Abstract
Objectives: A recently published retrospective analysis comparing two different active flowable hemostatic matrices (FLOSEAL and SURGIFLO Kit with Thrombin) showed significantly increased resource use and complications (surgery time, risk of blood product transfusion, and amount of matrix used) with SURGIFLO use compared to FLOSEAL in major spine surgery, and also significantly increased surgical time with SURGIFLO use in severe spine surgery. This analysis was developed as a follow-up to this prior analysis, to evaluate the cost-consequence of using FLOSEAL vs SURGIFLO in major and severe spine surgery.
Methods: A cost consequence model was constructed from a US hospital provider perspective. Model parameters combined clinical inputs from the published retrospective analysis with supplemental analyses on annual spine surgery volume using the 2012 National Inpatient Sample (NIS) database. Cost of hemostatic matrices, blood product transfusion, and operating room time were identified from published literature. Various one-way and probabilistic sensitivity analyses were performed.
Results: The base case for a medium volume hospital showed that, compared to SURGIFLO, patients receiving FLOSEAL required three fewer blood product transfusions and saved 27 h of OR time, resulting in annual savings of $151 per major and $574 per severe spine surgery. Additional scenarios for high and low volume hospitals supported cost savings in the base case. Probabilistic sensitivity analysis revealed FLOSEAL was cost-saving in 76% of simulations in major spine and 97% of iterations in severe spine surgery.
Conclusions: This economic analysis indicates that use of FLOSEAL instead of SURGIFLO hemostatic matrices to induce hemostasis in both major and severe spine surgery could potentially lead to sizable cost savings in US hospitals, regardless of spinal surgery case-mix.
Introduction
Inadequate hemostasis may lead to significant blood loss during surgery, which is associated with poor intra-operative and post-operative patient outcomes, including the need for blood product transfusions, the need for surgical revision, increased risk of infection and shock, and increased mortalityCitation1–5. Topical hemostatic agents have been developed and are indicated to control blood loss when traditional surgical methods, such as suture, cautery, or ligature, fail to control bleeding or are inappropriate to useCitation3.
In the US, two commonly used gelatin-based hemostatic matrices are FLOSEAL Hemostatic Matrix (Baxter Healthcare Corporation, Deerfield, IL)Citation6 and SURGIFLO Hemostatic Matrix Kit with thrombin (Ethicon Incorporated, Somerville, NJ)Citation7. Both agents combine passive and active hemostats in a single application productCitation3. Gelatin functions as a passive hemostat, inducing hemostasis via platelet activation and mechanical compression. Thrombin functions as an active hemostat, allowing for cleavage of fibrinogen to form fibrin. These flowable hemostat products act at the beginning and end of the coagulation cascade to promote physical contact activation of platelets and facilitate fibrin formation, respectively. Previous randomized clinical trials have demonstrated increased efficacy in achieving hemostasis for flowable hemostatic agents relative to conventional methods in multiple surgical specialtiesCitation8,Citation9.
FLOSEAL is comprised of a self-expandable gelatin matrix component and lyophilized human thrombinCitation6. SURGIFLO is comprised of a partially reconstituted porcine gelatin and is prepared with EVITHROM (Topical Human Thrombin)Citation7. Despite similar mechanisms of action, in-vitro and in-vivo animal studies comparing FLOSEAL and SURGIFLO have demonstrated faster and more effective hemostasis with FLOSEAL, due to its smooth gelatin matrixCitation10,Citation11. The gelatin within FLOSEAL is arranged with smooth distinct round particles, while the gelatin within SURGIFLO is arranged with stellate coalescing ribbon-like particlesCitation10. After being applied to the bleeding site, FLOSEAL gelatin granules will swell ∼10–20% upon contact with blood, restricting blood flow and providing tamponade, which has been shown to reduce bleedingCitation6,Citation10,Citation11.
There has been a dearth of publications assessing the comparative clinical effectiveness and cost of FLOSEAL and SURGIFLO in spinal surgery, but recent retrospective analyses have assessed population-based spinal surgery outcomes and costs using national hospital billing datasetsCitation4,Citation12. The David et al.Citation13 analysis evaluated outcomes and costs, but restricted to a patient population undergoing spinal fusions or refusions, which tend to be shorter and less complicated surgeries. Price et al.Citation4 evaluated comparative surgical outcomes in the full range of spinal surgery indications: fusions/refusions, resections, stabilizations and corpectomies, relevant to flowable hemostatic agent use. The Price et al.Citation4 study showed that, in major spine surgeries, SURGIFLO use was associated with significantly higher risk of blood product transfusion (OR = 2.56, p < 0.001), longer surgery time (8.84 min, p < 0.0001), and increased product usage (1.9 mL, p < 0.001) (FLOSEAL)Citation4. In severe spine surgeries, SURGIFLO was associated with significantly longer OR time (26.94 min, p < 0.001)Citation4.
This follow-up analysis aims to determine whether the clinically significant outcome differences in the Price et al.Citation4 study, which compared FLOSEAL and SURGIFLO, result in cost differentials for US payers and hospitals.
Methods
Overview
A cost consequence model was developed using Microsoft Excel (Redmond, WA) to estimate the annualized clinical and economic impact of FLOSEAL vs SURGIFLO with Thrombin, based on the average number of spinal surgeries performed in US hospitals, accounting for the difference in operating room time, blood product transfusions, and product volume used between the two products.
Multiple data sources were included in the model. Clinical inputs were taken from the Price et al.Citation4 retrospective analysis of the Premier Perspective hospital database (Charlotte, NC)Citation14. The Premier database captures approximately one of every four hospital discharges in the US, with date-stamped log including procedures, medications, laboratory, and diagnostic services encountered by a patient during a given hospital stay. Supplemental clinical analysis was conducted using the 2012 Healthcare Cost and Utilization Project’s (HCUP’s) National Inpatient Sample (NIS)Citation12. Cost inputs were included using wholesale acquisition costs and published literature.
Patient population
The Price et al.Citation4 study categorized spinal surgeries as either major or severe using ICD-9 procedure codes. Major spinal surgery (MSS) was defined based on the requirement of a fusion/revision-fusion of 2–3 vertebrae or a tumor resection. Severe spinal surgeries (SSS) were defined as one of the following: (1) fusion/revision-fusion of four-to-eight or nine or more vertebrae, (2) tumor resection with a corpectomy or fusion/revision-fusion of more than two vertebrae, (3) fracture stabilization with fusion/refusion of two or more vertebrae, and (4) fracture stabilization with corpectomy or osteotomy, with fusion/revision-fusion of two vertebrae. The Price et al.Citation4 spinal categorization is listed in .
Table 1. Primary or secondary ICD-9 procedural codes for spinal surgeries (from Price et al.Citation4).
Clinical inputs
All surgical outcomes found to be significant in the Price et al.Citation4 study comparing FLOSEAL and SURGIFLO were included in the model. In the MSS group, average length of surgery and percentage of surgical cases requiring blood product transfusions were included. In the SSS group, average length of surgery was included. No significant difference in surgical cases requiring transfusions was found in the SSS group. Additional analyses were conducted on the same Premier dataset to calculate adjusted rates of MSS and SSS cases requiring blood product transfusion, which were unpublished in Price et al.Citation4. summarizes the clinical inputs used in the model.
Table 2. Clinical inputs for MSS and SSS in the cost-consequence model.
Average spinal surgery volume was calculated using an analysis of the 2012 NIS. A hospital performing a medium volume of surgeries serves as the base case scenario for the model. Details of the hospital stratification for major and severe spine surgeries can be found in Appendix .
Cost inputs
Cost inputs were included based on wholesale acquisition costs, and published literature. All costs were converted to 2016 USD using the Medical Care component of the Consumer Price Index (CPI). The cost of product for the average surgical case was obtained by multiplying the average product volume identified in Price et al.Citation4 (from ) with publicly available product prices [Baxter Wholesale Acquisition Cost (WAC) for FLOSEAL and Average Sales Price (ASP) for SURGIFLO from the IMS Health Hospital Supply Index (HIS)]Citation15.
Operating room costs and blood product transfusion costs were taken from published literature. Cost of blood product transfusion was estimated based on Tackett et al.’sCitation1 study, which provides the incremental hospital cost of a single-unit allogenic blood transfusion, inclusive of adverse event and increased resource consumption costs associated with use of allogenic blood products. Blood transfusion costs were inflated in the model to 2016 USD. Using estimates from Chatterjee et al.Citation16, the operating room costs used in the model were estimated to be $478 for each 15-min interval. This estimate was calculated from an average per minute cost for operating room time across five different high-volume surgical proceduresCitation16, expressed in 2016 USD.
The costs associated with hemostatic matrices and surgical procedures are presented in .
Table 3. Cost inputs for MSS and SSS cost-consequence model.
Sensitivity analyses
One-way and probabilistic sensitivity analyses were performed to determine which inputs had the largest impact on the base case results. One-way sensitivity analyses were carried out by varying all model inputs one at a time using the lower and upper boundaries of the 95% confidence intervals or by ±20% if the 95% confidence interval was not available.
Probabilistic sensitivity analysis was conducted on the base case results using a Monte Carlo simulation. All key model inputs were varied at the same time by randomly selecting a value for each parameter within the 95% confidence interval of that parameter according to a distribution probability. All parameters, their 95% confidence interval, and the distribution probabilities can be found in Appendix .
To ascertain whether outcomes and costs varied by volume of spine surgeries performed, scenario analyses were conducted on hospitals performing an average low or high annual volume of spine surgeries.
Results
Base model
The base-case estimates that a hospital performing an average medium volume of major spine surgical procedures in a year may save 27 OR hours and avoid three transfusions with the use of FLOSEAL over SURGIFLO, which may yield net savings of $27,412 (or $151 per surgery). A hospital performing a medium volume of severe spine surgical procedures yearly may save 26 OR hours with FLOSEAL use over SURGIFLO for a net saving of $33,839 (or $574 per surgery) ().
Table 4. Summary of net cost savings—major and severe spine surgery, base case scenario.
Scenario analyses
Results were also calculated for hospitals performing on average more or fewer spine surgeries per year. A hospital performing a high volume of major spine surgery procedures yearly was estimated to save 74 OR hours and eight transfusions, resulting in net cost savings of $75,156 or $413 per surgery with FLOSEAL use. For severe spine surgeries, a hospital with a high volume of surgeries per year may expect to save an estimated 89 OR hours, which provides a net cost saving of $114,135, or $1,934 per surgery ().
Table 5. Summary of net cost savings—major and severe spine surgery, high volume scenario.
A hospital with a low yearly average volume of major spine surgeries was estimated to save 6 h of OR time and one transfusion with FLOSEAL use compared to SURGIFLO, resulting in net cost savings of $6,476, or $36 per surgery. A hospital performing a low average annual volume of severe spine surgeries was estimated to save 6 h of OR time and a reduction in costs of $8,030, or $136 per surgery, when using FLOSEAL compared to SURGIFLO ().
Table 6. Summary of net cost savings—major and severe spine surgery, low volume scenario.
Sensitivity analyses
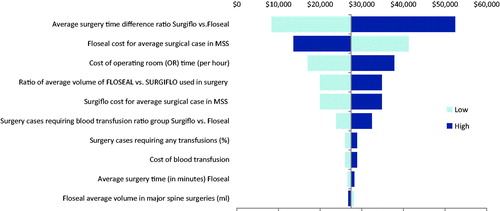
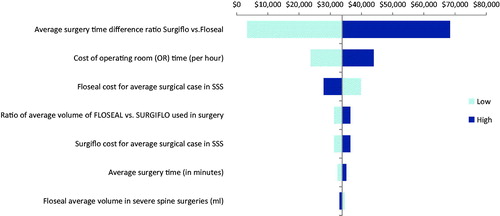
One-way sensitivity analyses were performed across all ranges of each parameter in the base case scenario. For major spine surgery, the cost differential varied between $8,348 and $52,426 (). In severe spine surgery, varying each parameter led to cost savings that ranged from $3,332 to $68,347 (). For all parameters in both major and severe spine surgery, FLOSEAL was always found to be cost-saving. The most influential cost drivers in both major and severe spine surgery were the ratio of the average surgery time using FLOSEAL vs SURGIFLO, the unit cost of FLOSEAL, and the cost of OR time (per hour).
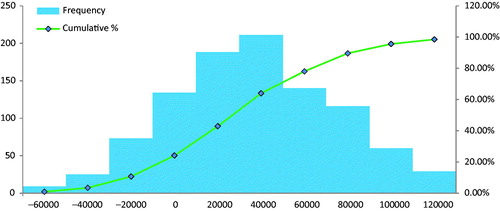
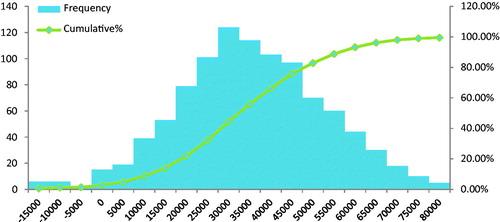
In the probabilistic sensitivity analysis, the base-case was modified by all parameters simultaneously. Approximately 76% of iterations were cost-saving for FLOSEAL in the major spine surgery group (), and 97% of iterations were cost-saving for FLOSEAL in the severe spine group (), supporting the robustness of the cost estimates.
Discussion
This study adds to the limited literature in spinal surgery cost and outcomes associated with the choice of flowable hemostatic agent. This analysis demonstrates cost savings associated with reductions in OR time and transfusions when using FLOSEAL over SURGIFLO as the hemostatic agent of choice during major and severe spine surgery for hospitals at three different volumes (low, medium, and high). We estimate that a US hospital performing a medium volume of spine surgeries on average annually (182 MSS and 59 SSS) may save 53 OR hours, avoid three transfusions, and achieve net savings of $61,250 per year by using FLOSEAL vs SURGIFLO. Despite the increased costs associated with FLOSEAL use, net cost savings of $151 per MSS and $574 per SSS were found. Transfusion rates were excluded from analysis in severe spinal surgery, as Price et al.Citation4 found no significant difference between FLOSEAL and SURGIFLO, and yet cost savings with FLOSEAL use in SSS persist. The most influential driver of cost, as detailed in the sensitivity analyses, was time spent in the OR performing surgery. This correlates with prior studies showing that FLOSEAL achieves hemostasis faster than SURGIFLOCitation3,Citation4.
The strength of our analysis is derived from the Price et al.Citation4 comparative effectiveness analysis of which it is based. To our knowledge, only two other studies comparatively evaluate FLOSEAL and SURGIFLO on surgical outcomes and cost in spine surgeriesCitation13. David et al.Citation13 compared both flowable hemostatic agents in a sample of patients undergoing spinal fusion/refusion surgeries, and found no significant differences in surgical outcomes defined as blood product transfusion procedures, diagnosis of hemorrhage or wound complications, and lower surgical costs with SURGIFLO compared to FLOSEAL. However, the David et al.Citation13 study population was restricted to less complicated spinal surgeries compared to the Price et al.Citation4 study, and did not include procedures with a high risk of bleeding, such as corpectomies and tumor removal. Additionally, David et al.Citation13 allowed for multiple hemostatic agents to be used, hence confounding the results, while Price et al.Citation4 did not. Landi et al.Citation17 compared FLOSEAL and SURGIFLO in lumbar and thoracic spinal surgeries, and found no significant differences between the two agents in time to hemostasis. The Landi et al.Citation17 analysis is hampered by a small patient sample, an exclusive focus on lumbar and thoracic spinal surgeries, and only a single clinical outcome, with no resource utilization measures on which to model an economic analysis.
The results from this model mirror those of a prior European publication evaluating spine surgery complication costs after hemostatic matrix use. Faivre et al.Citation18 reported annual cost savings of €56,000–€344,000 with FLOSEAL use in MSS, and €40,000–€179,000 with FLOSEAL use in SSS in an economic analysis derived from Price et al.Citation4 clinical inputs. Additionally, it is worth noting that our results are in agreement with a similar analysis of hemostatic matrix use in cardiac surgeryCitation3. Here, Tackett et al.Citation3 evaluated FLOSEAL and SURGIFLO in cardiac surgery cases using Premier data from 2006–2012, and found significantly higher risk of multiple adverse outcomes, including major (OR = 2.12, p = .001) and minor complications (OR = 1.84, p < .001); surgical revisions (OR = 2.01, p = .042); transfusions for any blood products (OR = 4.90, p < .001), and longer surgery times (adjusted mean difference = 64 min, p < .001) for surgeries performed with SURGIFLO. The cost consequence model based on the Tackett et al.Citation3 analysis estimated that, for an average hospital volume of 245 cardiac surgeries per year, FLOSEAL use would avoid 42 complications (11 major, 31 minor), nine surgical revisions, and 79 blood product transfusions, and reduce OR time by 260 h, with annual net cost saving of $1,426,688 compared to SURGIFLOCitation19.
This line of research has suggested that the differences between these flowable hemostatic agents results in different outcomes, some of which relate to patient safety, in both spine and cardiac procedures. Differences in outcomes and patient safety may result in substantial savings at the hospital level.
Limitations
All health economic models are a simplification of a complex healthcare decision and, as a result, can have inherent limitations. The clinical inputs that drive this model are derived from the Price et al.Citation4 study and, therefore, its limitations are applicable to our model. That said, for the reasons stated above, we believe Price et al.Citation4 has multiple strengths over the only other published outcome study available to the authors knowledge, that of David et al.Citation13. Additionally, it is noted that the cost inputs for OR time and blood product transfusion costs were taken from the published literature and updated to 2016 US dollars, as costs for these variables were not available in the source administrative datasets or from more recent sources. It is noted that the OR cost input is in line with estimates used in previous surgical studies of $15–34 per minuteCitation20,Citation21.
Conclusions
This study builds on a large, previously published retrospective study demonstrating an advantage in surgical outcomes advantage when using FLOSEAL rather than SURGIFLO during spine surgery. In addition to the clinical benefits, we estimate that FLOSEAL may provide annual cost savings for hospitals performing major and severe spinal surgery ($151 and $574 per surgery, respectively) relative to SURGIFLO. The average US hospital performing a medium number of spine surgeries (182 MSS and 59 SSS) may save 53 OR hours, avoid three transfusions, and realize a net saving of $61,251 per year by using FLOSEAL instead of SURGIFLO. Uncontrolled intra-operative blood loss during spinal surgery can be clinically catastrophic and costly, and use of FLOSEAL may lead to improved outcomes and reduced costs for US payers and hospitals.
Transparency
Declaration of funding
This study was funded by Baxter Healthcare Corporation.
Declaration of financial/other relationships
DM, MR, SI, and EK are all paid employees and stockholders at Baxter. JE and GN are employees at Stratevi, which was retained for this work. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
The research supporting this manuscript was presented in poster format at ISPOR’s 21st Annual International Meeting in Washington DC, 2016.
Acknowledgments
The authors would like to acknowledge Yan Xiong for providing additional analysis for this model.
References
- Tackett S, Sugarman R, Kreuwel H, et al. Hospital economic impact from hemostatic matrix usage in cardiac surgery. J Med Econ 2014;17:670-6
- Nasso G, Piancone F, Bonifazi R, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg 2009;88:1520-6
- Tackett S, Calcaterra D, Magee G, Lattouf OM. Real world outcomes of hemostatic matrices in cardiac surgery. J Cardiothorac Vasc Anesth 2014;28:1558-65
- Price JS, Tackett S, Patel V. Observational evaluation of outcomes and resource utilization from hemostatic matrices in spine surgery. J Med Econ 2015;18:777-86. Erratum in: J Med Econ 2016; 19:315-17
- Hu SS. Blood loss in spinal surgery. Eur Spine J 2004;13(Suppl 1):S3-S5
- FLOSEAL VH S/D Instructions for Use. Deerfield, IL: Baxter Healthcare Corporation; 2005
- SURGIFLO Instructions for Use. Somerville, NJ: J & J Wound Management; 2008
- Mozet C, Prettin C, Dietze M, et al. Use of floseal and effects on wound healing and pain in adults undergoing tonsillectomy: Randomised comparison versus electrocautery. Eur Arch Otorhinolaryngol 2012;269:2247-54
- Chapman WC, Singla N, Genyk Y, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surgeons 2007;205:256-65
- Lewis K, Atlee H, Mannone A, et al. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Invest Surg 2013;26:141-8
- Coenye KE, Bourgain C, Keibl C, et al. A qualitative morphological comparison of two haemostatic agents in a porcine liver trauma model. Surg Sci 2013;4:359-64
- HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 10, 2016
- David G, Lim S, Gunnarsson C, et al. Similar patient outcomes yet different hospital costs between flowable hemostatic agents. J Med Econ 2015;18:735-45
- Premier Inc: Premier PerspectiveTM Hospital Database. Charlotte, NC: Premier Inc.; 2012
- IMS Health. Hospital Supply Index. 2015. IMS America, Plymouth Meeting, PA
- Chatterjee A, Payette MJ, Demas CP, et al. Opportunity cost: a systematic application to surgery. Surgery 2009;146:18-22
- Landi A, Gregori F, Marotta N, et al. Efficacy, security, and manageability of gelified hemostatic matrix in bleeding control during thoracic and lumbar spine surgery: FloSeal versus Surgiflo. J Neurol Surg A Cent Eur Neurosurg 2016;77:139-43
- Faivre P, Laplante S, Kreuwel H. Multi European country cost consequence comparison of Floseal matrix and Surgiflo thrombin in major and severe spine surgeries. Value Health 2015;18:A370
- Makhija D, Rock M, Xiong Y, et al. Cost consequence analysis of two different active flowable hemostatic matrices in cardiac surgery patients (US hospital provider perspective). Poster Presentation at ISPOR 21st Annual International Meeting. Washington DC. May 23, 2016
- Bacchetta MD, Girardi LN, Southard EJ, et al. Comparison of open versus bedside percutaneous dilational tracheostomy in the cardiothoracic surgical patient: outcomes and financial analysis. Ann Thorac Surg 2005;79:1879-85
- Matin SF. Prospective randomized trial of skin adhesive versus sutures for closure of 217 laparoscopic port-site incisions. J Am Coll Surg 2003;196:845-53
Appendix
Table A1. Average number of surgeries per year in low, medium, and high volume hospitals.
Table A2. Probability distributions for clinical inputs.
Table A3. Probability distributions for cost inputs.