Abstract
Aims: Vitamin K antagonists (VKAs) are used for stroke prevention in patients with non-valvular atrial fibrillation (NVAF), but necessitate regular monitoring of prothrombin time via international normalized ratio (INR) testing. This study explores the economic burden of VKA therapy for Russian patients with NVAF.
Method: Cardiologists provided clinical characteristics and healthcare resource use data relating to the patient’s first year of treatment. Data were used to quantify direct medical costs (INR testing, consultations, drug costs). The same patients completed a questionnaire providing data on direct non-medical costs (travel/expenses for attendance at VKA appointments) and indirect costs (opportunity cost and reduced work productivity). Mean costs per patient per year are described (US dollars).
Results: Cardiologists (n = 50) provided data on 400 patients (mean age = 63, 47% female), and 351 patients (88%) completed the patient questionnaire. Patients had a mean of nine INR tests. Estimated direct medical costs totaled $151.06, and 18.5% of direct medical costs were attributable to drug costs. Estimated annual direct non-medical costs were $22.89 per patient, and indirect costs were $275.59 per patient.
Limitations: Included patients had been treated for 12–24 months, so are not fully representative of the broader treatment population.
Conclusion: Although VKA drugs costs are relatively low, regular INR testing and consultations drive the economic burden for Russian NVAF patients treated with VKA.
Introduction
In recent years, age-standardized rates of death due to atherosclerosis and coronary heart disease have decreased in developed countries as a result of modifiable risk factor management strategies, including the use of statins, strict control of hypertension, and changes in lifestyleCitation1–3. However, at the same time, the incidence of the most common arrhythmia—atrial fibrillation (AF), continued to increaseCitation4. The prevalence of AF in Russia was estimated at 5.6% among older adults (aged ≥60 years) in one community studyCitation5. Most cases of AF are non-valvularCitation6.
Atrial fibrillation can lead to significant complications including acute ischemic stroke and systemic embolismCitation7, heart failureCitation8, decreased quality-of-lifeCitation9, and prolonged and frequent hospitalizationsCitation10. The presence of AF is associated with a 5-fold increase in risk of stroke and a 3-fold increase in the risk of congestive heart failureCitation11. Compared with Western Europe, where stroke mortality decreased between 1990–2006, mortality associated with stroke has increased in Eastern EuropeCitation12. In 2002, age- and sex-adjusted stroke mortality rates per 100,000 persons for the Russian Federation were amongst the highest across the 35 European countries examined. For both males and females in Russia, the annual rate of change in stroke mortality between 1990–2006 was reported as ≥3 deaths per 100,000Citation12.
A key point in prevention of thromboembolic complications is adequate anticoagulation, which may be achieved with a vitamin K antagonist such as warfarin or with novel oral anti-coagulants (NOACs) such as factor Xa inhibitors and direct thrombin inhibitorsCitation13. Despite these options, evidence suggests under-use of anticoagulationCitation14. Although effective in preventing stroke, VKA treatment can be problematical as a result of the narrow therapeutic range and numerous drug–drug and drug–food interactionsCitation15,Citation16. Regular international normalized ratio (INR) testing is required throughout the duration of treatmentCitation17 and, in practice, achieving and maintaining the INR within the appropriate therapeutic range can be difficultCitation17. Studies are needed in order to understand the impact of monitoring on the cost of VKA treatment, and ensure that the correct treatment decisions are made.
Several studies have shown that INR monitoring is associated with notable healthcare resource utilization and costsCitation18–21. However, the majority of previous studies were conducted in North America and Western Europe. This data may be of limited relevance to other regions, as individual countries have evolved different approaches to managing careCitation20. In addition, few previous studies have assessed the impact of indirect costs, an important component of the overall cost of VKA treatment.
The present study sought to understand the costs associated with VKA treatment for stroke prevention in Russian patients with non-valvular atrial fibrillation (NVAF). The study adopted a collective perspective that encompassed direct medical cost, direct non-medical cost, and indirect costs.
Methods
Study population
The study recruited a sample of 50 cardiologists who were based in urban regions and were routinely responsible for stroke prevention in AF, including (but not limited to) experience of prescribing VKAs in this patient group for a minimum of 15 patients per month. In order to avoid recruiting newly qualified/close to retirement age physicians, inclusion criteria specified that physicians should have qualified between 1979–2012. Each physician completed a record form for the next eight eligible NVAF patients they saw, according to the following minimal criteria: the patient was aged 18 or over and the patient had received VKA treatment for a minimum of 12–24 months with no interruptions within the considered period to ensure sufficient data could be provided. As well as a physician completed record forms, eligible patients were invited to complete a patient questionnaire on a voluntary basis, which could be filled in with the assistance of a caregiver if needed.
Data collection
Physician reported data
Physicians completed an online record form that captured patient demographics, clinical characteristics, and healthcare resource use. Physicians provided data on the patient’s typical INR monitoring pathway, the number of INR tests they had, and VKA dose and dose adjustments for the first 12 months of treatment. Comorbidities and risk factors were reported and were used to calculate HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly (>65 years), Drugs/alcohol concomitantly)Citation22, and CHADS2-VASc risk scores (congestive heart failure, hypertension, age, diabetes, stroke, vascular disease, age, sex category)Citation23. The Charlson Comorbidity Index, predicting mortality from current comorbidities, was also calculatedCitation24. These same patients were invited to complete a patient questionnaire on a voluntary basis. Physician reported and patient reported data were anonymized, but could be linked via an ID number.
Patient reported data
Patients completed a paper-based questionnaire that assessed demographics, employment status, total loss of time due to their last VKA appointment, and specifically work time loss over the previous month due to VKA treatment. Loss of time, including worktime loss for a non-professional caregiver, was also collected if applicable. Patients also indicated mode of transport, distance travelled, and transport expenses associated with their last VKA appointment and any other expenses incurred over the last month (excluding travel) due to VKA treatment. In order to understand how frequently these costs were incurred, patients indicated the number of site visits relating to VKA treatment in the last 4 months to their main hospital. In addition, some patients reported that they sometimes visit an alternative hospital/clinic for INR testing. If this was the case, patients reported the number of visits to this site over the previous 4 months. For these patients, the same questions relating to loss of time, travel, and other expenses were repeated to collect relevant data associated with use of an alternative site for INR testing.
Analysis
Physician and patient reported-data were analysed to calculate direct medical costs, direct non-medical costs, and indirect medical costs. Direct medical costs were primarily reliant on physician-reported data, and direct non-medical and indirect costs were calculated from the patient-reported data.
Direct medical costs consisted of three elements: drug costs (dependent on dose), INR test costs (dependent on number of INR tests), and cost of appointments surrounding INR testing (dependent on the typical INR monitoring pathway, the healthcare professional seen and the estimated number of appointments).
Direct non-medical costs were calculated from the patient-reported data and included costs associated with attendance at the patient’s main hospital as well as an alternative site if applicable. Relevant variables included travel to and from appointments, the cost of meals and beverages while attending appointments, and any miscellaneous expenses. Consequently, the calculation of direct non-medical costs included all possible patient expenses in line with a previously published approachCitation21. Fuel costs were estimated based on distance for patients who attended appointments by car. Patient-reported data were provided either for the previous month or previous appointment; an annual estimate was calculated by multiplying by 12 or multiplying by the number of patient reported visits scaled up to 12 months. It should be noted that, although some patients reported paying for their own medication or to see a healthcare professional as part of their expenses, this was not included in the analysis to avoid double counts with the physician-reported data.
Indirect costs were calculated from data provided in the patient questionnaire and included worktime loss and opportunity loss for patients and, where applicable, a caregiver, following a similar analysis strategy in a previous VKA costing studyCitation21. Employed patients reported work time lost due to VKA treatment over the previous month. Patients similarly also reported any work time loss over the previous month for a caregiver if applicable. These data were multiplied by 12 and the median hourly wage in Russia, to derive an annual estimate. For any patient who reported being unemployed for 12 months or longer specifically due to their VKA treatment, the median annual wage was applied. For patients unemployed due to VKA treatment for a period less than 12 months, a proportion of the median annual wage was applied and, for the remaining proportion of the year, work time loss was imputed from the data supplied by employed patients. Even when patients are unemployed, there is an opportunity cost associated with lost leisure time when attending medical appointments. Opportunity loss was, therefore, calculated for patients who were unemployed when this was not due to VKA. This involved taking the patient reported loss of time for their last appointment (main hospital and an alternative site if applicable) multiplied by the patient reported number of visits to each site over the past 4 months scaled up to 1 year and multiplied by the national minimum wage to derive an annual estimated cost associated with opportunity loss. The same method was also used to calculate caregiver opportunity loss for instances where the patient said they were accompanied by a caregiver but did not report any specific worktime loss for that person. Applying the national minimum wage to loss of time experienced by out of work patients has previously been used to derive costs associated with opportunity lossCitation21.
Costs per INR test
In addition to the annual cost per patient, costs associated with a single INR test were calculated by simply dividing the estimated annual cost by the number of INR tests in the first 12 months of treatment, as reported by the physician. This cost per INR test could then be used to estimate costs under any assumed testing frequency. Specifically, as guidelines typically recommend INR tests at least once per monthCitation25, we estimated what the costs would have been if physicians/patients had adhered to this.
Unit costs
Unit cost data were gathered from external, country-specific sources, and the literature (see Supplementary material). Costs were identified in Russian rubles and converted into USD using an exchange rate of 1 ruble = 0.015 USD (www.xe.com, retrieved 2016).
Generalized linear model
Patient characteristics thought to be associated with higher direct medical costs were investigated using a Generalized Linear Model (GLM). A gamma distribution and a log link were used in the model, as these options are widely used for modeling cost outcomes. Variables included in the GLM were age, sex, body mass index (BMI), smoking status, disease duration, time to stability, CHA2DS2-VASC score, HAS-BLED score, and Charlson comorbidity index. It should be noted that ‘stability’ was based on the subjective opinion of the physician. All analyses were performed using STATA statistical software version 14.1 or higher (StataCorp, 2015. Stata statistical software: Release 14. StataCorp LP, College Station, TX).
Results
A total of 50 physicians (all cardiologists) from six major Russian cities participated in the study. Physicians provided data on 400 patients, 351 (87.8%) of whom completed a questionnaire. Most physicians (80%) reported treating patients primarily from urban areas; 10 physicians (20%) treated a mixed group of patients from urban and rural areas. The majority of physicians were based in clinics (n = 38; 76%); four (8%) were based in hospitals, and 10 (20%) treated patients in both settings.
Patient clinical and demographic data are summarized in . Patients had received uninterrupted VKA treatment for an average of 17.0 months (SD = 4.9). Physicians reported that it took a mean duration of 9.1 weeks (SD = 13.9) for 315 of the patient sample to become stable. They reported that an additional 18 patients were not yet stable (4.5%), and selected “don’t know” for 67 patients (16.8%). Of the 382 patients who were deemed to have become stable at some point (plus patients where the physician had responded “don’t know” when asked how long this had taken), 356 (93.2%) were considered to be currently well managed on their VKA treatment. For patients not currently stable or poorly managed patients, physicians provided reasons why they thought this was the case. This applied to 44 patients. Reasons are shown in (note, physicians were able to provide multiple reasons per patient).
Figure 1. Physician reported reasons for why patients were not currently stable or were considered to be poorly managed on VKA treatment. VKA: vitamin K antagonist.
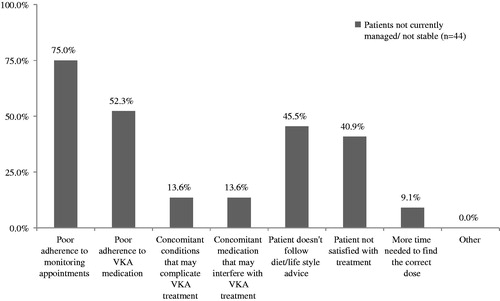
Table 1. Patient demographics and baseline characteristics.
Direct medical costs
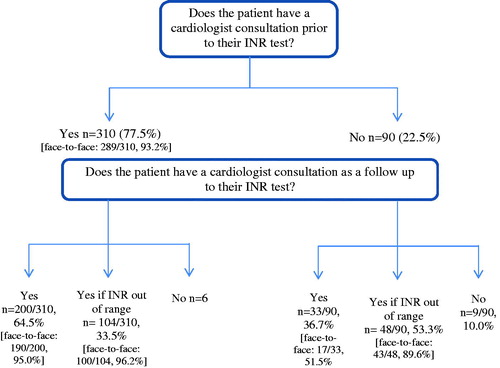
Patients had an average of nine INR tests over the first 12 months of their VKA treatment, which, according to their monitoring pathway, translated into a mean number of 21.4 face-to-face appointments and 0.8 telephone appointments with a cardiologist per year. This is depicted in , which summarizes the patient’s monitoring pathway.
The data collected from the present sample of patients found that INR testing is typically performed by a laboratory technician; however, patients usually consult with their cardiologist both before and after their INR test. Direct medical costs totaled $151.06, the majority of which was attributed to consultation time with a cardiologist. Direct medical costs in the first 12 months of treatment are summarized in , and the estimated cost per INR test was $19.21. By deriving a “cost per INR test”, we are also able to estimate what the direct medical costs would be under different scenarios. On this basis, we estimated that if physicians/patients adhered to 12 INR tests per year (one per month) rather than the reported mean of nine INR test per year, direct medical costs would be notably higher at $230.50 per patient per year.
Table 2. Direct medical costs associated with the first 12 months of VKA treatment
Direct non-medical costs
Direct non-medical costs, per patient per year, comprising travel/transport costs, meals/drinks, and miscellaneous expenses while attending appointments are shown in . Estimated direct non-medical costs per INR test were $3.05, which would translate to annual costs of $36.61 per patient per year if patients had one INR test per month.
Table 3. Direct non-medical costs associated with the first 12 months of VKA treatment.
Indirect non-medical costs
Indirect non-medical costs included worktime loss and opportunity loss for patients and caregivers. Patients reported their current employment status. A total of 143 patients (40.7%) were either in full or part-time employment, 205 (58.4%) were either retired or unemployed, and three patients (1.0%) did not respond. Employed patients reported taking an average of 4.9 hours (SD = 3.2) off work due to VKA treatment over the previous month. Of the patients who were out of work, a total of 10 patients reported being out of work due to VKA treatment, which was for a mean duration of 28 months for nine of these patients, and duration was not specified for one patient. From the total patient sample, based on the last appointments, 63 patients (17.9%) reported being accompanied by a caregiver to their usual hospital and, of the 59 patients who stated that they sometimes visit a different hospital, 13 patients (3.7%) had been accompanied by a caregiver. Overall mean patient reported worktime loss for caregivers was 5.7 h (SD = 3.5).
On average, patients reported 63.0 min (SD = 49.3) for traveling to and from appointments to their main hospital and an additional 62.1 min (SD = 42.8) were spent at the hospital. In addition, for the 59 patients stating that they sometimes went to a different hospital for INR testing, travel time and time spent at the hospital were 74.2 min (SD = 72.9) and 43.5 min (SD = 31.4), respectively.
Indirect costs incurred by patients and their carers over a 12-month period are shown in ; the estimated indirect cost per INR test was estimated at $35.88. On this basis, if physicians/patients adhered to one INR test per month, the estimated cost per patient per year would be $430.58.
Table 4. Indirect non-medical costs associated with the first 12 months of VKA treatment.
Generalized linear model
No patient characteristics were statistically significantly associated with direct medical costs in the GML, although a trend towards an association between CHA2DS2-VASc and cost was observed ().
Table 5. GLM exploring variables associated with direct medical costs.
Discussion
NVAF is a chronic condition that requires long-term medication in order to avoid potential consequences such as stroke. In terms of drug costs, VKAs are an inexpensive option for stroke prevention in patients with NVAF. However, regular monitoring of INR levels is needed in order to ensure coagulation is maintained within the narrow therapeutic window. Guidelines typically recommend that INR testing occurs at least once every 4 weeks for patients with stable INR levels, although a reduction in frequency may be possible where there are at least 3 months of consistent INR results, suggesting the patient has become stableCitation25. Consequently, INR testing, with its associated healthcare resource utilization, can add considerably to the cost of VKA treatment. Less widely recognized and quantified, however, are the costs incurred by patients, such as direct non-medical costs and indirect costs associated with a loss of worktime.
Assessing the broad, societal costs of VKA treatment in Russian patients was the goal of the present study. Taking into consideration direct medical costs of $151.06, direct non-medical costs of $22.89, and indirect costs of $275.59, the primary driver was consultation time with a cardiologist, with only a small percentage attributable directly to drug costs. This finding is in line with the results of a Canadian modeling study that reported drug costs to account for just 2–6% of VKA treatment costs, depending on the model usedCitation20. To the best of our knowledge, very few economic analyses of VKA treatment have included indirect costs, resulting in an under-estimation of these “hidden” costs in the management of patients with NVAF.
Results from the present study suggest that, in the real-world clinical setting in Russia, the frequency of INR monitoring was lower than recommended in treatment guidelines, which may indicate sub-optimal care of patients with NVAF. The low frequency of INR testing may be a result of the economic and/or humanistic burden to the patient in terms of cost or loss of time; alternatively, infrequent INR testing may arise due to inadequate patient and/or physician education regarding the importance of monitoring. The frequency of INR testing is known to vary worldwide. In the ROCKET AF study, Eastern European and Asian patients had significantly less frequent INR testing than North American and Western European patients (average interval 23 vs 19 and 20 days, respectivelyCitation26. Notably, the authors postulated that the INR test interval range is likely to be wider in regular clinical practice, as the study protocol mandated test intervals should not exceed 8 weeks.
Overall, the data suggests that the proportion of patients receiving anticoagulation therapy is increasing, as exemplified by the increase in such treatments from 57% to 71% of patients at baseline over the course of the GARFIELD AF studyCitation27. However, results from a variety of studies suggests that patient compliance with anticoagulant regimens is suboptimalCitation28, with a high proportion of INR tests being out of therapeutic rangeCitation26, thereby increasing the risk of stroke and major bleeding in patients with AF. Furthermore, complications associated with poor INR control may subsequently lead to an increase in the cost of VKA treatmentCitation29. Improving the management of patients with NVAF, either by increasing compliance with INR testing or using alternative anticoagulant medications, may be a means of generating significant cost savings for healthcare systems.
Some limitations of the present study warrant consideration. Our analyses were based in part on patient-reported data, and data were missing for some outcomes. Furthermore, the retrospective recall period for patients was limited to reduce bias. However, this meant that patient reported data for the previous month or previous appointment typically had to be marked up in order to obtain an annual estimate. In terms of the representativeness of the data, our study included only patients who had uninterrupted VKA therapy for the past 12–24 months and those under the care of a cardiologist. Specialist care from a cardiologist is perhaps the optimal scenario. However, many patients in Russia may have their VKA treatment managed in primary care or by an internist. The associated costs and quality of this care cannot be inferred from the data presented here.
In conclusion, this study has shown that, even for patients who have been treated with a VKA for 12–24 months with ample time to become stable, the true cost of VKA treatment for stroke prevention in Russian patients with NVAF cannot be appreciated by consideration of the drug costs alone. Additional direct medical, direct non-medical, and indirect costs are incurred as a result of INR monitoring. When determining the best course of treatment for stroke prevention in a patient with NVAF, the broader healthcare and patient cost implications should be considered.
Transparency
Funding
The study was sponsored by Bayer Pharma AG.
Declaration of financial/other relationships
JBB and KB are employees of Bayer Pharma AG. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
Some of the data presented in this manuscript was presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) conference 2016, Vienna.
Acknowledgments
We would like to thank Deirdre Carman for assistance with medical writing.
References
- Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333-41
- Townsend N, Nichols M, Scarborough P, et al. Cardiovascular disease in Europe—epidemiological update 2015. Eur Heart J 2015;36:2696-2705
- Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 2007;356:2388-98
- Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154-62
- Nguyen TN, Hilmer SN, Cumming RG. Review of epidemiology and management of atrial fibrillation in developing countries, Int J Cardiol 2013;167:2412-20
- Kirchhof P, Ammentorp B, Darius H, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events–European Registry in Atrial Fibrillation (PREFER in AF), Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol 2014;16:6-14
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke J Cereb Circ 1991;22:983-8
- Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure. Circulation 2009;119:2516-25
- Thrall G, Lane D, Carroll D, et al. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med 2006;119:448.e1-19
- Sheikh A, Patel NJ, Nalluri N, et al. Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future. Prog Cardiovasc Dis 2015;58:105-16
- Camm AJ, De Caterina R, Savelieva I, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation * Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47
- Redon J, Olsen MH, Cooper RS, et al. Stroke mortality and trends from 1990 to 2006 in 39 countries from Europe and Central Asia: implications for control of high blood pressure. Eur Heart J 2011;32:1424-31
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: Executive summary. A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:2246-80
- Buckingham TA, Hatala R. Anticoagulants for atrial fibrillation: Why is the treatment rate so low? Clin Cardiol 2002;25:447-54
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005;165:1095-106
- Boulanger L, Kim J, Friedman M, et al. Patterns of use of antithrombotic therapy and quality of anticoagulation among patients with non-valvular atrial fibrillation in clinical practice. Int J Clin Pract 2006;60:258-64
- Witt DM, Delate T, Clark NP, et al. Nonadherence with INR monitoring and anticoagulant complications. Thromb Res 2013;132:e124-e130
- Andersson S, Björholt I, Nilsson GH, et al. Resource consumption and management associated with monitoring of warfarin treatment in primary health care in Sweden. BMC Fam Pract 2006;7:67
- Chambers S, Chadda S, Plumb JM. How much does international normalized ratio monitoring cost during oral anticoagulation with a vitamin K antagonist? A systematic review. Int J Lab Hematol 2010;32:427-42
- Schulman S, Anderson DR, Bungard TJ, et al. Direct and indirect costs of management of long-term warfarin therapy in Canada. J Thromb Haemost JTH 2010;8:2192-200
- Walsh C, Murphy A, Kirby A, et al. Retrospective costing of warfarin. Ir Med J 2014;107:133-5
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100
- Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Clin Epidemiol 1987;40:373-83
- Wigle P, Hein B, Bloomfield HE, et al. Updated guidelines on outpatient anticoagulation. Am Fam Physician 2013;87:556-66
- Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global geographic region on time in therapeutic range on Warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc 2013;2:e000067
- Kakkar AK, Mueller I, Bassand J-P, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the International, Observational, Prospective GARFIELD Registry. PLoS One 2013;8:e63479
- Gomes T, Mamdani MM, Holbrook AM, et al. Persistence with therapy among patients treated with warfarin for atrial fibrillation. Arch Intern Med 2012;172:1687-9
- Biskupiak J, Ghate SR, Jiao T, et al. Cost implications of formulary decisions on oral anticoagulants in nonvalvular atrial fibrillation. J Manag Care Pharm JMCP 2013;19:789-98