Abstract
Objectives: BCR-ABL1 tyrosine kinase inhibitors (TKIs) are established treatments for chronic myelogenous leukemia (CML); however, they are associated with infrequent, but clinically serious adverse events (AEs). The objective of this analysis was to assess healthcare resource utilization and costs associated with AEs, previously identified using the FDA Adverse Event Reporting System (FAERS) in another study, among TKI-treated patients.
Methods: Adult patients with ≥1 inpatient or ≥2 outpatient ICD-9-CM diagnosis codes for CML and ≥1 claim for a TKI treatment between January 1, 2006 and September 30, 2012 were identified from the Commercial and Medicare MarketScan databases. The first claim for a TKI was designated as the index event. Patients were required to have no TKI treatment during a 12-month baseline period. Healthcare resource utilization and costs associated with select AEs having the strongest association with TKI treatment (femoral arterial stenosis [FAS], peripheral arterial occlusive disease [PAOD], intermittent claudication, coronary artery stenosis [CAS], pericardial effusion, pleural effusion, malignant pleural effusion, conjunctival hemorrhage) were evaluated during a 12-month follow-up period.
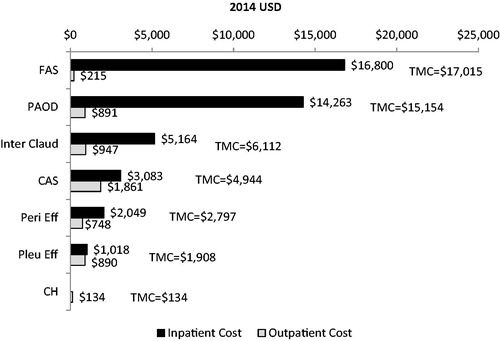
Results: The study sample included 2,005 CML patients receiving TKI therapy (mean age = 56 years; 56% male). Among all evaluated AEs, the highest mean inpatient healthcare costs were observed for FAS ($16,800 per patient) and PAOD ($14,263 per patient), which had total mean medical costs (inpatient + outpatient) of $17,015 and $15,154 per patient, respectively. Mean outpatient healthcare costs were highest for CAS ($1,861 per patient), followed by intermittent claudication ($947 per patient), PAOD ($891 per patient), and pleural effusion ($890 per patient). Total mean medical costs for fluid retention-related AEs, including pericardial effusion and pleural effusion, were $2,797 and $1,908 per patient, respectively.
Conclusions: The healthcare costs of AEs identified in the FAERS as having the strongest association with TKI treatment are substantial. Vascular stenosis-related AEs, including FAS and PAOD, have the highest cost burden.
Introduction
Chronic myelogenous leukemia (CML) is characterized by increased and unregulated growth of hematopoietic stem cells in the bone marrow with an incidence of 1–2 cases per 100,000 adultsCitation1. A reciprocal translocation leading to the formation of the Philadelphia chromosome results in a constitutively active BCR-ABL tyrosine kinase, which plays a central role in the pathogenesis of CMLCitation2,Citation3. Multiple targeted therapies inhibiting BCR-ABL tyrosine kinase activity have been developed, and their use is associated with marked improvements in the outcomes of patients with CMLCitation2–4.
The guidelines of the National Comprehensive Cancer Network (NCCN) recommend three TKIs, imatinib, dasatinib, and nilotinib, for first-line treatment of CML patientsCitation3, which have all been approved in the US and Europe for this indication. Although several clinical trials have demonstrated substantial clinical benefits for patients with CML treated with TKIs, there are some reports of infrequent, but clinically serious adverse events (AEs)Citation4,Citation5. The AEs that may be associated with TKI treatment include hematological, fluid retention, cardiac, metabolic, and gastrointestinal AEs, and can differ between the TKIs; however, some are likely related to effects of the drug classCitation4. For identifying AEs, clinical trial data are limited by the study sample size, entry criteria (e.g. exclusion of patients with comorbidities), and follow-up durationCitation5.
Cortes et al.Citation5 used the FDA Adverse Event Reporting System (FAERS), a large publicly accessible database that supports a post-marketing safety surveillance program for approved drugs and biologics, to identify potential AEs associated with treatment with imatinib, dasatinib, and nilotinibCitation5. The FAERS database includes AEs defined by the FDA as serious if they: (1) resulted in death, hospitalization, disability, or permanent damage; (2) were potentially life threatening; (3) may have caused a congenital anomaly or birth defect; (4) required medical or surgical intervention to prevent permanent damage; or (5) were considered important medical events that could have jeopardized the patient and/or required medical or surgical intervention to prevent one of the above-mentioned outcomesCitation5,Citation6. In the analysis of the FAERS database, Cortes et al.Citation5 used Multi-Item Gamma Poisson Shrinker (MGPS), an established and commonly-used method for identifying drug-event associationsCitation5–7. In brief, the MGPS disproportionality analysis was used to estimate the degree to which the reporting frequency of a drug-event pair is disproportionally higher than would be expected in cases with no drug-event associationCitation5. This estimate is termed the Empirical Bayesian Geometric Mean (EBGM), and an EBGM 90% confidence interval (EB05 ≥ 8) was used to identify events more likely to be clinically relevant and potentially attributable to drug therapy (i.e. meeting a threshold)Citation5. Based on this analysis of the FAERS database, the identified AEs potentially associated with imatinib treatment were bone marrow necrosis, conjunctival hemorrhage, and peritoneal fluid retention events; those associated with dasatinib treatment were related to hemorrhage and fluid retention, including pleural effusion and pericardial effusion; those associated with nilotinib treatment were mostly related to peripheral and cardiac vascular eventsCitation5.
The real-world healthcare and economic burden of the AEs associated with TKIs has not been well studied. To address this need, the objective of this analysis was to assess healthcare resource utilization and costs to a US payer associated with AEs identified with the strongest association in the study of Cortes et al.Citation5, irrespective of their causation, among TKI-treated CML patients using a large claims database analysis.
Methods
AE selection
FAERS adheres to international safety reporting guidance from the International Conference on Harmonization, and AEs are coded using preferred MedDRA termsCitation8. An AE from the Cortes et al.Citation5 analysis was included in our study when the corresponding International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes could be identified for the MedDRA preferred term for that AE. Such AEs identified as most highly associated with the specific TKI in the FAERs analysis were:
Imatinib: conjunctival haemorrhage;
Dasatinib: pleural effusion, malignant pleural effusion, pericardial effusion; and
Nilotinib: femoral arterial stenosis (FAS), peripheral arterial occlusive disease (PAOD), intermittent claudication, coronary artery stenosis (CAS).
Importantly, due to limitations of ICD-9-CM codes correlating with MedDRA terminology, it was not possible to assess the healthcare and economic burden in the claim database for some AE terms identified in the Cortes et al.Citation5 analysis, such as gastric antral vascular ectasia, chylothorax, bone marrow necrosis, tumor necrosis, and tumor hemorrhage.
Study population
Adults (≥18 years of age) with ≥1 inpatient or ≥2 outpatient claims with ICD-9-CM code 205.1, indicating a diagnosis of CML, and ≥1 claim for a TKI between January 1, 2006 and September 30, 2012 were identified from the Truven Health Analytics MarketScan data source. The claims data from the MarketScan databases includes inpatient and outpatient information reflecting real-world treatment patterns and costs. In compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA), the databases consist of fully de-identified data sets. Patient data were extracted from both the Commercial and Medicare Supplemental databases to maximize the representation of patients from different age groups.
The first claim for a TKI was designated as the index event. Patients were required to have continuous enrollment during 12-month periods before (baseline period) and after (follow-up period) the index event. Patients were also required to have received no prior TKI treatment in the baseline period (i.e. newly initiated TKI treatment).
Measurements
For each selected CML, patient demographics including age, gender, healthcare plan type, US geographic region of care, and Charlson Comorbidity Index (CCI) were determined during the baseline period. Healthcare resource utilization and costs associated with the select AEs, documented with primary ICD-9-CM codes on inpatient or outpatient healthcare claims, during the 12-month follow-up period were evaluated, irrespective of the TKI prescribed. All costs were inflation adjusted to 2014 cost levels using the Consumer Price Index: Medical Care. A cause and effect relationship between AEs and treatment was not assessed.
Results
Demographics and clinical characteristics
Patient demographics and clinical characteristics are presented in for the study population with CML treated with TKIs. The median age of the CML study population newly initiated on TKI treatment (n = 2,005) was 56.0 years, 55.7% were male, and the majority (56.9%) had a preferred provider organization health plan. Among the study population, 37.6% were from the South, 29.2% were from the North Central region, 16.9% were from the West, and 15.0% were from the Northeast. Mean CCI score was 2.9, with the majority (59.1%) of the study population having scores of 1–2.
Table 1. Demographics and clinical characteristics of the study population with chronic myelogenous leukemia.
AE-related healthcare resource utilization and associated costs
AE-related healthcare resource utilization and associated costs are shown in . During the 12-month follow-up period after initiation of TKI treatment, CAS (11.8%) occurred the most prevalently, followed by pleural effusion (4.7%), conjunctival hemorrhage (2.2%), PAOD (1.9%), intermittent claudication (1.1%), FAS (0.6%), and pericardial effusion (0.6%) among the study population with CML. No cases of malignant pleural effusion were observed in the study population. Of the select AEs, the greatest mean number of hospitalizations was observed for those with PAOD (0.24 per patient), followed by those with pericardial effusion (0.15 per patient), and CAS (0.09 per patient) (). Among patients with the select AE events, mean hospital LOS was longest for patients with FAS (3.0 days per patient) and PAOD (2.2 days per patient) (). Hospital LOS was much shorter for the other AEs. Use of outpatient medical services was greatest among patients with pericardial effusion, pleural effusion, and CAS.
Figure 1. Mean number of hospitalizations and length of stay (LOS) per patient for patients with select adverse events. FAS: femoral arterial stenosis; PAOD: peripheral arterial occlusive disease; Inter Claud: intermittent claudication; CAS: coronary artery stenosis; Peri Eff: pericardial effusion; Pleu Eff: pleural effusion; CH: conjunctival hemorrhage; LOS: length of stay.
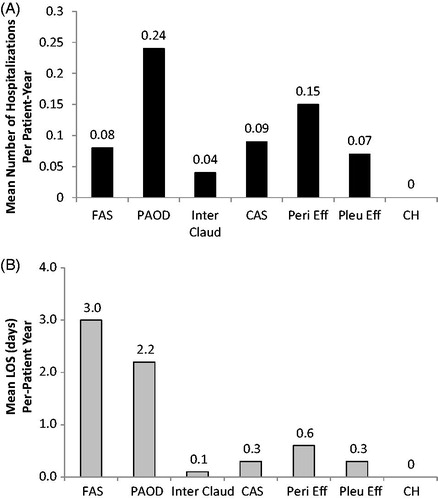
Table 2. Adverse event-related healthcare resource utilization and associated costs among patients with the corresponding select adverse events from the study population with chronic myelogenous leukemia
Among patients with the select AEs, the highest mean inpatient healthcare costs were among patients with FAS ($16,800 per patient) and PAOD ($14,263 per patient), which had total mean medical costs (inpatient + outpatient) of $17,015 and $15,154 per patient, respectively. Mean outpatient healthcare costs were highest for patients with CAS ($1,861 per patient), followed by those with intermittent claudication ($947 per patient), PAOD ($891 per patient), and pleural effusion ($890 per patient). Total mean medical costs for fluid retention-related AEs, including pericardial effusion and pleural effusion, were $2,797 and $1,908 per patient, respectively. In this analysis, patients with conjunctival hemorrhage received treatment in the outpatient setting only, with a total mean medical cost of $134 per patient. Total medical costs with the inpatient and outpatient costs breakdown for all analyzed AEs are shown in .
Figure 2. Total mean medical and inpatient and outpatient costs per patient for patients with select adverse events. FAS: femoral arterial stenosis; PAOD: peripheral arterial occlusive disease; Inter Claud: intermittent claudication; CAS: coronary artery stenosis; Peri Eff: pericardial effusion; Pleu Eff: pleural effusion; CH: conjunctival hemorrhage.
Discussion
According to this analysis of select AEs among CML patients treated with TKIs, irrespective of specific TKIs, inpatient utilization and consequently total medical costs are highest for those with vascular stenosis-related AEs, including FAS and PAOD. These vascular events were infrequent, with FAS and PAOD having prevalence rates of 0.6% and 1.9%, respectively, among the study population with CML. However, FAS and PAOD represent serious AEs with significant clinical manifestations, and consideration for patient risk should be carefully assessed in the decision-making process for TKI treatment. CAS (11.8%) and pleural effusion (4.7%) occurred at higher rates than FAS and PAOD, but were associated with less hospitalization and lower total medical costs per patient. Thus, monitoring for these conditions among CML patients treated with TKIs is warranted. Because of data source limitations, the prevalence rates of the evaluated AEs among CML patients in this study may not be reflective of other populations of CML patients in which age distribution and CML severity or phase differ. Furthermore, the prevalence rates of AEs and the associated estimated cost burdens are based on 1 year of follow-up, and future studies with a longer-term follow-up may reveal different rates over time and potentially higher costs. Also, it will be important in future investigations to evaluate the influence of certain co-morbid conditions on the risk for AEs among CML patients.
This study uniquely shows the costs of select AEs among CML patients treated with TKIs and few comparative data are available in the published literature. According to data from the Healthcare Cost and Utilization Project (HCUP), the mean cost per hospitalization for occlusion or stenosis of precerebral arteries was $8,000 in 2005 USDCitation9. A study of 2,137 US patients within an international registry who had peripheral arterial disease (2003–2004) reported 2-year hospitalization costs ranging from $7,000–$11,693Citation10. The greater costs observed in our study associated with similar events are likely related to several reasons, including more current study years, inclusion of all related costs in up to 1 year of follow-up, and that only CML patients were included.
From this analysis, a cause and effect relationship between AEs and treatment cannot be established. Also, specific TKI treatment related AEs were not determined. Furthermore, the selection of the AEs for this analysis was based on the Cortes et al.Citation5 study using the FAERS database, and may not be comprehensive due to the limitations of the FAERS database as previously described. Briefly, the data in the AE reports contains limited information on event severity, drug dose, and prior treatment history, reporting rates of AEs can change over time, and in the Cortes et al.Citation5 study, only three organ class terms were used. Despite these limitations of the data source, the selection of AEs in this study was based on what was reported in a peer reviewed publicationCitation5, and the results reflect the AEs that occurred among patients with CML treated with TKIs in the real-world setting. The study findings should provide healthcare decision-makers useful insights regarding the real-world economic burden of the AEs associated with TKI treatment.
Limitations
Healthcare claims in the MarketScan databases are submitted by healthcare providers to insurance companies for reimbursement, and such claims are subject to possible coding errors, coding for the purpose of rule-out rather than actual disease, and under-coding, without the possibility of verifying reported diagnoses. Although the MarketScan databases have a broad coverage across all geographic regions of the US, patient claims contained in the database do exhibit an uneven distribution among US regions, with more claims from the Southern and North Central regions. The data are primarily from large employers, i.e. medium and small firms are not well represented. Importantly, due to limitations of ICD-9-CM codes correlating with MedDRA terminology, it was not possible to assess the healthcare and economic burden in the claim database for some AE terms identified in the Cortes et al.Citation5 analysis, such as gastric antral vascular ectasia, chylothorax, bone marrow necrosis, tumor necrosis, and tumor hemorrhage. Additionally, more likely to occur in the outpatient setting, not severe AEs may have been coded less accurately and, thus, missed in this analysis. Approximately 24% of our study population was ≥65 years of age, and this proportion of elderly patients may not accurately reflect the age distribution of the CML population in the US. This limitation of the data source may have led to an under-estimate of the costs of AEs, since younger CML patients may potentially be less likely to experience AEs and when they do may have less costly events than older patients. Future studies focusing on patients more accurately reflecting the age distribution of the CML population are warranted to further examine the economic burden of these AEs.
Conclusion
The healthcare costs among patients who experienced AEs, as identified in the FAERS analysis as having the strongest association with TKI treatment, are substantial. Patients with vascular stenosis-related AEs, including FAS and PAOD, have the highest resource use and cost burden. Potential AE clinical and cost implications should be considered when treating patients with CML.
Transparency
Declaration of funding
This study and development of this manuscript was funded by Bristol-Myers Squibb.
Declaration of financial/other relationships
JL and MLS are employees of Novosys Health, which has received research funds from Bristol-Myers Squibb in connection with conducting this study and development of this manuscript. DM and RB are employees of Bristol-Myers Squibb and own stock in the company. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
Some aspects of this study were previously presented at the National Comprehensive Cancer Network 21st Annual Conference in Hollywood, FL, March 31–April 2, 2016, and the 58th American Society of Hematology Annual Meeting in San Diego, CA, December 3–6, 2015.
References
- Ramchandren R, Schiffer CA. Dasatinib in the treatment of imatinib refractory chronic myeloid leukemia. Biologics 2009;3:205-14
- Jabbour E, Cortes J, Kantarjian H. Long-term outcomes in the second-line treatment of chronic myeloid leukemia. Cancer 2011;117:897-906
- National Comprehensive Cancer Network Guidelines. Chronic myelogenous leukemia. v. 2.2017. January 19, 2017. Fort Washington, PA. https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf Accessed January 20, 2017
- Jabbour E, Deininger M, Hochhaus A. Management of adverse events associated with tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia 2011;25:201-10
- Cortes J, Mauro M, Steegmann JL, et al. Cardiovascular and pulmonary adverse events in patients treated with BCR-ABL inhibitors: Data from the FDA Adverse Event Reporting System. Am J Hematol 2015;90:E66-72
- US Food and Drug Administration. What is a serious adverse event? Silver Spring, MD. http://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm Accessed January 20, 2017
- Harpaz R, DuMouchel W, LePendu P, et al. Performance of pharmacovigilance signal detection algorithms for the FDA Adverse Event Reporting System. Clin Pharmacol Ther 2013;93:539-46
- MedDRA Maintenance and Support Services Organization. Introductory guide to MedDRA Version 15.0. Publication no. MSSO-DI-6003-15.0.0. Chantilly, VA: International Federation of Pharmaceutical Manufacturers and Associations; 2012
- Russo CA, Andrews RM. Healthcare Cost and Utilization Project. Statistical Brief #51. Hospital stays for stroke and other cerebrovascular diseases, 2005. May 2008. Rockville, MD. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb51.jsp Accessed January 20, 2017
- Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes 2010;3:642-51
