Abstract
Introduction Anaplastic lymphoma kinase (ALK) targeting drugs provide an important option for advanced non-small cell lung cancer patients with this distinct tumor type; however, there is considerable uncertainty as to which drug provides the optimal value after crizotinib treatment. This study estimated the cost-utility of alectinib vs ceritinib from a US payer perspective.
Methods A cost-utility model was developed using partition survival methods and three health states: progression-free (PF), post-progression (PP), and death. Survival data were derived from the key clinical trials (alectinib: NP28761 & NP28673, ceritinib: ASCEND I and II). Costs included drugs, adverse events, and supportive care. Utilities were based on trial data and the literature. One-way and probabilistic sensitivity analyses (PSA) were performed to assess parameter uncertainty.
Results Treatment with alectinib vs ceritinib resulted in increases of 2.55 months in the PF state, 0.44 quality adjusted life-years (QALYs), and $13,868, yielding a mean cost/QALY of $31,180. In the PSA, alectinib had a 96% probability of being cost-effective at a willingness-to-pay of $100,000/QALY. Drivers of model results were drug costs and utilities in the PF health state. The ICER ranged from $10,600–$65,000 per QALY in scenario analyses, including a sub-group analysis limited to patients with prior chemotherapy and crizotinib treatment.
Conclusions Treatment with alectinib in ALK + crizotinib-treated patients increased time progression-free and QALYs vs ceritinib. The marginal cost increase was driven by longer treatment durations with alectinib. This model demonstrates that alectinib may be considered a cost-effective treatment after progression on crizotinib.
Introduction
Advances in our understanding of the etiology and molecular characteristics of cancer have improved our ability to target therapy according to the unique molecular characteristics of tumor tissueCitation1–4. Notably, there have been many developments in the treatment of non-small cell lung cancer (NSCLC) with a number of targeted drugs developed, approved, and being used to improve patients’ length and quality-of-life. Specifically, the development of agents targeting anaplastic lymphoma kinase fusion-gene positive (ALK+) tumors has provided an important option for patients with advanced stage ALK + NSCLC. Approximately 4% of advanced NSCLC patients have ALK + tumors, which translates into 2,300 new patients (previously treated with an ALK inhibitor) expected in 2016Citation5–7. Crizotinib is approved for treatment of ALK + metastatic NSCLCCitation8. However, the majority of patients progress within the first year of treatment or develop intoleranceCitation9–11. Two recently approved agents, alectinib and ceritinib, have been shown to provide clinical benefit for patients who progressed on or were intolerant to crizotinibCitation12–16. These next-generation ALK inhibitors fill an unmet clinical need for ALK + patients. A comparative evaluation of these two agents can inform subsequent treatment decisions for patients in this setting.
Alectinib is a selective inhibitor of ALK that received approval in 2015 by the US Food and Drug Administration (FDA) for the treatment of patients with ALK + metastatic NSCLC who have progressed on or are intolerant of crizotinib treatment. Two recent phase II studies (NP28761 & NP28673) in advanced ALK + NSCLC whose disease progressed on crizotinib (600 mg orally twice daily) demonstrated the efficacy and safety of alectinib in these patientsCitation12,Citation13,Citation17. The NP28761 study was a single-arm, open-label, multi-center trial that evaluated 87 patients, and the NP28673 study was a single-arm, open-label, multi-center trial that evaluated 138 patients. Both studies met their primary end-points with objective response rates (ORR) of 52% (95% CI = 40–65) and 51% (95% CI = 42–60) (at the time of the updated data cut-off), respectivelyCitation12,Citation13,Citation17,Citation18. Median progression-free survival for these two trials were 8.1 (95% CI = 6.2–12.6) and 8.9 (95% CI = 5.6–12.8) (see )Citation13,Citation17,Citation19. Median overall survival (OS) was not reached for either trial after 21 months, but the overall survival at 12 months was 71% (95% CI = 61–81) in the NP28761 studyCitation13.
Table 1. Comparison of alectinib and ceritinib trial data.
Ceritinib was approved in 2014 based on the results of a multi-center, single-arm, open-label clinical trial enrolling a total of 246 advanced ALK + NSCLC patients, 163 of whom had progressed on or were intolerant of crizotinibCitation16. Another similar trial, ASCEND II, was a single-arm, open-label, multi-center phase II study of ceritinib in adult patients with ALK + non-small cell lung cancer previously treated with chemotherapy and crizotinib that enrolled 140 patientsCitation14. All patients received ceritinib at a dose of 750 mg once daily. The ORR for the ASCEND I prior crizotinib sub-group was 46% (95% CI = 38–54)Citation15,Citation16. ASCEND II had an ORR of 49% (95% CI = 39–59)Citation14. Progression-free and overall survival results were also reported with median PFS estimates of 7.0 (95% CI = 5.7–8.7) and 7.2 (95% CI = 5.4–9.0), respectivelyCitation14,Citation15. Median OS was 16.7 (95% CI = 14.8–NR) months for the prior crizotinib sub-group in ASCEND I, with an overall survival at 12 months of 67% (95% CI = 59–74)Citation16. ASCEND II reported a median OS estimate of 14.9 (95% CI = 13.5–NR) months (see )Citation14,Citation15.
Alectinib and ceritinib are both approved for the same indication, have been evaluated in similar patient populations, and are recommended by clinical guidelines as options after crizotinibCitation20. However, they have not been compared head-to-head in clinical trials and, therefore, it is not yet clear which option is likely to provide the optimal choice for patients after crizotinib progression. Our objective was to estimate the clinical and economic impact of treatment with alectinib vs ceritinib from the US payer perspective.
Materials and methods
Markov model overview
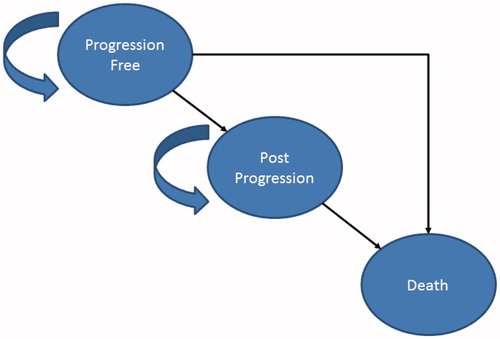
We developed a model in Microsoft Excel (Redmond, WA) with three health states: progression-free (PF), post-progression (PP), and death (). Time in each health state was estimated using partition survival methods (i.e. area under the survival curves). We applied a discount rate of 3% and used a US payer perspective. We used a cycle length of 1 month. Costs are reported in 2016 US dollars. For the cost-effectiveness analysis, the results are reported in terms of incremental cost per life year (LYs) gained and incremental costs per quality-adjusted life years (QALYs) gained.
Clinical inputs
There are no clinical trials directly comparing the efficacy of alectinib and ceritinib. A review of the clinical trial populations and outcomes suggest that a naïve comparison, although limited, may still be informative given the similarity of the patient populations and the clinical setting in which these agents are expected to be used. We, therefore, performed a naive comparison (i.e. a comparison of treatment outcomes across separate trials) using available clinical trial data. Data for alectinib and ceritinib progression-free survival (PFS) and overall survival (OS) were derived from the key clinical trials for these drugs in this setting (alectinib: pooled data from NP28761 & NP28673, ceritinib: ASCEND I and II)Citation12–14,Citation16,Citation17,Citation19,Citation22. We used the Kaplan Meier (KM) curves until the end of the study and extrapolated beyond the end of study using a Weibull parametric function (selected based on AIC values, visual fit, and clinical plausibility) (See Appendix ). The estimates for the parametric functions used to extrapolate the alectinib curves were derived from clinical trial dataCitation23. The estimates for ceritinib were derived using the available KM data and the methodology described previouslyCitation14,Citation16,Citation22,Citation24. In the base case, we used data from ASCEND I for ceritinib PFSCitation16,Citation22. As there is not yet publicly available KM OS data from ASCEND I, we used data from ASCEND II for ceritinib OSCitation14. Although the ASCEND II population includes all patients with prior chemotherapy as well as prior crizotinib treatment, this population is still considered suitable for comparison given the similarity in the PFS data (ASCEND I: prior ALK inhibitor patients = median PFS of 6.93 months vs ASCEND II: median PFS of 7.2 months)Citation14,Citation16,Citation22. However, a sub-group analysis was also conducted which limited the alectinib PFS and OS data to comparable patients with both prior chemotherapy and prior crizotinib treatment and compared outcomes to a ceritinib treated population using ASCEND II data.
Adverse events
Adverse event rates were based on the clinical trial data and package insertsCitation23,Citation25. Grade 3 and 4 adverse events (AEs) occurring in greater than 5.0% of patients or those considered to be resource intensive (i.e. cost per event greater than $1,000) were included. We did not include any AE costs related to alanine aminotransferase or aspartate aminotransferase laboratory levels. The costs of managing each adverse event were estimated using treatment assumptions based on clinical guidelines and expert opinion and drug and medical services unit costsCitation20,Citation26–29. The AE rates and costs are provided in . Treatment assumptions are provided in Appendix .
Table 2. Grade 3/4 adverse event ratesTable Footnotea and costsCitation20,Citation26–29.
Table 3. Model inputs.
Quality-of-life inputs
Health-related quality-of-life was incorporated into the model using utility values (). The PF utility estimates were calculated from patient level data for alectinib and were obtained from publicly available data for ceritinib. Both estimates were calculated by mapping EORTC data from the clinical trials for alectinib and ceritinib to EQ-5DCitation23,Citation30. The PF utility estimates were 0.79 for alectinib and 0.73 for ceritinibCitation23,Citation30. We also evaluated a scenario with equal utilities as part of the sensitivity analysis. The utility of the progression health state was the same for all comparators, and estimated at 0.46 based on Zhou et al.Citation31 and Naffees et al.Citation32.
Cost inputs
We included costs related to drug treatment, adverse events, and supportive care (). Treatment costs per month are calculated using dosing schedules and unit costs for the drugs based on the wholesale acquisition costCitation23,Citation25,Citation33. The model calculates the total cost of treatment assuming that patients are treated until progression or death. Alectinib and ceritinib are both oral drugs and, therefore, no administration costs are included.
Supportive care costs were also included in the progression-free and post-progression health states. The additional cost of care for patients in the progression-free health state was assumed to be $261 per month based on routine monitoring of metastatic NSCLC patients (i.e. one physician visit per month, one chest CT scan every 8 weeks, and one brain/head CT scan every 8 weeks)Citation20. The monthly cost during progression is based on a study by Mariotto et al.Citation34, which estimated the cost of NSCLC in the initial, continuing, and terminal care (last 12 months of life) phases. The cost is highest for the terminal care phase and, therefore, the cost per month in progression is treatment-specific, depending on the average time spent in the post-progression health state, i.e. the longer the time spent in progression beyond 12 months, the lower the per month cost in the progression health state. The monthly cost of the progression-free and post-progression health states is then multiplied by the proportion of patients in each health state in each month in the model and then summed to calculate the total cost.
Sensitivity analysis
The uncertainty in the model was evaluated using one-way and probabilistic sensitivity analysis (PSA). Ranges were based on 95% confidence intervals or plausible ranges, and distributional assumptions were based on recommended guidelinesCitation35. In addition, we performed the following scenario analyses: (1) Alectinib PFS and OS data limited to patients with prior exposure to both platinum-based therapy and crizotinib; (2) Utility values in the PF health state are equal for both alectinib and ceritinib; and (3) ceritinib overall survival is assumed to be the same as alectinib.
Results
In the base case, treatment with alectinib vs. ceritinib resulted in an increase of 2.55 months in the PF state, an additional 0.72 life years, an additional 0.44 quality adjusted life-years (QALYs), and an increase of $13,868. This yielded a mean incremental cost per life-year gained of $19,313, and incremental cost per QALY gained of $31,180 ().
Table 4. Results for cost utility analysis.
Sensitivity analyses
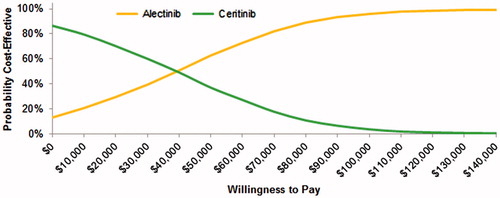
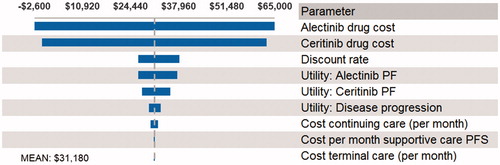
A probabilistic sensitivity analysis was performed (5,000 simulations) for the base-case analysis, and the cost-effectiveness acceptability curve is shown in . The PSA demonstrated that alectinib has a 96% probability of being cost-effective at a willingness-to-pay of $100,000/QALY. In one-way sensitivity analysis, the main model drivers were drug costs and utilities in the PF health state (see ).
In the scenario analysis which used alectinib data limited to patients with prior chemotherapy and prior crizotinib treatment vs ASCEND II data, the ICER was $10,644/QALY. In the scenario with equal utility values (0.79) for alectinib and ceritinib in the PF health state, the ICER was $34,758/QALY. Finally, in the scenario in which the overall survival for ceritinib was set equal to alectinib, the ICER was $64,562/QALY.
Discussion
There is an important unmet need for ALK + metastatic NSCLC patients that progress or are intolerant to treatment with crizotinib. Two agents have been approved in this setting; however, no direct comparisons exist in terms of their clinical and economic impact. We evaluated alectinib vs ceritinib for the treatment of ALK + advanced NSCLC patients to estimate the incremental QALYs and treatment costs from the US payer perspective. We estimated that alectinib is likely to increase time progression free, increase quality adjusted life years, and moderately increased costs. At an estimated cost per QALY of $31,000, alectinib may be considered a cost-effective option compared to ceritinib in the US.
Continued innovation in the treatment of advanced NSCLC is improving outcomes for patients with this serious condition. Crizotinib was the first treatment option available specifically for patients with ALK + tumors. Although this treatment option continues to improve outcomes for these patients compared to traditional chemotherapy, there are limitations with its use, including acquired resistance and the ability to penetrate the blood–brain barrier, which results in modest efficacy preventing or shrinking brain metastasesCitation38. Both alectinib and ceritinib have demonstrated the ability to meaningfully impact central nervous system (CNS) tumors (), however only Alectinib has data on CNS efficacy in its FDA approved prescribing informationCitation39–41. The ability to impact CNS tumors is another advance in the treatment of advanced ALK + NSCLC patients and may confer additional improvements to the length and quality of patients’ lives. Also in this context, these agents may have a role earlier in the treatment sequence—a hypothesis currently under investigation in ongoing clinical trialsCitation42. For example, recent data from the J-ALEX study found a progression-free hazard ratio of 0.34 (95% CI = 0.17–0.70, p < .0001) for alectinib vs crizotinib in Japanese ALK + treatment-naïve NSCLC patientsCitation43. A larger global study is due to be completed in 2017.
There is limited existing data on the cost-effectiveness of alectinib or ceritinib. However, in one analysis reported at ISPOR 2015, Zhou et al.Citation44 found that ceritinib improved outcomes vs untargeted chemotherapy agents, pemetrexed and docetaxel. Importantly, these results are consistent with, and externally validate, the life expectancy and QALY estimates for ceritinib from our model (1.67 life years and 0.98 QALYs vs 1.77 life years and 0.94 QALYs, respectively). Another study evaluated the cost-effectiveness of certinib in Canada and used the same model structure and many of the same assumptions as we have herein. The authors did not, however, include alectinib, which is an appropriate comparator for ALK + patients after crizotinib. We note that the modeled life years and QALYs for ceritinib in their analysis are very similar to those in our model (life years = 1.61, QALYs = 0.86; life years = 1.67, QALYs = 0.98, respectively)Citation45. With the lack of cost-effectiveness data, our study provides the first clinical and economic comparison of alectinib and ceritinib.
There are several limitations to this model worth noting. First, there is no direct evidence comparing alectinib to ceritinib in this setting, and, therefore, we do not have information as to the relative clinical efficacy, safety, and resource utilization for the interventions in the same population. This issue was further complicated by the lack of Kaplan Meier data on overall survival for ceritinib from the ASCEND I trial. We, therefore, performed a naïve comparison and evaluated a number of different scenarios in our sensitivity analysis, including a sub-group analysis using the most comparable data to ASCEND 2 available from the alectinib trials, those with prior exposure to both crizotinib and chemotherapy. Across all the scenario analyses, the cost per QALY was below $100,000 per QALY. Our model results also align well with the 12-month overall survival rate provided in updated results from the ASCEND-1 trial (trial results: 67% vs modeled results: 65%)Citation16. Ultimately, direct comparative trials are needed to determine if there are statistically significant or clinically meaningful differences in progression-free and overall survival between the treatment strategies. Another limitation was the absence of data on the actual costs of care in the progression health state. In the absence of this data, we used data from a widely cited retrospective cancer costing study that utilized SEER-Medicare claims dataCitation34.
In conclusion, we estimated that treatment with alectinib in ALK + crizotinib-treated NSCLC patients increased time in the PF health state, increased life-years, and increased QALYs vs ceritinib. The marginal increase in costs was driven by longer treatment durations with alectinib. The results remained consistent across a number of scenarios. This model demonstrates that alectinib may be considered a cost-effective treatment after patients have progressed on crizotinib according to commonly used thresholds in the US (i.e. < $100,000 to $150,000/QALY).
Transparency
Declaration of funding
This work was funded by Genentech, Inc.
Declaration of financial/other relationships
JC has been a consultant for Genentech, Pfizer, and Seattle Genetics. WC has been a consultant for Astra Zeneca, MedImmune, and the National Pharmaceutical Council. AR is an employee of Genentech. WW is an employee of Genentech. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82
- Mani S. UGT1A1 polymorphism predicts irinotecan toxicity: evolving proof. AAPS PharmSci 2001;3:2
- Tan BR, McLeod HL. Pharmacogenetic influences on treatment response and toxicity in colorectal cancer. Semin Oncol 2005;32:113-9
- Krynetski EY, Evans WE. Pharmacogenetics as a molecular basis for individualized drug therapy: the thiopurine S-methyltransferase paradigm. Pharm Res 1999;16:342-9
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6
- Shaw AT, Solomon BJ. Anaplastic lymphoma kinase (ALK) fusion oncogene positive non-small cell lung cancer. The Netherlands: Wolters Kluwer; Alphen aan den Rijn 2016. http://www.uptodate.com/contents/anaplastic-lymphoma-kinase-alk-fusion-oncogene-positive-non-small-cell-lung-cancer. Accessed March 27, 2016
- XALKORI® (crizotinib) capsules, for oral use. New York: Pfizer, Inc; 2016. http://labeling.pfizer.com/showlabeling.aspx?id = 676. Accessed March 1, 2016
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82
- Katayama R, Friboulet L, Koike S, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res 2014;20:5686-96
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77
- Ou S-HI, Ahn JS, De Petris L, et al. Efficacy and safety of the ALK inhibitor alectinib in ALK + non-small-cell lung cancer (NSCLC) patients who have failed prior crizotinib: an open-label, single-arm, global phase 2 study (NP28673). Paper presented at the ASCO Annual Meeting Proceedings; Chicago, June 2-6 2015
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2015;17:234?42
- Mok T, Spigel D, Felip E, et al. ASCEND-2: A single-arm, open-label, multicenter phase 2 study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung vancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ). Paper presented at the American Society of Clinical Oncology (ASCO); June 2015; Chicago, IL.
- Felip E, Kim DW, Mehra R. Efficacy and safety of ceritinib in patients with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC): an update of ASCEND-1. Paper presented at the European Society of Medical Oncology (ESMO); 26 Sep - 30 Sep 2014; Madrid, Spain
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452?63
- Ou SI, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol 2015;34:661-68
- Barlesi F, Dingemans A-MC, Ou S-HI. Updated efficacy and safety results from a global phase II, open-label, single-arm study (NP28673) of alectinib in crizotinib-refractory ALK + non-small-cell lung cancer (NSCLC). Paper presented at the European Society of Medical Oncology; 25 Sep - 29 Sep 2015
- Gadgeel S, Shaw A, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pre-treated ALK + NSCLC. Paper presented at WCLC; Sept 6-9 2015; Denver, Colorado
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology non-small cell lung cancer. Fort Washington: NCCN, Inc.; 2016
- Shaw AT, West H, Socinski MA. Updated efficacy/safety data from the phase II NP28761 study of alectinib in ALK + NSCLC. World Conference on Lung Cancer (WCLC); Sept. 6-9 2015; Denver, CO
- Felip E, Kim D-W, Mehra R, et al. Efficacy and safety of ceritinib in patients with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC): An update of ASCEND-1. Paper presented at the European Society of Medical Oncology (ESMO); September 2014; Madrid, Spain
- Genentech. Data on File. 2015
- Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Method 2011;11:139
- Novartis Pharmaceuticals Corporation. ZYKADIA™ highlights of prescribing information. Basel, Switzerland: Novartis Pharmaceuticals Corporation 2015
- First DataBank, Inc. Analy$ource Online: the online resource for drug pricing and deal information. South San Francisco, CA: First DataBank, Inc. 2016
- Center for Medicare and Medicaid Services. Acute inpatient prospective payment system. Baltimore, MD; 2015; http://www.cms.hhs.gov/AcuteInpatientPPS. Accessed March 1, 2016
- Centers for Medicare & Medicaid Services. Physician fee schedule national payment amount. Centers for Medicare & Medicaid Services; 2015
- Amgen Inc. EPOGEN® (Epoetin alfa) FOR INJECTION. Thousand Oaks, CA; 2016
- The Scottish Medicines Consortium. Ceritinib 150 mg hard capsules (Zykadia®) SMC No. (1097/15). Glasgow, Scotland: The Scottish Medicines Consortium 2015. https://www.scottishmedicines.org.uk/files/advice/ceritinib__Zykadia__FINAL_Nov_2015_for_website.pdf. Accessed December 20, 2015
- Zhou ZY, Zhang J, Morga A, et al. Cost-effectiveness of ceritinib in the treatment of previously treated anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer in the United Kingdom. Paper presented at the ISPOR 18th Annual European Meeting; November 2015; Milan, Italy
- Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes 2008;6:84
- First DataBank, Inc. Analy source Online: the online resource for drug pricing and deal information. South San Francisco, CA: 2016
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103:117-28
- Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006
- Riley W, Smalley B, Pulkrabek S, et al. Using lean techniques to define the platelet (PLT) transfusion process and cost-effectiveness to evaluate PLT dose transfusion strategies. Transfusion 2012;52:1957-67
- Center for Medicare and Medicaid Services. Physician fee schedule and acute inpatient prospective payment system. Baltimore, MD: Center for Medicare and Medicaid Services 2007. http://www.cms.hhs.gov/PhysicianFeeSched/PFSFRN/list.asp and http://www.cms.hhs.gov/AcuteInpatientPPS. Accessed May 5, 2007
- Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 2015;33:1881-8
- Shi W, Dicker AP. CNS metastases in patients with non-small-cell lung cancer and ALK gene rearrangement. J Clin Oncol 2016;34:107-9
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73
- Genentech. ALECENSA® (alectinib) highlights of prescribing information. South San Francisco, CA: Genentech 2016
- ClinicalTrials.gov. Bethesda, MD: 2016. https://clinicaltrials.gov/. Accessed March 7, 2016
- Hiroshi Nokihara TH, Masashi K, Young HK, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK + NSCLC): Primary results from the J-ALEX study. Paper presented at the 2016 ASCO Annual Meeting; 2016; Chicago, IL
- Zhou Z, Zhang J, Morga A, et al. Cost-effectiveness of ceritinib in the treatment of previously treated anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer in the United Kingdom. Paper presented at the ISPOR 18th Annual European Meeting; 2015; Milan, Italy
- Hurry M, Zhou ZY, Zhang J, et al. Cost-effectiveness of ceritinib in patients previously treated with crizotinib in anaplastic lymphoma kinase positive (ALK+) non-small cell lung cancer in Canada. J Med Econ 2016;19:936-44
Appendix
Table A1. Healthcare resources and costs to treat adverse eventsCitation20,Citation26–29.
Table A2. Parameter inputs.