor of vascular endothelial growth factorCitation11. Both drugs in combination with chemotherapy are indicated as first-line treatment of adult patients with wild-type RAS mCRC in clinical practice guidelines and the recommendations of Spanish (Spanish Society of Medical Oncology) and European [European Society for Medical Oncology (ESMO)] scientific societies and the current literatureCitation4,Citation12–15.
These recommendations have been updated as a result of the evidence observed in recent studies where not only KRAS exon 2 mutations, but also KRAS mutations in exons 3 and 4 and NRAS mutations in exons 2, 3, and 4, are considered negative predictors of treatmentCitation4,Citation9,Citation15. Accordingly, current ESMO guidelines on mCRC indicate that RAS testing should be carried out in all patients at diagnosis and is mandatory before treatment with EGFR monoclonal antibodies. It should include at least KRAS exons 2, 3, and 4 (codons 12, 13, 59, 61, 117, and 146) and NRAS exons 2, 3, and 4 (codons 12, 13, 59, 61, and 117)Citation4,Citation15.
The PEAK Phase II clinical trial showed that patients with wild-type RAS (KRAS and NRAS in exons 2, 3, and 4) treated with panitumumab plus mFOLFOX6 chemotherapy regimen (panitumumab + mFOLFOX6) for the first-line treatment of mCRC had greater progression-free survival (PFS) compared with patients treated with bevacizumab plus mFOLFOX6 (bevacizumab + mFOLFOX6) [13.0 months vs 9.5 months; hazard ratio (HR) = 0.65; 95% confidence intervals (CI) = 0.44–0.96; p = .029]Citation16.
Cost-effectiveness analysis (CEA) is one of the main tools in health economic evaluation that allow the analysis of both the cost of a specific medical intervention and the therapeutic or social value a drug providesCitation17,Citation18. At present, some of the available CEA between these two treatments are now outdated, as they analysed only patients with wild-type KRASCitation19. Consequently, they did not consider clinical outcomes in the current population for which panitumumab is indicated, namely patients with wild-type RASCitation19. Moreover, few published CEA have evaluated wild-type RAS population, and none of them considered the Spanish National Health System (NHS) perspectiveCitation20–23.
The aim of this study was to evaluate the efficiency of panitumumab + mFOLFOX6 vs bevacizumab + mFOLFOX6 as first-line treatment of patients with wild-type RAS mCRC in the Spanish setting.
Patients and methods
Population
The model analysed a subset of patients with wild-type RAS from the PEAK clinical trial, the only available trial that evaluated the first-line treatment of mCRC with panitumumab + mFOLFOX6 vs bevacizumab + mFOLFOX6. The subset included patients aged ≥18 years diagnosed with wild-type RAS mCRC who had not received prior chemotherapy, anti-EGFR therapy, or bevacizumab therapyCitation16.
Comparators
The mCRC treatment comparators were:
Panitumumab administered every 2 weeks plus mFOLFOX6 chemotherapy regimen.
Bevacizumab administered every 2 weeks plus mFOLFOX6 chemotherapy regimen.
The mFOLFOX6 chemotherapy regimen for both treatment options was that used in the PEAK trialCitation16: oxaliplatin administered on day 1 of each cycle as an intravenous infusion of 85 mg/m2; 5-fluorouracil administered on days 1 and 2 of each cycle as an intravenous bolus of 400 mg/m2 on day 1, followed by a 2,400 mg/m2 intravenous infusion administered over 46 h on day 2; and leucovorin 400 mg/m2 administered on day 1 of each cycle. It was considered that patients were treated with this chemotherapy regimen every two weeks after receiving treatment with panitumumab or bevacizumab.
Type of analysis
The efficiency of panitumumab + mFOLFOX6 vs bevacizumab + mFOLFOX6 as first-line treatment was evaluated using a CEA of the data from the primary analysis of the PEAK trialCitation16. A semi-Markov model programmed in Microsoft Excel and adapted to the Spanish context was used to compare the effects on health and the costs associated with the two therapeutic options. This model has been used previously in other published economic evaluations between these two treatments in the settings of the Czech Republic, Greece, France, and MexicoCitation20–23. Semi-Markov models allow us to consider time dependency for state transitions; whereas in standard Markov models the state transitions remain constant over time, since they are based on the premise that state transitions are independent of any knowledge from the past (such as time since entry into a certain health state)Citation24–29. The incremental cost-effectiveness ratio (ICER) was calculated using the following equation:
where CostPmab and CostBmab represent the costs associated with treatment with panitumumab and bevacizumab in combination with mFOLFOX6, respectively, while EffectivenessPmab and EffectivenessBmab represent the clinical consequences in terms of quality-adjusted life years (QALY) and life-years gained (LYG). The indicators of cost effectiveness were the incremental cost per QALY gained with panitumumab + mFOLFOX6 vs bevacizumab + mFOLFOX6, and per LYG.
The perspective of the Spanish NHS was used, including only direct healthcare costs (indirect costs were not considered). The time horizon was the lifetime of patients with mCRC (assuming a maximum of 20 years of life after the start of treatment). Costs were expressed in 2015 euros and an annual discount rate of 3% for both costs and health outcomes was applied, in line with Spanish health economic evaluation recommendationsCitation17.
Model description
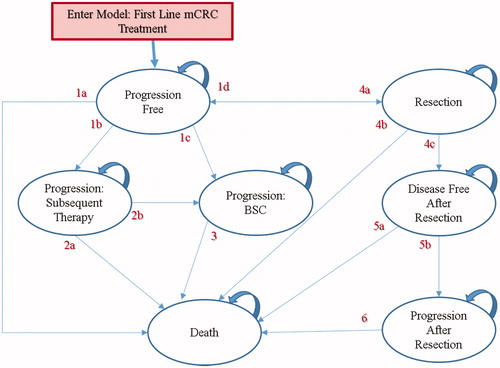
The model simulated a cohort of patients from the initiation of first-line treatment of mCRC until death. The model consists of mutually-exclusive health states, with patients transitioning from one state to another, simulating the course of the disease: Progression free; Progressive disease: treat with best supportive care (BSC); Progressive disease: treat with subsequent active therapy; Attempted resection of metastases; Disease free after metastases resection; Progressive disease: after resection and relapse; and Death (). Patients entered the model in the progression free state and were randomly assigned to first-line treatment with panitumumab + mFOLFOX6 or bevacizumab + mFOLFOX6. When the disease progressed after first-line treatment, patients were treated with an active second-line treatment or BSC. It was assumed that as active second-line treatment patients would receive the monoclonal antibody not received in first-line, combined with FOLFIRI chemotherapy regimen (irinotecan, 5-fluorouracil, and leucovorin). The model also included resection of tumor metastases in eligible patients.
Figure 1. Semi-Markov model structure. BSC: best supportive care; mCRC: metastatic colorectal cancer. 1a: Progression Free to Death; 1b: Progression Free to Progressive Disease: Treat with Subsequent Active Therapy; 1c: Progression Free to Progressive Disease: Treat with Best Supportive Care; 1d: Progression Free to Attempted Resection of Metastases; 2a: Progressive Disease: Treat with Subsequent Active Therapy to Death; 2b: Progressive Disease: Treat with Subsequent Active Therapy to Progressive Disease: Treat with Best Supportive Care; 3: Progressive Disease: Treat with Best Supportive Care to Death; 4a: Attempted Resection of Metastases to Progression Free; 4b: Attempted Resection of Metastases to Death; 4c: Attempted Resection of Metastases to Disease Free After Metastases Resection; 5a: Disease Free After Metastases Resection to Death; 5b: Disease Free After Metastases Resection to Progressive Disease: After Resection and Relapse; 6: Progressive Disease: After Resection and Relapse to Death.
The duration of the Markov cycles (time in which patients remain in a specific health state) in the model was 2 weeks, which corresponds with the frequency of administration of the monoclonal antibody in wild-type RAS patients in the PEAK trial. At the end of each cycle patients transitioned to another health state or remained in the same state, depending on the transition probabilities described below.
Transition probabilities
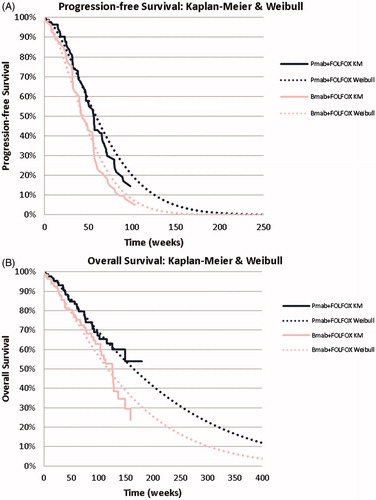
Transition probabilities for the states of disease progression and death for the two study options were estimated according to the parametric extrapolation of data from the PEAK trialCitation16 using a Weibull distribution. This distribution was selected as the best-fit to the Kaplan-Meier curves, based upon both the Akaike information criterion and the graphic representation (). The parametric survival modeling was coded in SAS (version. 9.3; Cary, NC) using the LIFEREG procedure.
Figure 2. Survival curves. (a) Progression-free survival; (b) Overall survival. Bmab: bevacizumab; Pmab: panitumumab.
The probabilities of the PEAK trial related to resection were also incorporated; attempted resection (13.64% of patients with panitumumab and 10.98% of patients with bevacizumab) and successful resection (66.7% for panitumumab and 77.8% for bevacizumab)Citation20. In patients with successful resection, probabilities for PFS and overall survival (OS) were extrapolated from the data in the study by Adam et al.Citation30 using the log-logistic distribution that best adjusted to the parametric curves.
When disease progressed after first-line treatment, patients received active second-line treatment (61.4% in the panitumumab group, 69.5% in the bevacizumab group) or BSC (38.6% for panitumumab, 30.5% for bevacizumab)Citation31. Based on the PEAK trial, this transition was calculated taking into account the likely progression in each cycle according to the percentage of patients receiving each type of treatment. In the context of this analysis, it was considered that the second-line therapies received did not affect OS, and only had an impact on costs and the quality-of-life. The duration of active second-line treatment was determined from the PFS shown in the literatureCitation32,Citation33. Upon disease progression, the model assumed that patients received BSC until death.
Costs
The direct costs evaluated were: drug costs, cost of drug administration, and other medical costs (medical visits, diagnostic tests, adverse events, resection, and end of life). The costs and resource use included in the model were validated by Spanish clinical experts in mCRC in order to be consistent with Spanish clinical practice. Thus, inputs provided included active subsequent lines, BSC, resection of metastasis, medical visits, management of adverse events, end of life costs and utilities.
Drug cost and cost of administration
Drug costs for panitumumab and bevacizumab were expressed as ex-factory price (wholesale price) including mandatory discounts according to Royal Decree Law 8/2010 (an urgent measure enacted by the Spanish Government to control public spending)Citation34 (). Both the total consumption of vials (mean number of vials used and treatment cycles per patient) and the doses used were from the PEAK study. After model adaptation to routine clinical practice in Spain, an analysis per milligram was carried as base caseCitation35,Citation36.
Table 1. Drug acquisition and administration costs.
Drug costs associated with chemotherapy were established according to the use observed in the PEAK trial of mFOLFOX6 as frontline therapy and for subsequent lines with FOLFIRI other studies were usedCitation32,Citation33. The cost of chemotherapy administration was estimated according to Spanish day hospital costsCitation37.
Drug costs associated with BSC were defined considering Spanish clinical practice and were validated by the clinical experts consulted. BSC drug costs included were the cost of painkillers (morphine, metamizole, fentanyl, tramadol, and midazolam), anti-cachexia agents (megestrol), anti-emetics (ondansetron), steroids (dexamethasone and prednisone), non-steroidal anti-inflammatory drugs (ibuprofen and naproxen), and low molecular weight heparin (tinzaparin).
Other medical costs
Other medical costs included in the model were: cost of RAS testing, cost of outpatient visits, cost of diagnostic tests, cost of serious adverse events management, and cost of resection ().
Table 2. Incidence/costs of serious adverse events and other medical costs.
Utilities
The impact of the disease development on the quality-of-life was assessed using utilities, through the different health states in the model. Utilities were expressed on a numerical scale from 0 (death) to 1 (perfect health) and based on the EuroQol-5 Dimension (EQ-5D) questionnaire. EQ-5D responses from patients with wild-type RAS from the PRIME studyCitation20,Citation38 were used for the progression-free state (since PEAK trial did not collect this data) and calculated according to Dolan’sCitation39 algorithm (). Given the lack of specific data for patients with wild-type RAS, for subsequent lines of treatment the utilities observed in trials of panitumumab as second- and third-line treatment in patients with KRAS were usedCitation20,Citation32,Citation40. Like other previous studiesCitation20, it was assumed that the utility of patients with wild-type RAS was the same to that reported by patients with KRAS. Thus, for active treatment, the utility observed in trials of panitumumab as second-line line treatment was usedCitation32, for BSC, the utility observed in trials of third-line treatmentCitation40, and the mean of these two utilities was used for the utility associated with recurrence of the disease. The utilities used in the model were validated by local clinical experts in mCRC.
Table 3. Utility weight values.
Sensitivity analysis
Scenario analyses were made after variation of the following parameters: adjustment of the drug cost per vial (wastage of vial remainder) and tolerance (using a new vial only if the calculated dose exceeded 30 mg); the assumption that all patients received BSC after progression of the disease in subsequent lines of therapy; no consideration of the drug costs, BSC, and end of life costs after disease progression in later lines of treatment; reduction of the cost of bevacizumab by 7.5%, 10%, 12.5%, 15%, 17.5%, and 20%.
Additionally, a probabilistic sensitivity analysis (PSA) was run analyzing 10,000 iterations through a Monte Carlo simulation where all parameters evaluated in the model were modified simultaneously. A normal multivariate distribution was assigned for the PFS and OS curves, a normal distribution for the number of vials consumed and the mean number of treatments observed, a Dirichlet distribution for distribution of subsequent therapy, a beta distribution for the probabilities of toxicity and utilities, and a gamma distribution for costs and hospital days.
Results
The results of the CEA showed that first-line treatment of RAS wild type mCRC with panitumumab + mFOLFOX6, based on efficacy from the PEAK trial, was more effective than bevacizumab + mFOLFOX6, with a QALY gain of 2.753 and 2.107, respectively (). Treatment with panitumumab had a higher overall cost than with bevacizumab: €72,203 and €57,485, respectively. The ICER between treatments was €22,794/QALY gained.
Table 4. Base case results of the cost-effectiveness analysis.
Panitumumab + mFOLFOX6 resulted in more LYG vs bevacizumab + mFOLFOX6: 3.685 and 2.796, respectively, resulting in an incremental cost per LYG of €16,567.
The majority of the assessed scenarios showed an ICER below €30,000/QALY gained, reaching a maximum of €31,842/QALY gained in the scenario that considered that all patients received BSC in subsequent lines of treatment, therefore demonstrating the robustness of the results of the base case ().
Table 5. Scenario analyses results.
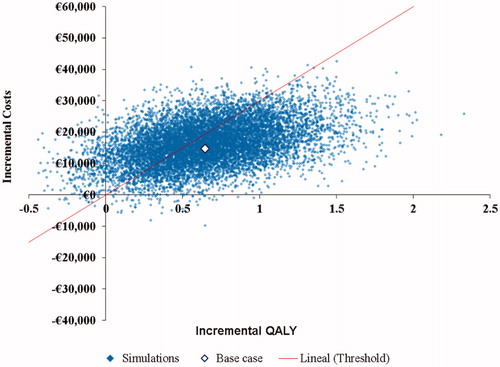
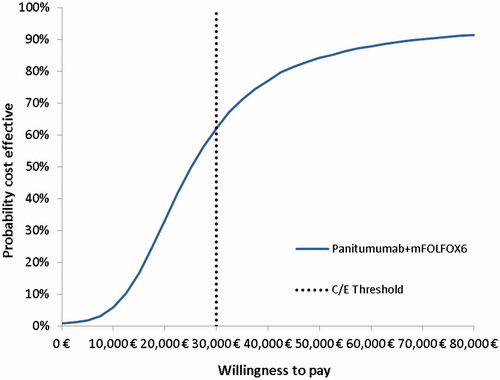
The PSA results showed that the ICER between treatments was below €30,000/QALY gained in 62% of the generated iterations ( and ).
Discussion
In recent years, the high levels of mortality of CRC have been reduced as a result of the incorporation of new treatment alternatives, the individualization of treatment with the use of molecular biomarkers, the introduction of screening programs, and reductions in the prevalence of risk factorsCitation1,Citation8. In order to improve the current management of the disease, it is essential to incorporate in the treatment paradigm the options that proved effectiveness, safety, and efficiency of treatmentCitation1,Citation8,Citation41.
Based on these grounds, this CEA took into account recent information on the use of anti-EGFR therapies in patients with wild-type RAS, comparing first-line treatment with panitumumab vs bevacizumab using data from the PEAK trial, as best available evidenceCitation16. The results showed that panitumumab + mFOLFOX6 provided increased survival and more QALY gained than treatment with bevacizumab + mFOLFOX6 (increase of 0.646). Although panitumumab + mFOLFOX6 had a higher total cost than bevacizumab + mFOLFOX6 (difference of €14,718), this increase in cost was due to a greater PFS in the PEAK trial, hence greater drug costsCitation16, together with a greater percentage of patients undergoing tumor resectionCitation16, which increased cost of treatment.
When health outcomes and costs were compared, first-line management of mCRC with panitumumab + mFOLFOX6 may be considered a cost-effective option vs bevacizumab + mFOLFOX6 in the Spanish setting, as the ICER of €22,794/QALY gained is below the commonly accepted threshold of efficiency in Spain (≤30,000€/QALY gained)Citation42,Citation43. Furthermore, the incremental cost per LYG was €16,567. The robustness of these results was confirmed in the PSA, where panitumumab + mFOLFOX6 was cost-effective from the Spanish NHS perspective (ICER ≤€30,000/QALY) in 62% of cases. Besides, the sensitivity analysis of scenarios evaluating the uncertainty of the parameters included in the model (such as variation in the consumption of monoclonal antibodies, which may depend on the patient's tolerance of treatment or the optimization of the vials during administrationCitation35,Citation36) confirmed the robustness of the results. Even in the scenario that considered a 20% reduction of the cost of bevacizumab, the first-line treatment with panitumumab remained as a cost-effective option within the Spanish NHS.
The results obtained are in line with current literature analyzing the efficiency of the first-line management of mCRC with anti-EGFR compared with bevacizumab in patients with wild-type RASCitation20–23,Citation44. For example, the study by Graham et al.Citation20, made from the French NHS perspective, showed that treatment with panitumumab + mFOLFOX6 was cost-effective at a willingness-to-pay of between €40,000–€60,000/QALY gained. This was a result of a greater number of QALY gained vs bevacizumab + mFOLFOX6, 2.68 vs 2.05, respectively, together with a higher total cost due largely to increased survival. Similar conclusions highlighting the improved efficiency of panitumumab have been demonstrated in studies from the Czech RepublicCitation21, GreeceCitation23, and MexicoCitation22, which used the same model as that of the current analysis. All these studies were based on PEAK first-line results and included the same health states, disease evolution rationale, and cost-effectiveness outcome (cost per LYG and cost per QALY)Citation20,Citation21,Citation23. Furthermore, all the studies were adapted to the characteristics of each setting, including treatments received as second-line, costs, and temporal discountsCitation20–23. As a result, comparison of the results obtained should be made with caution.
Other publications have also evaluated the economic consequences of panitumumab for the first-line treatment of mCRC in combination with FOLFOX in patients with wild-type RAS vs cetuximab plus FOLFIRICitation45. In a cost minimization analysis carried out in the US setting, panitumumab resulted in savings of $23,125 (11.4%) in comparison with cetuximab, due to the lower acquisition and management costs and a lower cost of adverse eventsCitation45. This is in line with another Spanish study in patients with wild-type RAS, with first-line treatment with panitumumab resulting in savings of €5,801 (18%) compared with treatment with cetuximab, both in combination with FOLFOX4Citation46. Although cetuximab was not the comparator in the current analysis, these results show the cost differences between these two EGFR monoclonal antibodies, and suggest that the results of the present analysis cannot be extrapolated to cetuximab.
Other cost-effectiveness studies have examined panitumumab vs bevacizumab in first-line mCRC, but only in wild-type KRAS populations, which are not the current populations for which panitumumab is indicatedCitation19,Citation47,Citation48. As explained previously, RAS testing is mandatory before treatment initiation with EGFR targeted monoclonal antibodies, since only wild-type RAS patients can be treated with panitumumabCitation9,Citation10. Therefore, it is critically important to have economic evaluations in this population. Thus, in light of the latest available evidence, these analyses in wild-type KRAS populations would be less relevant, given that the determination of RAS is required for panitumumab administration in its current indicationCitation9,Citation10. In addition, these studies are not comparable with the current analysis, as the efficacy evaluated did not come from directly-comparable clinical trials, and the studies also included different health states and time horizons. Similarly, they cannot be extrapolated to the Spanish context given the large variability in drug costs between countriesCitation49 and the differences in the cost categories included and the therapeutic algorithms applied.
Currently, the indication of anti-EGFR is subject to the state of the RAS gene. This is of such importance that some major international drug agencies that include economic evaluation in their assessments are updating their clinical practice guidelines on this basisCitation50,Citation51. Consequently, the present study represents an advance in the evaluation of the efficiency associated with the first-line management of mCRC with panitumumab, given the current need for studies in this areaCitation52,Citation53.
As noted before, an increase in long-term survival in patients with mCRC has been observed in recent evidenceCitation1,Citation7,Citation8,Citation54. In consequence, the current analysis considered a lifetime horizon of 20 years. This time horizon was considered as appropriate to collect the main clinical and economic outcomes related to the disease evolution, due to the fact that Markov modeling simulation showed that 99% of the patients in each arm would be deceased by this time.
The study has some limitations. First, the inherent limitations of a simulation model, which means the results should be interpreted with caution. Nonetheless, this model has been used in other mCRC studiesCitation20, and, furthermore, the use of simulation models is one of the only alternatives available to represent the natural history of a disease and assess the long-term impact of interventions, and they have also been widely used to study the efficiency of therapies available for the management of mCRCCitation19,Citation47,Citation48,Citation55. To overcome this limitation, the model was adapted to Spanish clinical practice based on the available literature and validated with clinical experts in the treatment of mCRC in Spain. Due to the Spanish cost perspective and considering the large variability in drug costs between countries, the results of this CEA cannot be directly extrapolated to other settings, such as the USCitation49.
Another limitation might be the use of a phase 2 clinical trial (PEAK). However, this was the best available evidence and the only available direct comparison between the study treatments for the studied indication. CEAs in other oncological diseasesCitation56 have also been based on phase II trials, although the majority are not. Therefore, further studies including phase III results are desirable. Nevertheless, some recent indirect comparisons have shown results in line with those of the PEAK trial, where panitumumab had greater efficacyCitation14,Citation57. Khattak et al.Citation14 found that patients with wild-type RAS mCRC who received first-line with EGFR monoclonal antibodies vs VEGF had longer OS with a HR of 0.77 (95% CI = 0.63–0.95; p = .016), whereas the HR in the meta-analysis by Pietrantonio et al.Citation57 was 0.80 (95% CI = 0.69–0.92; p = .003).
This CEA did not model effectiveness in OS associated with subsequent therapy lines. Although it is expected that newer therapies would increase the total cost of treatment, it would not modify the conclusions of the present analysis given the first-line focus and the fact that it will affect both arms.
Conclusions
In conclusion, based on the PEAK Phase II clinical trial and taking into account Spanish costs, the results of this analysis showed that first-line treatment of mCRC with panitumumab + mFOLFOX6 could be considered a cost-effective treatment option vs bevacizumab + mFOLFOX6 for the Spanish NHS.
Transparency
Declaration of funding
The study was financed by AMGEN, S.A. AMGEN, S.A. carried out the analysis with the help of Oblikue Consulting, which received financing from AMGEN, S.A.
Declaration of financial/other relationships
FR and MV participated as independent assessors and have received honoraria from AMGEN for participation in local and international advisory boards. SG works for AMGEN, S.A. NLM works for Oblikue Consulting. The other authors report no conflicts of interest. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
The authors would like to acknowledge Miriam Fernández from AMGEN, S.A. and Ferran Pérez from Oblikue Consulting for their support and insights during all the stages of this project.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108
- Sociedad Española de Oncología Médica (SEOM). Las cifras del cáncer en España 2014. Madrid: SEOM, 2014. www.seom.org/seomcms/images/stories/recursos/Las_cifras_del_cancer_2014.pdf. Accessed August 6, 2015
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer, 2013. globocan.iarc.fr/Default.aspx. AccessedAugust 6, 2015
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(Suppl 3):iii1-9
- Peeters M, Kafatos G, Taylor A, et al. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: A pooled analysis of randomised controlled trials. Eur J Cancer 2015;51:1704-13
- Corral J, Borràs JM, Chiarello P, et al. Estimation of hospital costs of colorectal cancer in Catalonia. Gac Sanit 2015;29:437-44
- Binefa G, Rodríguez-Moranta F, Teule A, et al. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol 2014;20:6786-808
- Vera R, Alonso V, Gállego J, et al. Current controversies in the management of metastatic colorectal cancer. Cancer Chemother Pharmacol 2015;76:659-77
- Navarro S, Musulén-Palet E, Cuatrecasas M, et al. Actualización de la recomendación para la determinación de biomarcadores en el carcinoma colorrectal. Consenso Nacional de la Sociedad Española de Anatomía Patológica y de la Sociedad Española de Oncología Médica. Rev Esp Patol 2015;48:14-24
- Vectibix® Summary of product characteristics. London: European Medicines Agency, 2015. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000741/WC500047710.pdf. Accessed August 6, 2015
- Avastin® Summary of product characteristics. London: European Medicines Agency, 2015. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf. Accessed August 6, 2015
- Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479-516
- Casado-Saenz E, Feliu J, Gomez-España MA, et al. SEOM clinical guidelines for the treatment of advanced colorectal cancer 2013. Clin Transl Oncol 2013;15:996-1003
- Khattak MA, Martin H, Davidson A, et al. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer 2015;14:81-90
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422
- Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014;32:2240-7
- López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac Sanit 2010;24:154-70
- Puig J, Oliva J, Trapero M, et al. Guía y recomendaciones para la realización y presentación de evaluaciones económicas y análisis de impacto presupuestario de medicamentos en el ámbito del CatSalut. Barcelona: Generalitat de Catalunya, Departament de Salut, Servei Català de la Salut, 2014. catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/medicaments_farmacia/farmaeconomica/caeip/documents/gaeip_publica_castellano_octubre2014_catsalut.pdf. Accessed June 22, 2015
- Lawrence D, Maschio M, Leahy KJ, et al. Economic analysis of bevacizumab, cetuximab, and panitumumab with fluoropyrimidine-based chemotherapy in the first-line treatment of KRAS wild-type metastatic colorectal cancer. J Med Econ 2013;16:1387-98
- Graham CN, Hechmati G, Hjelmgren J, et al. Cost-effectiveness analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer 2014;50:2791-801
- Fínek J, Skoupá J, Jandová P. Cost effectiveness analysis of panitumumab plus mFOLFOX6 compared to bevacizumab plus mFOLFOX6 for first-line treatment of patients with wildtype RAS metastatic colorectal cancer - Czech Republic model adaptation. Klin Onkol 2015;28:265-72
- Vargas JJ, Alva ME. Cost-effectiveness analysis of panitumumab + FOLFOX in RAS-WT mCRC. GAMO 2015;14:250-8
- Kourlaba G, Boukovinas I, Saridaki Z, et al. Cost-effectiveness analysis of panitumumab + mFOLFOX over bevacizumab + mFOLFOX as a first-line treatment for metastatic colorectal cancer patients with Wild-Type RAS in Greece. Value Health 2014;17:A633
- Stahl JE. Modelling methods for pharmacoeconomics and health technology assessment. An overview and guide. Pharmacoeconomics 2008;26:131-48
- Culyer JA. The dictionary of health economics. 3rd edn. Cheltenham: Edward Elgar Publishing, 2014
- Cao Q, Buskens E, Feenstra T, et al. Continuous-time semi-Markov models in health economic decision making: an illustrative example in heart failure disease management. Med Decis Making 2016;36:59-71
- Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force–3. Value Health 2012;15:812-20
- Hettle R, Posnett J, Borrill J. Challenges in economic modeling of anticancer therapies: an example of modeling the survival benefit of olaparib maintenance therapy for patients with BRCA-mutated platinum-sensitive relapsed ovarian cancer. J Med Econ 2015;18:516-24
- Casciano R, Chulikavit M, Perrin A, et al. Cost-effectiveness of everolimus vs sunitinib in treating patients with advanced, progressive pancreatic neuroendocrine tumors in the United States. J Med Econ 2012;15(Suppl1):55-64
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-58
- Karthaus M, Schwartzberg L, Rivera F, et al. Updated overall survival analysis of novel predictive KRAS/NRAS mutations beyond KRAS exon 2 in PEAK: A 1st-line phase 2 study of FOLFOX6 plus panitumumab or bevacizumab in metastatic colorectal cancer. Eur J Cancer 2013;49(Suppl 2):S1-S1028
- Peeters M, Price TJ, Cervantes A, et al. Final results from a randomized phase 3 study of FOLFIRI {+/−} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:107-16
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44
- BOT Plus 2.0 [Internet]. Madrid: Consejo General del Colegio Oficial de Farmacéuticos, 2015. botplusweb.portalfarma.com. Accessed May 12, 2015
- Magaña M. Panitumumab en CCRm que exprese EGFR con KRAS no mutado. Grupo de Evaluación de Novedades, EStandarización e Investigación en Selección de medicamentos (GÉNESIS). Palma de Mallorca, Spain: Sociedad Española de Farmacia Hospitalaria, 2009. gruposdetrabajo.sefh.es/genesis/genesis/Documents/PANITUMUMAB_Hsll_0309.pdf. Accessed October 15, 2015
- Castillo MA, Ubago R, Navarro JA, et al. Anticuerpos monoclonales asociados a quimioterapia en el tratamiento de primera línea del cáncer colorrectal metastásico: eficacia, seguridad y eficiencia comparadas. Sevilla: Agencia de Evaluación de Tecnologías Sanitarias de Andalucía, 2013. www.juntadeandalucia.es/salud/servicios/contenidos/nuevaaetsa/up/AETSA_6_2011_MAB_CaColorrectal.pdf. Accessed October 15, 2015
- Spanish Health Costs Database: eSalud [Internet]. Barcelona: Oblikue Consulting, S.L., 2007. www.oblikue.com/bddcostes/. Accessed February 1, 2015
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34
- Dolan P. Modeling valuations for EuroQol Health States. Med Care 1997;35:1085-108
- Van Cutsem E, Siena S, Humblet Y, et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol 2008;19:92-8
- Corral MJ, Clopès A, Navarro M, et al. Impact on budget of new drugs for colorectal cancer treatment. Med Clin (Barc) 2007;129:134-6
- Sacristán JA, Oliva J, Del Llano J, et al. ¿Qué es una tecnología sanitaria eficiente en España? Gac Sanit 2002;16:334-43
- Rodríguez Barrios JM, Pérez Alcántara F, Crespo Palomo C, et al. The use of cost per life year gained as a measurement of cost-effectiveness in Spain: A systematic review of recent publications. Eur J Health Econ 2012;13:723-40
- Shankaran V, Ortendahl JD, Purdum AG, et al. Cost-effectiveness of cetuximab as first-line treatment for metastatic colorectal cancer in the United States. Am J Clin Oncol 2015: published online September 22, 2015, http://journals.lww.com/amjclinicaloncology/Abstract/publishahead/Cost_Effectiveness_of_Cetuximab_as_First_line.99114.aspx doi: 10.1097/COC.0000000000000231
- Graham CN, Hechmati G, Fakih MG, et al. Cost-minimization analysis of panitumumab compared with cetuximab for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. J Med Econ 2015;18:619-28
- Grávalos C, Fernández M, Gea S. Análisis de minimización de costes de panitumumab frente a cetuximab en combinación con quimioterapia en primera y segunda línea de tratamiento del cáncer colorrectal metastásico RAS no mutado en España. 3r Congreso de Oncología Médica y Farmacia Oncológica, Toledo (España); November 26–28, 2015
- Ewara EM, Zaric GS, Welch S, et al. Cost-effectiveness of first-line treatments for patients with KRAS wild-type metastatic colorectal cancer. Curr Oncol 2014;21:e541-50
- Riesco MC, Berry SR, Ko YJ, et al. Cost-effective analysis of the use of EGFR inhibitors for wild-type KRAS unresectable metastatic colorectal cancer. J Clin Oncol 2013;31(Suppl):abstract 6552
- Lien K, Berry S, Ko YJ, et al. The use of EGFR inhibitors in colorectal cancer: is it clinically efficacious and cost-effective? Expert Rev Pharmacoecon Outcomes Res 2015;15:81-100
- Huxley N, Crathorne L, Varley-Campbell J, et al. The clinical effectiveness and cost-effectiveness of cetuximab (review of TA176) and panitumumab (partial review of TA240) for previously untreated metastatic colorectal cancer: a systematic review and economic evaluation. PenTAG, University of Exeter Medical School (Report for NICE), 2015. www.nice.org.uk/guidance/GID-TAG470/documents/colorectal-cancer-metastatic-cetuximab-review-ta176-and-panitumumab-part-review-ta240-1st-line-id794-assessment-report2. Accessed October 20, 2015
- Pan-Canadian Oncology Drug Review. Initial Clinical Guidance Report Panitumumab (Vectibix) for Metastatic Colorectal Cancer. Toronto: Canadian Agency for Drugs and Technologies in Health, 2015. www.cadth.ca/sites/default/files/pcodr/pcodr_panitumumab_vectibix_mcrc_in_cgr.pdf. Accessed October 20, 2015
- Lange A, Prenzler A, Frank M, et al. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer 2014;50:40-9
- Antoñanzas F, Juárez-Castelló CA, Rodríguez-Ibeas R. Some economics on personalized and predictive medicine. Eur J Health Econ 2015;16:985-94
- Sorbye H, Cvancarova M, Qvortrup C, et al. Age-dependent improvement in median and long-term survival in unselected population-based Nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol 2013;24:2354-60
- Marsh K, Phillips CJ, Fordham R, et al. Estimating cost-effectiveness in public health: a summary of modelling and valuation methods. Health Econ Rev 2012;2:17
- Shiroiwa T, Fukuda T, Shimozuma K, et al. Cost-effectiveness analysis of capecitabine compared with bolus 5-fluorouracil/l-leucovorin for the adjuvant treatment of colon cancer in Japan. Pharmacoeconomics 2009;27:597-608
- Pietrantonio F, Cremolini C, Petrelli F, et al. First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2015;96:156-66