Abstract
Purpose: To evaluate the insulin wastage and associated acquisition costs when switching from individual patient supply (IPS) of 3-mL pens of rapid-acting insulin (RAI) aspart to floor stock (FS) dispensing of 3-mL vials of RAI lispro, and with conversion from IPS of 3-mL pens to centralized unit dose (CUD) of 10-mL vials of basal insulin detemir.
Methods: Data from September 2010 to December 2012 from three hospitals in the Roper St. Francis Healthcare (RSFH) were used: Roper Hospital (368 beds), Bon Secours St. Francis Hospital (204 beds), and Roper St. Francis Mt. Pleasant Hospital (85 beds). Insulin wastage and associated acquisition costs were estimated using regression models.
Results: The conversion from IPS of 3-mL pens of insulin aspart to FS of 3-mL vials of lispro was associated with a significant decrease in insulin wastage (204,042 IUs; p < .001) and equated to an average savings of $106.40 per patient at all three hospitals combined (p < .001). For basal insulin, conversion from IPS of 3-mL pens of insulin detemir to CUD of 10-mL vials was associated with a significant decrease in insulin wastage at Roper and St. Francis Hospitals (p < .001). For Mt. Pleasant Hospital, the decrease was not statistically significant. The predicted average reduction in insulin wastage per month was 52,542.9 IUs (p < .001) at all three hospitals combined.
Conclusions: Switching RAI from IPS of 3-mL pens of insulin aspart to one-time unit dose insulin lispro dispensed from FS 3-mL vials as needed significantly reduced insulin wastage and associated acquisition costs at the three combined hospitals. Conversion of basal insulin from IPS of 3-mL pens of insulin detemir to CUD of 10-mL vials of insulin detemir was associated with a significant reduction in insulin wastage and associated acquisition costs at three hospitals combined.
Introduction
Diabetes patients account for up to 26% of all patient admissions and ∼25% of total inpatient days in US hospitalsCitation1. In addition, it has been estimated that 12% of US hospital patients have admission hyperglycemia, despite no prior history of diabetes, resulting in a total of 38% of hospital patients requiring treatment for hyperglycemia during their stayCitation2. In 2015, expenditures for diabetes therapies in US non-federal hospitals were 412 million dollars, comprising 1.2% of total pharmaceutical expenditures, and representing a 5.8% increase compared with the previous yearCitation3. Insulins are the predominant class of diabetes therapies in the US, accounting for 62% of total sales of diabetes treatmentsCitation4.
One potential path to reducing the pharmaceutical costs of treating hospital patients with admission hyperglycemia is to reduce insulin wastage. The primary driver of insulin wastage in hospitals is the unused insulin remaining in a pen or vial that is disposed of when a patient is discharged or when the pen or vial reaches the end of its in-use period. Insulin wastage may be reduced by using more efficient methods of insulin delivery to patientsCitation5–8.
Common insulin products are available in the US as either 3-mL pens, 10-mL vials, or 3-mL vials. Insulin is delivered in hospitals using the following methods: (1) individual patient supply (IPS) of pens or vials, (2) distribution by nursing staff who withdraw individual doses from floor stock (FS) vials distributed to hospital departments or automated dispensing machines (ADM), or (3) as centralized unit doses (CUD) prepared by the pharmacy staffCitation9.
Few studies have evaluated insulin wastage and economic outcomes in hospitals. One study estimated the wastage across all insulin types resulted in annual acquisition costs of $360,000 for all hospitals in Ontario, CanadaCitation5. Another hospital study projected that replacing 10-mL vials with 3-mL pens distributed by IPS would save $36 per patientCitation6. In a subsequent review paper of insulin pen usage in hospital settings, it was noted that the 3 mL vial may be associated with less wastage than the 10 mL vial, and recommended that further studies be conducted to investigate potential cost savings associated with a 3 mL vialCitation1.
A budget impact model was developed to estimate the economic outcomes of converting from pens (IPS) or 10-mL vials (IPS or FS) to 3-mL vials (IPS or FS)Citation7. Conversion to FS of 3-mL vials was associated with less wastage and lower costs for all scenarios. Furthermore, the delivery mode and the device volume impacted wastage and costs, but the delivery mode had a larger effect.
Edmondson et al.Citation8 reported a naturalistic study of a US hospital that converted from FS of 10-mL vials to IPS of 3-mL pens and vials across multiple classes of insulins. This study found that the total volume of insulin purchased and total acquisition costs decreased after the conversion. The authors note that these findings conflict with the results of the budget impact analysis by Lee et al.Citation7, and hypothesize that, in this naturalistic study, the benefits of the smaller sizes of the pens and vials used after the conversion outweighed the relative inefficiencies of IPS compared with FS.
To date, no study has assessed the economic impact of converting the insulin distribution method from IPS to multi-user delivery methods (FS and CUD) in a US hospital that has undergone such a conversion. This study was designed to address this gap by assessing insulin wastage and acquisition costs in a US hospital that simultaneously converted from IPS of 3-mL pens to FS of 3-mL vials of rapid acting insulin (RAI) and from IPS of 3-mL pens to CUD of 10-mL vials of basal insulin.
Methods
Setting
In this retrospective study to compare insulin wastage and pharmacy acquisition costs, information was used from Roper St. Francis Healthcare (RSFH), a healthcare system in Charleston, SC, composed of three hospitals: Roper Hospital (368 beds), Bon Secours St. Francis (St. Francis) Hospital (204 beds), and Roper St. Francis Mt. Pleasant Hospital (Mt. Pleasant; 85 beds).
In April 2012, all three hospitals changed their delivery methods for RAI and basal insulin. For RAI, hospitals converted from IPS of 3-mL pens of insulin aspart to FS of ADM-sequestered 3-mL vials of insulin lispro. For basal insulin, hospitals converted from IPS of 3-mL pens of insulin detemir to CUD of 10-mL vials of insulin detemir. RAI analogs are available in 3-mL pens or 10-mL vials; however, only insulin lispro was available in 3-mL vials.
Conversion periods
The study was divided into conversion, pre-conversion, and post-conversion periods, using data from September 2010 to December 2012. The study period was designed to capture a minimum of 6 months immediately following and prior to the conversion period, plus an additional 12 months prior to serve as a control period. Regression analyses indicated that there were no significant differences in the patient population or insulin wastage between the 6 months immediately prior to the conversion compared with the preceding 12-month control period, and, thus, the entire 18-month period prior to the conversion was used in the final analyses to improve statistical power.
For RAI, data were collected before (September 2010 to February 2012) and after conversion (July 2012 to December 2012) from IPS of 3-mL pens to FS of 3-mL vials of RAI analog. The hospitals retained the same insulin protocol prior to and after the change in process. For basal insulin, data were collected before (September 2010 to February 2012) and after conversion (June 2012 to December 2012) from IPS of 3-mL pens to CUD of 10-mL vials.
The conversion periods were withheld from the analysis of insulin wastage to avoid spurious effects related to preparations for, and stabilization of practices after, the conversion. The months of the pre-conversion period that aligned with the months of the conversion period were also withheld from the regression analysis to control for seasonal effects.
End-points
The main outcome variables were the following: (1) the difference in RAI wastage between the pre-conversion period (IPS of 3-mL pens) and post-conversion period (FS of 3-mL vials) and (2) the difference in basal insulin wastage between the pre-conversion period (IPS of 3-mL pens) and post-conversion period (CUD of 10-mL vials). Wastage was defined as the difference between the total insulin dispensed by the pharmacy and the total insulin administered to patients. For the pre-conversion period (IPS of 3-mL pens), the number of pens dispensed was estimated from administrative billing records, and the volume of insulin administered to patients was estimated from electronic medical records. To estimate RAI wastage during the post-conversion period (FS of 3-mL vials), the volume of RAI dispensed (stocked in ADMs) and the volume of RAI administered to patients were estimated from ADM transaction records. To estimate basal insulin wastage during the post-conversion period (CUD of 10-mL vials), the volume of basal insulin administered to patients during the post-conversion period was estimated from electronic medical records. However, since no data were available regarding the number of vials put in use or the volume of basal insulin disposed, the volume of basal insulin purchased was estimated from pharmacy purchasing invoices as a proxy estimate of the volume of basal insulin dispensed.
Other variables for both insulin types included the acquisition costs associated with insulin wastage, and the average volume of insulin wastage per patient.
Variables
Study variables were calculated from pharmacy-level invoices, billing data (including patient demographic characteristics, admission/discharge dates, ICD-9 diagnosis codes, charges for insulin pens dispensed to patients), Electronic Medical Records (EMR), and ADM transactions.
The patient load data was the number of patients treated, which included those who received ≥1 dose of insulin, and the time on treatment was the number of days on which the patient was treated. The length of stay was the number of days during which the patient was in the hospital. The Charlson comorbidity index (CCI) was calculated from patient age and ICD-9 diagnosis codesCitation10.
The insulin administered was calculated from electronic medical records data for IPS and CUD, and from ADM transaction data for FS. Insulin dispensed was calculated from administrative billing data for pens, and from ADM inventory data for FS vials. Pharmacy acquisition costs were calculated as the product of insulin volume and current average wholesale price (AWP). Costs were estimated from AWP rather than actual costs expended by RSFH in order to make the results more readily generalizable, as actual prices may vary across different healthcare systems.
The net total insulin purchased per month was calculated from pharmacy purchasing invoices. The AWPs per pen or vial were obtained from REDBOOKTM, although for RAI and basal insulin, the access dates for the source were different. For RAI, the pre-conversion costs were calculated using the AWP package price of $453.07, equivalent to a price of $90.61 per insulin aspart pen or $0.3020 per insulin unit (IU)Citation11. Post-conversion costs were calculated using the AWP package price of $72.94, equivalent to a price of $0.2431 per IU for an insulin lispro 3-mL vial. For basal insulin, the pre-conversion costs were calculated using the AWP package price of $519.66, equivalent to a price of $103.93 per pen or $0.3464 per IUCitation12. Post-conversion costs were calculated using the AWP package price of $298.21, equivalent to a price of $0.2982 per IU for an insulin detemir 10-mL vial.
Analysis
The primary analysis was conducted using linear regression models to test the hypotheses that the conversion from 3-mL pens to small (3-mL) vials of RAI results in a reduction in insulin wastage, and that the conversion from IPS of 3-mL pens to CUD of 10-mL vials of basal insulin detemir results in a reduction in insulin wastage. Controlling for population covariates in the regression model enables the analysis to quantify the differences in wastage associated with the conversion itself, rather than due to differences in the populations of patients treated during the pre-conversion period vs the post-conversion period.
The response variable was the volume of insulin wastage for a specific hospital and month. For hypothesis testing, 2-sided tests were used with a significance level of .05.
Covariates included the number of patients treated during the month, the mean time on treatment per patient treated, the mean daily dose per patient, and the mean CCI per patient.
The regression sample was composed of all observations during the pre-conversion and post-conversion periods (excluding the calendar months that aligned with the conversion period, as discussed above). A binary indicator variable was used to distinguish pre-conversion observations from post-conversion observations.
The regression model included main effects, which represented the mean wastage per month, and interaction effects between the conversion effect and the other predictor variables. All covariates were centered about the hospital mean, where the mean was calculated across all months. The main effects included the time period effect (pre-conversion vs post-conversion), and hospital effect (Mt. Pleasant as reference hospital). The incremental effects of increasing or decreasing the covariates of number of patients treated, mean time on treatment, mean daily dose, and mean CCI and the interaction of time period with hospitals and covariates were analyzed.
Results
Demographics
The demographic and clinical characteristics of patients eligible for RAI and basal insulin during the pre- and post-conversion periods at all three hospitals combined are displayed in . For RAI during the pre-conversion and post-conversion periods, the mean number of study patients per month was 751.9 vs 685.7, and the mean number of patients treated per month was 654.3 vs 647.8, respectively. The mean time on treatment per patient was 4.9 days vs 4.5 days, and the mean daily dose per patient was 10.5 IUs vs 9.5 IUs, respectively. For basal insulin during the pre-conversion and post-conversion periods, the mean number of study patients per month was 301.4 vs 307.6, and the mean number of patients treated per month was 269.5 vs 275.6, respectively. The mean time on treatment per patient was 4.9 days vs 4.5 days, and the mean daily dose per patient was 28.5 IUs vs 26.8 IUs, respectively. For both RAI and basal patients, the gender, age, average length of stay, mean CCI, and percentages of patients by diabetes diagnosis were comparable during the pre-conversion and post-conversion periods.
Table 1. Demographic and clinical characteristics of patients eligible for RAI or basal insulin (pooled across all hospitals).
RAI
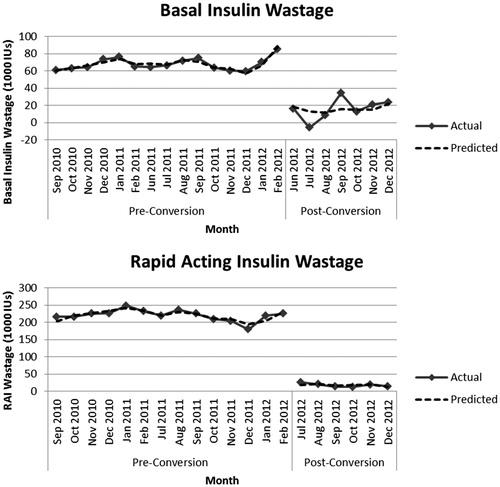
The regression model for RAI (R2 = .996; adjusted R2 = .995; p < .001) showed that the conversion had a significant negative effect on average insulin wastage per month (). The conversion had a significant negative effect on average insulin (IUs) wastage per month, indicating reduced total insulin wastage at all three hospitals combined (−204,041.6; 95% CI = −215,757.8, −192,325.4; p < .001). compares the regression model used for hypothesis testing with the actual wastage incurred each month at all three hospitals combined.
Table 2. Main effects of conversion on wastage per month.
The effect of the conversion on insulin wastage yields average monthly savings in acquisition costs of the wasted insulin of $62,742.35 at all three hospitals combined (). This corresponds to a per-patient savings of 346.03 IUs (1.15 pens) and $106.40 in associated acquisition costs.
Table 3. Predicted mean wastage per month and associated acquisition costs.
The mean units of RAI administered per patient at all three hospitals combined was 46.8 IUs during the pre-conversion period compared with 44.1 IUs during the post-conversion period, for a reduction of 2.7 IUs (5.8%). The mean units of RAI dispensed per patient at all three hospitals combined was 400.8 IUs during the pre-conversion period compared with 73.9 IUs during the post-conversion period, for a reduction of 326.9 IUs (81.6%) ().
Table 4. Average insulin usage per patient.
The average total volume of RAI purchased per month at all three hospitals combined was 240,583.3 IUs during the pre-conversion period compared with 53,950.0 IUs during the post-conversion period, for a reduction of 186,633.3 IUs (77.6%). The average total RAI acquisition costs per month at all three hospitals combined were $72,667.39 during the pre-conversion period compared with $13,117.04 during the post-conversion period, for a savings of $59,550.35 (81.9%) ().
Table 5. Average monthly insulin purchases.
Basal insulin
The regression model for basal insulin (R2 = .961; adjusted R2 = .950; p < .001) showed that the conversion had a significant negative effect on average insulin wastage per month (). The conversion produced a reduction in average insulin (IUs) wastage per month at all three hospitals combined (−52,542.9; 95% CI = −60,353.8, −44,732.1; p < .001). compares the regression model used for hypothesis testing with the actual wastage incurred each month at all three hospitals combined.
The effect of the conversion on insulin wastage yielded average monthly savings in acquisition costs of the wasted insulin of $19,007.80 at all three hospitals combined (). The reductions in wastage measured on a per-patient basis were 200.65 IUs (0.67 pens) and $72.59 in associated acquisition costs.
The mean units of basal insulin administered per patient at all three hospitals combined was 131.5 IUs during the pre-conversion period, compared with 115.2 IUs during the post-conversion period, for a reduction of 16.3 IUs (12.4%). The mean units of basal insulin dispensed per patient at all three hospitals combined was 412.0 IUs during the pre-conversion period, compared with 175.1 IUs during the post-conversion period, for a reduction of 236.8 IUs (57.5%) ().
The average total volume of basal insulin purchased per month at all three hospitals combined was 101,166.7 IUs during the pre-conversion period, compared with 45,857.1 IUs during the post-conversion period, for a reduction of 55,309.5 IUs (54.7%). The average total basal insulin acquisition costs per month at all three hospitals combined were $35,048.18 during the pre-conversion period, compared with $13,675.06 during the post-conversion period, for a saving of $21,373.12 (61.0%) ().
Discussion
For RAI, the conversion from IPS of 3-mL pens of insulin aspart to FS of one-time unit dose insulin lispro dispensed from 3-mL vials as needed reduced insulin wastage at all three hospitals in the study. For basal insulin, the conversion from IPS of 3-mL pens to CUD of 10-mL vials of basal insulin reduced insulin wastage at Roper Hospital, St. Francis Hospital, and the RSFH system as a whole. Insulin wastage was also reduced at Mt. Pleasant hospital, but it did not achieve statistical significance (p = .515). Note that the direct beneficiary of the savings in acquisition costs depends on the payment structure under which the hospital is reimbursed.
Per-patient reductions in insulin wastage ranged from 0.89–1.25 pens per patient for RAI, and 0.50–0.71 pens per patient for basal insulin. As expected, larger hospitals obtained a greater benefit from the conversion with respect to reduced wastage.
In IPS, three key drivers influence insulin wastage. First, a minimum of one pen was dispensed to every patient to whom insulin was administered, regardless of the patient’s total insulin consumption. Second, pens were distributed to diabetic patients who did not require treatment during the hospital stay. Third, pens were lost, most commonly when patients were transferred between hospital departments.
Converting from IPS to FS or CUD of insulin avoids these three key drivers of wastage. For FS and CUD, the key driver of wastage is the total aggregate insulin consumption from the vial. The savings in wastage were more pronounced at hospitals with larger populations, and thus greater aggregate insulin consumption. Converting from IPS to FS of RAI and CUD of basal insulin enabled the pharmacy to more efficiently manage its inventory, leading to a reduction in wastage and associated acquisition costs.
The focus of other research examining insulin wastage was wastage as a proportion of insulin used. In this study, RAI wastage at all three hospitals combined represented 88.3% and 40.3% of the RAI insulin dispensed, with the associated annual acquisition costs of the wasted RAI of $806,800 and $51,189 for the pre-conversion period and post-conversion periods, respectively. For basal insulin, the observed wastage at all three hospitals combined represented 68.1% and 34.2% of the insulin dispensed, with the annual acquisition costs of the wasted basal insulin of $290,408 and $56,127 during the pre-conversion period and post-conversion periods, respectively.
Rosenbloom et al.Citation5 estimated that wastage across all types of insulin represented 34.1% of insulin used, resulting in estimated annual acquisition costs of $360,000 for all hospitals in Ontario, Canada. The proportional wastage reported by Rosenbloom et al.Citation5 is similar to the post-conversion wastage observed in the current study. Differences in acquisition costs between the Ontario study and the current study of US hospitals are influenced by differences in pricing, inflation, and the patient population.
In the budget-impact model of RAI developed by Lee et al.Citation7, the estimated proportional wastage was 67% with IPS of 3-mL pens, 15% with FS of 3-mL vials, and 25% with FS of 10-mL vials. The Lee et al.Citation7 model also estimated that converting from IPS of 3-mL pens to FS of 3-mL vials was associated with 87% less wastage and 69% lower acquisition costs. In the current study, the proportional wastage of RAI was substantially higher than the estimates reported by Lee et al.Citation7: 88.3% vs 67% with IPS of 3-mL pens, and 40.3% vs 15% with FS of 3-mL vials. However, the reduction in wastage associated with converting from IPS of 3-mL pens to FS of 3-mL vials was also higher in this study than the results reported by Lee et al.Citation7: a 91.5% vs 87% reduction in the insulin volume wastage, and a 93.2% vs 69% reduction in associated acquisition costs. Note that, in the Lee et al.Citation7 study, the estimates of expected wastage with IPS of 3-mL pens and FS of 3-mL vials were based on the opinions of experts who were extrapolating from the wastage observed in a visual census of FS use of 10-mL vials. In contrast, the current study is based on empirical data of actual wastage associated with IPS of 3-mL pens and FS of 3-mL vials. Therefore, differences between the two studies are because of the assumptions used by the experts in the Lee et al.Citation7 study to extrapolate the wastage observed with FS use of 10-mL vials to expected wastage with IPS use of 3-mL pens and FS use of 3-mL vials.
The results of Lee et al.Citation7 also indicated that both the delivery mode and the device volume impacted wastage and costs, but the delivery mode had a larger effect. The current study was limited in its ability to separately measure the effects of delivery mode and device size on the reduction in wastage.
In contrast, the study by Edmondson et al.Citation8 assessed the economic impact of the conversion from FS use of 10-mL vials to IPS use of 3-mL vials or pens in a US hospital that underwent such a conversion. The results of this study indicated that the conversion from FS 10-mL vials to either IPS 3-mL vials or IPS 3-mL pens was associated with a reduction in the volume of insulin purchased. Edmondson et al.Citation8 also noted that, during the pre-conversion period when FS use of 10-mL vials was in effect, an estimated 36.4% of the 10-mL vials were wasted, with an average 9 mL wasted per vial. The key difference between the Edmondson et al.Citation8 study and the current study is the size of the insulin vials used in the floor stock mode: the current study of RAI focused on FS use of smaller 3-mL vials rather than the 10-mL vials used in the Edmondson et al.Citation8 study. The differences in the results of the two studies suggest that the size of the vial used in FS mode has a substantial impact on economic outcomes, and that FS use of 3-mL vials may be more efficient than FS use of 10-mL vials.
Limitations
The analyses were subject to limitations related to the data and study design. The first was data censoring, which was caused by the discretization of the data into months. Insulin dispensed during 1 month may have been administered the following month; while, conversely, insulin administered in a given month may have been dispensed the prior month. These errors did not impact total wastage because, over the course of the analysis, the dispensed insulin was included. The accounting may have increased the sample variance, although it is anticipated that the effect would render the hypothesis tests more conservative.
Second, these studies focused exclusively on differences in acquisition costs based on differences in insulin wastage. Other costs may have been associated with the conversion, such as increased labor costs associated with centralized dispensing and distribution, that offset cost savings associated with reduced insulin wastage. Other potential costs of conversion were not considered.
Additionally for the basal insulin analysis, data showing the number of vials opened or the insulin volume discarded in CUD mode were not available. In the absence of these data, the volume of insulin dispensed in CUD mode was estimated by using the volume of insulin purchased as a proxy variable, which assumes all insulin purchased was used and did not have to be discarded, which could have introduced measurement error.
Furthermore, no data were available for this study regarding medication error rates or staff and patient satisfaction with insulin vials as opposed to insulin pens. Prior research has found that insulin vials are associated with higher error rates than insulin pens, and that hospital staff and patients express higher levels of satisfaction with insulin pensCitation1,Citation6. In addition, the RAI conversion in the current study entailed a change from insulin aspart to insulin lispro. While both insulin aspart and insulin lispro are considered safe and effective, they are not medically equivalent. Therefore, it is recommended that formulary decisions should balance economic and clinical considerations.
Finally, while one advantage of the present research is the ability to explore the economic impact at multiple hospitals of different sizes, all of the hospitals were part of the same healthcare system. The analysis cannot rule out the possibility that the results were related to factors specific to the Roper St. Francis Healthcare system that are not present in other healthcare systems. Additional research is needed to confirm the generalizability of the findings.
Conclusions
Switching from an individual patient supply of 3-mL pens of insulin aspart to as needed unit dose ADM sequestered 3-mL vials of insulin lispro significantly reduced insulin wastage and associated acquisition costs. Conversion from IPS of 3-mL pens to CUD of 10-mL vials of basal insulin detemir in a hospital setting resulted in lower insulin wastage.
Transparency
Declaration of funding
This study was funded by Eli Lilly and Company.
Declaration of financial/other interests
KK and SP are employees of Medical Decision Modeling Inc. MPN, HF, and XP are employees of Eli Lilly and Company. MPN and HF are also stockholders of Eli Lilly and Company. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
A sub-set of the results for rapid acting insulin was previously published as an abstract/poster at the ASHP 2015 Midyear Clinical Meeting. This manuscript contains a full report of the results for both rapid acting insulin and basal insulin.
Acknowledgments
The authors would like to acknowledge Laura Bean Warner for her assistance in the preparation of this manuscript.
References
- Davis EM, Foral PA, Dull RB, et al. Review of insulin therapy and pen use in hospitalized patients. Hosp Pharm 2013;48:396-405
- Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978-82
- Schumock GT, Li EC, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2016. Am J Health Syst Pharm 2016;73:1058-75
- IMS Health. Disease insights: Type 1 and Type 2 diabetes – United States. December 2014 London:IMS Health. http://www.imshealth.com/en_US
- Rosenbloom D, Scime J, Elviss OD, et al. Measurement of insulin waste in five Ontario hospitals. Can J Hosp Pharm 1994;47:5-7
- Davis EM, Christensen CM, Nystrom KK, et al. Patient satisfaction and costs associated with insulin administered by pen device or syringe during hospitalization. Am J Health Sys Pharm 2008;65:1347-57
- Lee LJ, Smolen LJ, Klein TM, et al. Budget impact analysis of insulin therapies and associated delivery systems. Am J Health Syst Pharm 2012;69:958-65
- Edmondson G, Criswell J, Krueger L, et al. Economic impact of converting from 10-mL insulin vials to 3-mL vials and pens in a hospital setting. Am J Health-Syst Pharm 2014;71:1485-9
- Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: Dispensing and administration–2011. Am J Health Syst Pharm 2012;69:768-85
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9
- Rapid-Acting Insulin. REDBOOKTM Online. Micromedex Healthcare Series [database online]. Greenwood Village, CO: Truven Health Analytics. http://micromedex.com/products/product-suites/clinical-knowledge/redbook 2015 Accessed January 1, 2015
- Basal Insulin. REDBOOKTM Online. Micromedex Healthcare Series [database online]. Greenwood Village, CO: Truven Health Analytics. http://micromedex.com/products/product-suites/clinical-knowledge/redbook 2015 Accessed March 12, 2015