Abstract
Background and aims: IDegLira, a fixed ratio combination of insulin degludec and glucagon-like peptide-1 receptor agonist liraglutide, utilizes the complementary mechanisms of action of these two agents to improve glycemic control with low risk of hypoglycemia and avoidance of weight gain. The aim of the present analysis was to assess the long-term cost-effectiveness of IDegLira vs liraglutide added to basal insulin, for patients with type 2 diabetes not achieving glycemic control on basal insulin in the US setting.
Methods: Projections of lifetime costs and clinical outcomes were made using the IMS CORE Diabetes Model. Treatment effect data for patients receiving IDegLira and liraglutide added to basal insulin were modeled based on the outcomes of a published indirect comparison, as no head-to-head clinical trial data is currently available. Costs were accounted in 2015 US dollars ($) from a healthcare payer perspective.
Results: IDegLira was associated with small improvements in quality-adjusted life expectancy compared with liraglutide added to basal insulin (8.94 vs 8.91 discounted quality-adjusted life years [QALYs]). The key driver of improved clinical outcomes was the greater reduction in glycated hemoglobin associated with IDegLira. IDegLira was associated with mean costs savings of $17,687 over patient lifetimes vs liraglutide added to basal insulin, resulting from lower treatment costs and cost savings as a result of complications avoided.
Conclusions: The present long-term modeling analysis found that IDegLira was dominant vs liraglutide added to basal insulin for patients with type 2 diabetes failing to achieve glycemic control on basal insulin in the US, improving clinical outcomes and reducing direct costs.
Introduction
In 2012 it was estimated that 29.1 million people in the US were living with diabetes mellitus (including diagnosed and undiagnosed patients), a prevalence of 9.3%Citation1. This is projected to increase to over 35 million by 2040Citation2. The disease represents the seventh leading cause of death in the US, listed as the underlying cause of death on 69,071 death certificates, and mentioned as a cause of death in a total of 234,051 death certificates in 2010Citation1. However, studies suggest that this may be an under-estimate, as only 35–40% of patients with diagnosed diabetes who have died have the condition mentioned on their death certificate, and patients with diabetes may not be diagnosed at allCitation1. Diabetes is also associated with a large morbidity burden, with increased risk of cardiovascular disease, renal disease, blindness, and amputation compared to the general population. Data from long-term studies in the US has shown that the event rates for diabetes-related complications have fallen from 1990 to 2010 (myocardial infarction by 67.8%, stroke by 52.7%, amputation by 51.4%, and end-stage renal disease by 28.3%)Citation3. Over this 20-year time period, better management of risk factors, such as serum lipid levels (using statins), blood pressure (using renin-angiotensin-system-acting agents), and smoking status are likely to have resulted in reduced incidence of cardiovascular disease, end-stage renal disease, and amputation. Improvements in the management of patient blood sugar levels may have led to reductions in the incidence of end-stage renal disease, amputation, and, to a lesser extent, cardiovascular disease.
Lifestyle intervention and oral anti-diabetic therapies are recommended as initial treatments following diagnosis of type 2 diabetes, but many patients require additional treatment (such as glucagon-like peptide-1 [GLP-1] receptor agonists) and later initiate insulin therapy due to the progressive nature of the diseaseCitation4. Basal insulin alone is often used as the first injectable treatment regimen, and doses are titrated with the aim of bringing patients to a recommended glycated hemoglobin target of 7%Citation5. However, data suggest that 67% of patients with type 2 diabetes receiving basal insulin in the US are not achieving this treatment targetCitation6. Patients failing to achieve glycemic targets on basal insulin are likely to receive additional interventions as part of treatment intensification. Current injectable options for treatment intensification include addition of up to three times daily prandial insulin (such as insulin aspart) or addition of a GLP-1 receptor agonist (such as liraglutide) to basal insulinCitation4. IDegLira represents an alternative treatment option for patients with type 2 diabetes not achieving treatment targets on basal insulin.
IDegLira is a fixed ratio combination of insulin degludec and GLP-1 receptor agonist liraglutide in a single injection device. The fixed-ratio combination was developed to take advantage of the combined effects of a basal insulin and a GLP-1 receptor agonist on glycemic control through their complementary mechanisms of action. The combination of a basal insulin and a GLP-1 receptor agonist may reduce the increased risk of hypoglycemia and weight gain often associated with insulin therapy, and slower titration of the GLP-1 receptor agonist as part of the fixed ratio combination may reduce nausea ratesCitation7,Citation8. The safety and efficacy of IDegLira has been assessed in the DUAL trial programCitation9–13.
The DUAL VII trial comparing IDegLira with basal-bolus insulin therapy has completed and will be reported in mid-2017. However, to date, no head-to-head clinical trials comparing IDegLira with liraglutide added to basal insulin have been conducted. The aim of the present analysis was to assess the long-term cost-effectiveness of IDegLira vs liraglutide added to basal insulin, for patients with type 2 diabetes not achieving glycemic control on basal insulin in the US setting, based on a published indirect comparison.
Methods
Model description
The analysis was performed using the IMS CORE Diabetes Model (IMS Health, Basel, Switzerland), the architecture, assumptions, features, and capabilities of which have been previously publishedCitation14. The model is a validated, non-product specific diabetes policy analysis tool, and is based on a series of inter-dependent sub-models that simulate the complications of diabetes (angina, myocardial infarction, congestive heart failure, stroke, peripheral vascular disease, diabetic retinopathy, macular edema, cataract, hypoglycemia, ketoacidosis, lactic acidosis, nephropathy including end-stage renal disease, neuropathy, foot ulcer and amputation, and non-specific mortality). Each sub-model has a semi-Markov structure and uses time, state, time-in-state, and diabetes type-dependent probabilities derived from published sources. Monte Carlo simulation using tracker variables overcomes the memory-less properties of the standard Markov model, and allows interconnectivity and interaction between individual complication sub-models. Long-term outcomes projected by the model have been validated against real life data in 2004 and, more recently, in 2014Citation15,Citation16.
In the present analysis, a simulated cohort of 1,000 patients was run through the model 1,000 times for each simulation (base case and sensitivity analyses). Mean values and standard deviations were generated for long-term outcomes (life expectancy, quality-adjusted life expectancy, cumulative incidence of diabetes-related complications, time to onset of diabetes-related complications, direct medical costs). The time horizon was set to patient lifetimes in the base case (50 years) to capture all relevant long-term complications and associated costs, thereby assessing their impact on life expectancy and quality-adjusted life expectancy. This approach is in line with guidance on the assessment of the cost-effectiveness of diabetes interventionsCitation17. Future costs and clinical benefits were discounted symmetrically by 3% per annum, in line with published health economic guidance for the USCitation18.
Clinical inputs
To date, no head-to-head clinical trial comparing IDegLira with liraglutide added to basal insulin has been conducted. In light of this, an indirect comparison was conducted, with patient-level statistically modeled to account for differences in baseline characteristics across the trial populationsCitation19. The indirect comparison was performed using a robust methodology (utilizing patient-level data and, therefore, providing a high degree of reliability) aligned with the European Network for Health Technology Assessment guidelines on how to conduct indirect analyses, and has been used previously for indirect comparisons of trials of interventions for diabetesCitation20,Citation21. Treatment effects applied in the first year of the analysis are shown in .
Table 1. Treatment effects applied in the first year of the analysis.
Data to populate the IDegLira arm of the pooled analysis conducted by Freemantle et al.Citation19 were taken from the DUAL II study (NCT01392573)Citation22. DUAL II was a 26-week, randomized, double-blinded trial comparing IDegLira with insulin degludec (capped at 50 international units [IU]) in people with type 2 diabetes uncontrolled on basal insulin in combination with metformin ± sulfonylurea/glinides. Sulfonylureas and/or glinides were discontinued at randomization. Data to inform the pooled analysis and, therefore, present health economic analysis was taken from all patients in the IDegLira arm of the study.
The liraglutide added to basal insulin arm was based on the LIRA-ADD2BASAL study (NCT01617434)Citation23. In this 26-week, double-blinded, randomized trial, patients previously receiving basal insulin were randomly allocated to receive either liraglutide 1.8 mg or placebo. In this study, 66.7% of patients were receiving insulin glargine, and 33.3% of patients were receiving insulin detemir, and the present health economic analysis takes this into account.
The baseline cohort was based on the characteristics of patients receiving IDegLira in the DUAL II studyCitation22. Mean age, duration of diabetes, glycated hemoglobin (HbA1c), and body mass index (BMI) were 56.8 years, 10.3 years, 8.7%, and 33.6 kg/m2, respectively.
Treatment intensification and long-term parameter progression
Patients receiving IDegLira or liraglutide added to basal insulin were assumed to receive that treatment for 5 years before intensifying treatment to basal-bolus insulin therapy. This assumption recognizes that intensification to basal-bolus insulin therapy will likely be required to maintain glycemic control over the long-term due to the progressive nature of the disease (with continued decline of beta-cell function and increased insulin resistance over time). Following application of the treatment effects based on the data from the indirect comparison in the first year of the analysis, systolic blood pressure, and serum lipids were assumed to follow the natural progression algorithms built into the IMS CORE Diabetes Model, based on the UK Prospective Diabetes Study (UKPDS) or Framingham data (as described by Palmer et al.Citation14). Benefits in terms of HbA1c were assumed to persist for the 5 years patients received initial treatments and were abolished on treatment switching. This approach was chosen as there is growing evidence from long-term studies that, in patients with type 2 diabetes mellitus, particularly those receiving some form of insulin therapy, HbA1c remains stable and does not increase over timeCitation24–28. Studies suggest that HbA1c can remain stable for 5 years in both conventional and intensive treatment arms, and therefore assumptions around HbA1c used in the present analysis are likely to reflect clinical practice. These assumptions were varied in sensitivity analyses.
Costs and utilities
Diabetes medication resource use was based on the trial data from which the treatment effects were taken for the two interventions, and was adjusted in the indirect comparisonCitation19. In the IDegLira arm patients were assumed to use one needle per day, with patients in the liraglutide added to basal insulin arm using two needles per day. Costs of self-monitoring of blood glucose (SMBG) testing were based on an analysis of insurance claims in the USCitation29. Annual costs were calculated by multiplying the daily cost by 365.25 ().
Table 2. Annual treatment costs.
The modeling analysis captured the cost of treating diabetes-related complications in the year of the event and in subsequent years. Costs of treating diabetes-related complications were based on a literature reviewCitation30–34. Costs were inflated to 2015 values if necessary. As diabetes progresses, patients develop complications that influence their overall health-related quality-of-life. Utilities associated with each of the modeled complications were taken from published sourcesCitation14,Citation35–38.
Sensitivity analyses
The extrapolation of clinical results by modeling the long-term consequences is associated with uncertainty. Sensitivity analyses were performed on key parameters in the model to assess the robustness of the base case findings. The influence of time horizon on the outcomes projected by the model was investigated by running analyses over 10 and 20 years. It should be noted that a time horizon of 50 years was required for all modeled patients to have died, and therefore shorter time horizons did not capture all potential late-stage complications and costs. To examine the effect of discounting on cost-effectiveness outcomes, simulations were performed with (symmetric) discount rates of 0% and 6% annually. Five simulations were run to assess the key drivers of clinical benefits associated with IDegLira. In the IDegLira arm, changes in HbA1c, systolic blood pressure, serum lipids, BMI, and hypoglycemia were set to the value in the liraglutide added to basal insulin arm in turn. This allowed the contribution of individual clinical effects to long-term health economic outcomes to be assessed. A further analysis was prepared with only statistically significant differences between the treatment arms applied ().
Two alternative approaches to HbA1c progression were explored. In the first, no HbA1c changes were applied following the treatment effects applied in the first year of the analysis. This attempts to capture the legacy effect, where an early improvement in HbA1c has a benefit in the later years of life, even if the HbA1c difference no longer persists. In the second, the UKPDS HbA1c progression equation was applied in both arms of the simulation. HbA1c increases over time in both arms of the analysis, with the HbA1c benefit in the IDegLira arm gradually reduced. Analyses were run with the upper and lower 95% confidence interval of the HbA1c change seen in the IDegLira arm of the pooled analysis applied, with all other parameters in the IDegLira and liraglutide added to basal insulin arm remaining unchanged. The base case analyses assumed that the BMI difference between the treatment arms was abolished on treatment switching, and an alternative to this was explored in a sensitivity analysis, with the difference maintained for the duration of the analysis.
To investigate the effect of the timing of treatment switching on cost-effectiveness, simulations were performed with the year of treatment switch to basal-bolus therapy brought forward to the end of year 3 in the IDegLira and liraglutide added to basal insulin arms, pushed back to the end of year 7 in the IDegLira and liraglutide added to basal insulin arms, and no treatment switching.
The effect of over- or under-estimating the direct cost of treating diabetes-related complications was investigated in two scenarios. In the first, the cost of treating complications was increased by 10%, and in the second the cost was reduced by 10%. The impact of applying alternative disutilities for severe and non-severe hypoglycemic events was assessed by using the values published by Currie et al.Citation39 (–0.0118 per severe hypoglycemic event and –0.0035 per non-severe hypoglycemic event). Additionally, a scenario was investigated in which 28% of patients receiving liraglutide added to basal insulin were assumed to require twice daily insulin, incurring the cost of a further needle for subcutaneous injection, based on a 5-year parallel group study of insulin glargine vs neutral protamine Hagedorn (NPH) insulinCitation40. Insulin doses in the comparator arm were conservatively assumed to remain unchanged in this analysis, To further investigate the impact of consumables on cost-effectiveness outcomes, scenarios were evaluated with costs of needles and SMBG testing excluded. The effect of the cost of basal insulin was investigated in analyses with the cost of the comparator basal insulin replaced with the cost of NPH insulin.
In February 2014, an update to the IMS CORE Diabetes Model incorporating data from the UKPDS 82 was released, and an analysis using this version of the model has been conducted. While a validation study of the revised model has been published, the model proprietors suggest that the update is used in a sensitivity analysis, with the previous version being used in the base caseCitation16. Similarly, version 9.0 of the IMS CORE Diabetes Model was released in summer 2015, and this was used in a sensitivity analysis. To date, no validation studies or user guide for the updated version of the model have been released, and, therefore, it was not considered appropriate to use version 9.0 of the model for the base case analysis.
Probabilistic sensitivity analysis (PSA) was performed using the pre-defined function in the IMS CORE Diabetes Model. Cohort characteristics, treatment effects, and complication costs and utilities were sampled from distributions, and the simulation was run using a second order Monte Carlo approach. Cohorts of 1,000 patients were run through the model 1,000 times for the PSA, as results were not subject to random statistical variation with these settings.
Results
Base case analysis
In the base case analysis, IDegLira was associated with improvements in discounted life expectancy of 0.02 years and discounted quality-adjusted life expectancy of 0.03 quality-adjusted life years (QALYs) vs liraglutide added to basal insulin (). These small but statistically significant improvements (95% confidence intervals for the differences in life expectancy and quality-adjusted life expectancy, 0.01–0.04 years and 0.02–0.05 QALYs, respectively) were due to minor reductions in the incidence of diabetes-related complications, including myocardial infarction, stroke, amputation, end-stage renal disease, and blindness. IDegLira was also associated with a small increase in the time to onset of diabetes-related complications, with mean time free of all complications increased by 0.2 years.
Table 3. Long-term cost-effectiveness outcomes.
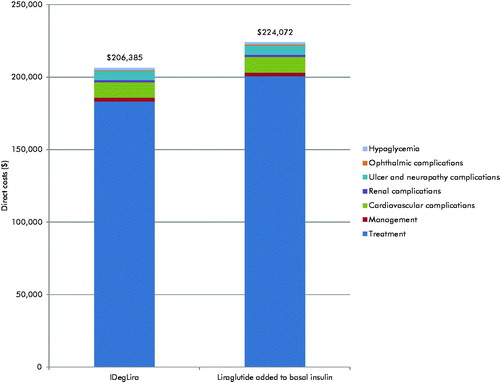
IDegLira was associated with mean cost savings of $17,687 per patient vs liraglutide added to basal insulin over patient lifetimes (). The cost saving was driven predominantly by the lower acquisition cost of IDegLira compared with liraglutide added to basal insulin over the first 5 years of the analysis (annual cost savings of $3,990 per patient). Lower acquisition costs resulted from the lower dose of liraglutide received as part of IDegLira (37.80 dose steps of IDegLira contains 1.36 mg of liraglutide, vs 1.8 mg used in the LIEA-ADD2BASAL trial). Additional cost savings were made due to diabetes-related complications avoided, most notably ulcer and neuropathy complications (mean cost savings of $209 per patient) and cardiovascular complications (mean cost saving of $138 per patient). IDegLira was associated with a small improvement in clinical outcomes and reduced costs compared with liraglutide added to basal insulin, and, therefore, was considered dominant, with no calculation of an incremental cost-effectiveness ratio required ().
Sensitivity analyses
IDegLira remained dominant or associated with equivalent efficacy, but lower costs compared with liraglutide added to basal insulin in all sensitivity analyses prepared (). Shortening the time horizon of the analysis had a notable impact on the calculated outcomes, with the clinical benefit reduced at shorter time horizons. Surprisingly, cost savings were greater at 10 years than at 50 years, which is likely to be due to the survival paradox, where greater survival in the IDegLira arm results in greater costs in later years. Altering the discount rates reflected the long-term benefits associated with IDegLira, with clinical benefits and cost savings increasing when a discount rate of 0% was used, and decreasing when a discount rate of 6% was used.
Table 4. IDegLira vs liraglutide added to basal insulin: Sensitivity analyses.
Abolishing each of the changes in physiological parameters associated with IDegLira identified that the key driver of improved clinical outcome was the greater reduction in HbA1c. Abolishing this difference between the treatment arms resulted in equivalent quality-adjusted life expectancy (although IDegLira remained less costly). Applying only the statistically significant differences did not change the conclusion of the analysis that IDegLira was dominant over liraglutide added to basal insulin. Applying an alternative HbA1c progression with no increases applied at any stage of the analysis in both arms (attempting to replicate the legacy effect) resulted in an increased clinical benefit and increased cost savings in the IDegLira arm, while application of the UKPDS HbA1c progression equation resulted in only small changes in the incremental quality-adjusted life expectancy benefit and cost savings, with IDegLira remaining dominant over basal insulin plus liraglutide.
When the upper 95% confidence interval of the HbA1c change was applied in the IDegLira arm, clinical benefits and cost-savings with IDegLira were found to increase. Conversely, when the lower 95% confidence interval was applied, IDegLira and basal insulin plus liraglutide were associated with equivalent efficacy, but IDegLira was associated with lower costs. Maintaining the BMI difference between the arms (i.e. a small benefit in the basal insulin plus liraglutide arm) resulted in a small reduction in the quality-adjusted life expectancy benefit associated with IDegLira, with only a small change in the difference in costs.
Changing the assumptions around treatment switching had a notable impact on the calculated health economic outcomes. Maintaining patients on IDegLira and basal insulin plus liraglutide for longer increased the incremental clinical benefit and cost saving associated with IDegLira. Assuming that patients remained on IDegLira or basal insulin plus liraglutide for patient lifetimes led to a quality-adjusted life expectancy benefit of 0.15 QALYs and a cost saving of $57,739. Use of alternative hypoglycemia disutilities resulted in increased quality-adjusted life expectancy in both arms of the analysis, but incremental differences showed only small changes due to the similar hypoglycemia rates in both arms. The conclusion that IDegLira was dominant over basal insulin plus liraglutide did not change.
Increasing the cost of complications resulted in increased cost savings with IDegLira, while reducing the cost of complications had the converse effect. Assuming that 28% of patients receiving basal insulin plus liraglutide required twice daily basal insulin resulted in increased cost savings with IDegLira, due to the increased needle costs in the basal insulin plus liraglutide arm. Conversely, removing the costs of needles and SMBG testing resulted in smaller cost savings with IDegLira. In the conservative scenario, with the cost of NPH insulin applied in the basal insulin plus liraglutide arm, IDegLira remained dominant, although cost savings were reduced.
Variation in the risk equations applied and the version of the IMS CORE Diabetes Model used resulted in increased quality-adjusted life expectancy and costs in both arms, but incremental differences showed only minor changes, with IDegLira remaining dominant over basal insulin plus liraglutide in both analyses. PSA suggested that, at a willingness-to-pay threshold of $100,000 per QALY gained, there was a 95.8% probability that IDegLira would be considered cost-effective vs liraglutide added to basal insulin.
Discussion
Based on clinical efficacy data from the DUAL II and LIRA-ADD2BASAL trials, the present study found that, in the US, IDegLira was associated with small improvements in clinical outcomes compared with liraglutide added to basal insulin. Sensitivity analyses identified that the key drivers of superior clinical outcomes with IDegLira was the greater improvement in HbA1c. IDegLira was associated with cost savings as a result of lower daily acquisition costs of treatment and reduced costs of treating diabetes-related complications. Therefore, IDegLira was considered dominant over liraglutide added to basal insulin.
IDegLira may have a number of advantages compared with alternative treatment intensification strategies for patients with type 2 diabetes not achieving glycemic control on basal insulin. It is administered in a single injection device and requires only a single daily injection, compared to up to four daily injections with a basal-bolus regimen and two daily injections with liraglutide added to basal insulin. This lower injection burden is likely to be attractive to patients with type 2 diabetes, as studies show a preference for reduced frequency of injectionCitation41. The single device may also be attractive to general practitioners treating patients with diabetes, as it allows two agents to be titrated simultaneously. This avoids the complexity of titration of basal and prandial insulin doses, aiming to manage overall glycemic control (as measured by HbA1c), post-prandial glycemic excursions, and hypoglycemic events (both nocturnal and diurnal).
To date, no head-to-head trials have been performed comparing IDegLira directly with liraglutide added to basal insulin. In the absence of such head-to-head data, a statistical indirect pooled analysis was performed using robust methodology in line with the European Network for Health Technology Assessment guidelines on how to conduct indirect analysesCitation19,Citation20. A meta-analysis using equivalent methodology to assess the efficacy of diabetes interventions has been previously publishedCitation42. That the comparison was not based on head-to-head studies could be considered a shortcoming of the present analysis. However, selection of the most appropriate comparator was the first priority in the analysis and the use of evidence synthesis, using robust methodologies, is becoming increasingly important and accepted for health technology assessment globallyCitation43.
The present analysis used wholesale acquisition costs to calculate annual treatment costs associated with IDegLira and liraglutide added to basal insulin, and this may represent a limitation of the study. Costs of treatment may have been over-estimated, as potential contracting and rebate structures between the healthcare plans and manufacturers were not captured. Negotiations around these reductions in costs are almost always confidential and, as such, are difficult to capture in cost-effectiveness analyses. The present analysis conducted extensive sensitivity analyses around the pack prices and doses used to calculate annual treatment costs, with IDegLira remaining dominant over liraglutide added to basal insulin in all scenarios. Therefore, the conclusions of the analysis are likely to hold true when rebates are applied, although the magnitude of cost savings may vary.
Another limitation, common to many health economic analyses, particularly those of diabetes interventions, was the reliance on relatively short-term clinical trial data to make long-term projections. Every effort was made in the present analysis to minimize this, primarily by using a model of diabetes that has been extensively published and validated against real-life data, both on first publication and recently following a series of model updatesCitation15,Citation16. Projecting outcomes over patient lifetimes is recommended in guidelines for economic evaluation of interventions for patients with diabetes mellitusCitation17.
Conclusions
The present long-term modeling analysis found that IDegLira was dominant vs liraglutide added to basal insulin for patients with type 2 diabetes failing to achieve glycemic control on basal insulin in the US, improving clinical outcomes and reducing direct costs. The improvements in risk factors associated with IDegLira resulted in a small reduction in the rates of diabetes-related complications over patient lifetimes. Cost savings were identified as a result of avoided diabetes complications and lower costs of treatment. IDegLira is highly likely to be considered cost-effective from a healthcare payer perspective in the US setting.
Transparency
Declaration of funding
The present cost-effectiveness analysis was supported by funding from Novo Nordisk A/S and Novo Nordisk Inc.
Declaration of financial/other relationships
BH and WV are employees of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S and Novo Nordisk Inc. to support preparation of the analysis. MM is an employee of Novo Nordisk Inc. JL is an employee and shareholder of Novo Nordisk A/S. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
No assistance in the preparation of this article is to be declared.
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, US: Centers for Disease Control and Prevention, 2014. http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html. Accessed July 14, 2016
- International Diabetes Federation. IDF Diabetes Atlas, 7th edn. Brussels, Belgium: International Diabetes Federation; 2015
- Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 2014;370:1514-23
- American Diabetes Association. 7. Approaches to glycemic treatment. Diabetes Care 2016;39:S52-S9
- American Diabetes Association. 5. Glycemic targets. Diabetes Care 2016;39:S39-S46
- Langer J, Tian Y, Weng W, et al. Assessing unmet needs for type 2 diabetes patients treated with basal insulin in the United States. Endocrine Prac 2016;22(S2):78-9
- Gough SC, Jain R, Woo VC. Insulin degludec/liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab 2016;11:7-19
- Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014;2:885-93
- Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014;2:885-932
- Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014;37:2926-33
- Linjawi S, Bode B, Chaykin L, et al. Efficacy and safety of IDegLira (combination of insulin degludec + liraglutide), in insulin-naïve patients with T2D uncontrolled on GLP-1 receptor agonist (GLP-1RA) therapy. Diabetes 2015;64(Suppl.1):A255. abstract 1002-P
- Rodbard HW, Bode B, Harris S, et al. IDegLira in insulin-naïve patients with type 2 diabetes (T2D) inadequately controlled on sulfonylureas (SU) alone or in combination with metformin: the DUAL IV study. Diabetes. 2015;64(Suppl.1):A255-A256. abstract 1003-P
- Lingvay I, Manghi FP, García-Hernández P, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA 2016;315:898-907
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (Types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(Suppl1):5-26
- Palmer AJ, Roze S, Valentine W, et al. Validation of the CORE diabetes model against epidemiological and clinical studies. Curr Med Res Opin 2004;20(Suppl1):27-40
- McEwan P, Foos V, Palmer JL, et al. Validation of the IMS CORE diabetes model. Value Health 2014;17:714-24
- American Diabetes Association Consensus Panel. Guidelines for computer modeling of diabetes and its complications. Diabetes Care 2004;27:2262-5
- Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996
- Freemantle N, Mamdani M, Vilsbøll T, et al. IDegLira versus alternative intensification strategies in patients with type 2 diabetes inadequately controlled on basal insulin therapy. Diabetes Ther 2015;6:573-91
- EUnetHTA. European Network for Health Technology. Guideline—comparators & comparisons: direct and indirect comparisons. Diemen: Netherlands, February 2013. http://5026.fedimbo.belgium.be/sites/5026.fedimbo.belgium.be/files/Direct%20and%20indirect%20comparisons.pdf [Last accessed 14 July 2016]
- Zinman B, Schmidt WE, Moses A, et al. Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme. Diabetes Obes Metab 2012;14:77-82
- Buse JB, Vilsbøll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014;37:2926-33
- Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab 2015;17:1056-64
- Wright A, Burden AC, Paisey RB, et al. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002;25:330-6
- Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736-47
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129-39
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72
- Yeaw J, Lee WC, Aagren M, et al. Cost of self-monitoring of blood glucose in the United States among patients on an insulin regimen for diabetes. J Manag Care Pharm 2012;18:21-32
- Yeaw J, Halinan S, Hines D, et al. Direct medical costs for complications among children and adults with diabetes in the US commercial payer setting. Appl Health Econ Health Policy 2014;12:219-30
- Foos V, Varol N, Curtis BH, et al. Economic impact of severe and non-severe hypoglycemia in patients with type 1 and type 2 diabetes in the United States. J Med Econ 2015;18:420-32
- Ramulu PY, Do DV, Corcoran KJ, et al. Use of retinal procedures in Medicare beneficiaries from 1997 to 2007. Arch Ophthalmol 2010;128:1335-40
- Department of Health and Human Services. Questionable billing by suppliers of lower limb prostheses. Washington, DC: Department of Health and Human Services, 2011. https://oig.hhs.gov/oei/reports/oei-02-10-00170.pdf [Last accessed 14 July 2016]
- Carroll K. Older adults can thrive as prosthesis users. Senior Step. Manassas, VA: National Limb Loss Information Center, 2004. http://www.amputee-coalition.org/senior_step/older_prosthesis_users.html [Last accessed 14 July 2016]
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637
- Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes 2013;11:90
- Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ 2005;14:217-30
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523-34
- Rosenstock J, Fonseca V, McGill JB, et al. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: a long-term, randomised, open-label study. Diabetologia 2009;52:1778-88
- Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ 2011;12:219-30
- Zinman B, Schmidt WE, Moses A, et al. Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme. Diabetes Obes Metab 2012;14:77-82
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: Introduction to evidence synthesis for decision making; 2011; last updated April 2012. http://www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf [Last accessed 14 July 2016]