Abstract
Objectives: This study investigated the cost per responder and number needed to treat (NNT) in type 2 diabetes mellitus (T2DM) patients for lixisenatide compared to insulin intensification regimens using composite endpoints in the UK, Italy, and Spain.
Methods: Efficacy and safety outcomes were obtained from GetGoal Duo-2, a 26-week phase 3 trial comparing lixisenatide vs insulin glulisine (IG) once daily (QD) and three times daily (TID). Response at week 26 was extrapolated to 52 weeks, assuming a maintained treatment effect, based on long-term evidence in other T2DM populations. Responders were defined using composite end-points, based on an HbA1c threshold and/or no weight gain and/or no hypoglycemia. The HbA1c threshold was varied in sensitivity analyses. Annual treatment costs were estimated in euros (1 GBP = 1.26 EUR), including drug acquisition and resource use costs. Cost per responder was computed by dividing annual treatment costs per patient by the proportion of responders.
Results: Lixisenatide was associated with the lowest cost per responder for all composite end-points that included a weight-related component. For the main composite end-point of HbA1c ≤7.5% AND no weight gain AND no symptomatic hypoglycemia, cost per responder results were: UK: 6,867€, 8,746€, and 12,410€; Italy: 7,057€, 9,160€, and 12,844€; Spain: 8,370€, 11,365€, and 17,038€, for lixisenatide, IG QD, and TID, respectively. The NNT analysis showed that, for every 6.85 and 5.86 patients treated with lixisenatide, there was approximately one additional responder compared to IG QD and TID, respectively.
Limitations: A limitation of the clinical inputs is the lack of 52-week trial data from GetGoal Duo-2, which led to the assumption of a maintained treatment effect from week 26 to 52.
Conclusions: This analysis suggests lixisenatide is an efficient economic resource allocation in the UK, Italy, and Spain.
Introduction
The economic burden of diabetes is well understood, affecting an estimated 9.1% of the adult population in EuropeCitation1. Of the adult population with diabetes in high-income countries, it is estimated 91% have type 2 diabetes mellitus (T2DM)Citation1. This translates to 2.60 million, 3.19 million, and 3.26 million patients with T2DM in the UK, Italy, and Spain, respectivelyCitation1. The International Diabetes Federation (IDF) estimates diabetes accounts for 11.6% of total health expenditure worldwide. The UK, Italy, and Spain spend 12.5 billion, 12.1 billion, and 10.2 billion USD a year, respectively, with the IDF placing the UK and Italy in the top 10 countries for diabetes-related expenditure in 2015Citation1. With the number of people being diagnosed with T2DM rapidly growing, it is expected this expenditure will continue to rise in future years, and, therefore, it is essential the most cost-effective interventions are at the forefront of diabetes management.
Conventional therapy for T2DM has focused on glycemic control with glycated hemoglobin (HbA1c) thresholds. When patients have failed treatment on oral antidiabetic (OAD) medicinal products and/or basal insulin in combination with diet and exercise, an insulin intensification regimen with rapid-acting insulin (RAI) would often be recommended to manage the HbA1c level of a patient. However, insulin intensification regimens with RAI have been shown to be associated with increased hypoglycemic events and weight gainCitation2. Recent guidelines in the UK, Italy, and Spain reflect the importance of these short-term outcomes in diabetes management by including them as key drivers of treatment managementCitation3–6. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), such as lixisenatide, have been shown to reduce weight and rates of hypoglycemia in patientsCitation7. GLP-1RAs are indicated in overweight patients, and, therefore, weight gain is a particularly important factor when determining treatment response in these patients and a focus in treatment guidelines. Furthermore, a reduction in weight has been shown to improve glycemic control and lead to an improved cardiovascular risk profileCitation8. European guidelines now recommend GLP-1RAs, such as lixisenatide, as another option to RAI at this point in the treatment pathwayCitation2.
Lixisenatide is a once-daily injectable, GLP-1RA for the treatment of T2DM in adults. It is indicated for adult patients who have failed treatment on OADs and/or basal insulin, in combination with diet and exercise. The efficacy and safety of lixisenatide was investigated in an insulin-exposed population in a phase III, randomized trial, and compared to insulin intensification regimens with RAICitation7.
The aim of this analysis was to assess the economic benefit of lixisenatide compared to insulin intensification regimens with RAI by calculating a cost per responder and number needed to treat (NNT) using composite end-points reflecting the multi-faceted approach to diabetes management.
Methods
Study design
The cost-effectiveness of lixisenatide vs insulin intensification regimens was evaluated in terms of cost per responder and number needed to treat (NNT) for the UK, Spain, and Italy. Lixisenatide was compared to insulin glulisine (IG) once daily (QD) and three times daily (TID). A successfully treated patient was defined by two composite end-points; HbA1c threshold ≤7.5% AND no weight gain and HbA1c threshold ≤7.5% AND no weight gain AND no symptomatic hypoglycemia. These composite end-points reflect treatment management guidelines in the three country settings of the analysis. Weight gain was defined as any increase in weight over the trial for an individual patient. Documented, symptomatic hypoglycemic events were included in the analysis, defined by an event with clinical symptoms that are considered to result from a hypoglycemic episode with an accompanying plasma glucose of <60 mg/dL or associated with a prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration. Sensitivity analyses were performed using HbA1c thresholds of ≤7.0%, ≤8.0%, ≤8.5%, and a reduction of HbA1c in ≥1% as well as for the end-points of HbA1c threshold AND no symptomatic hypoglycemia, and no weight gain AND no symptomatic hypoglycemia.
Clinical data
The proportions of responders for all analyses were obtained from the GetGoal Duo-2 study. GetGoal Duo-2 (NCT01768559) was a phase III, randomized, open-label, active controlled, 26-week trial investigating the efficacy and safety of lixisenatide vs IG on top of insulin glargine, with or without metformin in type 2 diabetic patientsCitation7. Patients aged 18 years and older treated with basal insulin for at least 6 months, with or without OADs, were randomized to receive lixisenatide (starting dose 10 mcg, increased to 20 mcg maintenance dose after 2 weeks), IG once a day (individual titration, injected before breakfast or dinner), or IG three times a day (individual titration, injected before breakfast, lunch, and dinner). The patients included in the GetGoal Duo-2 trial had a mean duration of diabetes of 12.2 years with a baseline BMI of 32 kg/m2 (see the supplementary material Table 6 for more details on the baseline characteristics from the GetGoal Duo-2 study).
The 26-week clinical trial data were extrapolated to 52 weeks to estimate the annual cost per responder. An assumption of a maintained treatment effect from week 26 to week 52 was implemented. For example, 46.1% of patients were observed to achieve the composite end-point of HbA1c threshold ≤7.5% AND no weight gain in the lixisenatide arm over 26 weeks, and it was assumed that the same proportion of patients (46.1%) would have reached this end-point at week 52. This assumption was based on longer-term evidence from other lixisenatide trials. In lieu of 52-week evidence for lixisenatide in an insulin-exposed population, lixisenatide trials longer than 26 weeks in any T2DM population were identified to determine if there was a significant difference in the number of HbA1c responders at a ≤7% threshold at 24 weeks and 52 weeks in the lixisenatide arm. An HbA1c threshold of ≤7% was the closest primary or secondary outcome of the trials to the base case threshold in these analyses of 7.5%. There were six trials identified that extended to 52 weeks, which included eight lixisenatide arms in total (GetGoal-F1Citation9, GetGoal-MCitation10, GetGoal-LCitation11, GetGoal-PCitation12, GetGoal-SCitation13, GetGoal-XCitation14). Chi-squared tests were performed. No significant difference was found in the proportion of responders in seven of the eight lixisenatide arms at the 95% confidence level. For the morning injection of lixisenatide arm in GetGoal-MCitation10, there was a significant difference found; however, the percentage of responders increased, rather than decreased, from week 24 to week 52.
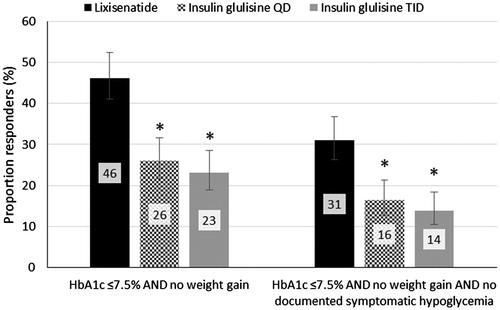
The evidence from GetGoal Duo-2 showed lixisenatide was associated with a statistically significant higher proportion of responders (p < .05) compared to both insulin intensification regimens for both composite end-points (). For the composite end-point of HbA1c threshold AND no weight gain, there was a 2.43- and 2.86-times higher odds of being a responder for a patient receiving lixisenatide compared to IG QD and TID, respectively. When no symptomatic hypoglycemia was added to the composite end-point, patients receiving lixisenatide had a 2.29- and 2.78-times higher odds of being a responder compared to IG QD and TID, respectively. The confidence intervals were calculated using the Wilson Score Interval method for binary dataCitation15. The supplementary material contains the proportion of responders for the sensitivity analyses (Supplementary Table 7).
Figure 1. Proportion of responders for the composite end-points by treatment arm. Error bars represent the confidence intervals calculated using the Wilson score method. * Indicates a statistically significant difference in the number of responders compared to lixisenatide at the 95% confidence level.
Cost inputs
The cost per patient in each treatment arm was calculated over 52 weeks. Costs included pharmacy and resource use costs. The pharmacy costs included the cost of lixisenatide or IG, with all arms having a cost for insulin glargine and metformin. In all country settings Lantus was the brand used to cost insulin glargine, whereas the most commonly used brand specific to the country setting was used for IG; Apidra, Lispro, and Aspart for the UK, Italy, and Spain, respectively. Drug costs were sourced from country-specific sources for 2016 (see ); the British National Formulary for the UK, BotPlus for Spain, and the Gazette Ufficiale for Italy. Resource use was sourced from the GetGoal Duo-2 trial, and included the cost of lancets, needles, and self-monitoring blood glucose test strips. Resource use costs were sourced from official country-specific sources where available or communication with pharmacists. The UK costs were converted to euros to allow for comparison across the country settings (exchange rate 1 GBP = 1.26 EURCitation16). See for the drug and resource unit costs.
Table 1. Drug and resource use unit costs (€).
These drug costs were combined with dosing and resource usage from the GetGoal Duo-2 trial () to calculate a treatment cost for each arm over a 52-week period (). No discount rate was applied to cost.
Table 2. Daily dosages and resource consumptions (from GetGoal Duo-2).
Table 3. Treatment costs per patient over 52 weeks (€).
Cost per responder
The responder analysis using the clinical efficacy data from the GetGoal Duo-2 trial was combined with the 52-week treatment costs to determine an annual cost per responder for each treatment arm. This was computed by dividing annual treatment costs per patient by the proportion of responders. The confidence intervals calculated for the binary outcomes in the responder analysis were used to generate a sensitivity range for the cost per responder (the costs were held constant).
Number needed to treat
The NNT was calculated to determine the average number of patients needed to be treated in order to gain one additional successful responderCitation31. In lieu of a placebo arm in the GetGoal Duo-2 trial, the NNT was calculated using the IG QD and TID arms as the control. Confidence intervals were calculated using the Wilson Score methodCitation31.
Results
Cost per responder
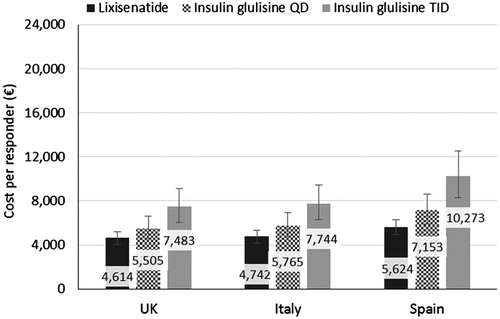
Lixisenatide was associated with a lower cost per responder for the composite end-point of HbA1c threshold AND no weight gain (). The largest difference in the cost per responder was seen in the Spanish setting, with lixisenatide 1,529€ and 4,649€ lower than IG QD and TID, respectively. Lixisenatide had a similar cost per responder in the UK and Italy, 4,614€ and 4,742€, respectively.
Figure 2. Cost per responder base case results across the country settings for the composite end-point of HbA1c threshold ≤7.5% AND no weight gain. Error bars represent the confidence intervals calculated using the Wilson score method.
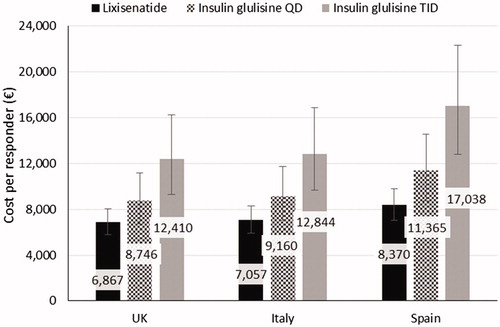
When no symptomatic hypoglycemia was added to the composite end-point definition, lixisenatide remained the intervention with the lowest cost per responder (). For this end-point, lixisenatide had a cost per responder of 6,867€, 7,057€, and 8,370€for the UK, Italy, and Spain, respectively.
Figure 3. Cost per responder base case results across the country settings for the composite end-point of HbA1c threshold ≤7.5% AND no weight gain AND no hypoglycemia. Error bars represent the confidence intervals calculated using the Wilson score method.
The results of the sensitivity analyses are presented in . The sensitivity analyses showed lixisenatide remained the lowest cost per responder across the HbA1c thresholds of ≤7.0%, ≤8.0%, ≤8.5%, and a reduction in ≥1% investigated for the composite end-points of HbA1c threshold AND no weight gain, and HbA1c threshold AND no weight gain AND no symptomatic hypoglycemia. As the threshold increased, the difference in responder rates between lixisenatide and the insulin intensification regimens increased and remained statistically significant.
Table 4. Cost per responder (€) sensitivity analysis results.
For the end-points considering different HbA1c thresholds AND no symptomatic hypoglycemia, lixisenatide was associated with the highest responder rate compared to both insulin intensification regimens for the HbA1c thresholds of ≤7.0%, ≤7.5%, ≤8.0% and ≤8.5%. Although lixisenatide is more effective, the difference in proportion of responders was not sufficiently high to balance the higher daily cost of lixisenatide compared to IG QD and TID in the UK and Italian settings. In the Spanish setting lixisenatide is associated with a higher cost per responder than IG QD for all HbA1c thresholds, and it is higher compared to IG TID for the thresholds of ≤7.5% and a reduction in ≥1%.
When the composite end-point including the safety end-points of no weight gain AND no symptomatic hypoglycemia was considered, lixisenatide was associated with a significantly higher proportion of responders and the lowest cost per responder compared to both insulin intensification regimens.
Number needed to treat
The NNT analysis showed that for every 4.98 and 4.34 patients treated with lixisenatide, there was approximately one additional responder compared to IG QD and TID, respectively, for the composite end-point of HbA1c threshold ≤7.5% AND no weight gain (). When the additional end-point of no symptomatic hypoglycemia is added to the composite endp-oint, the NNT increased to 6.85 and 5.86 for lixisenatide compared to IG QD and TID, respectively. The sensitivity analysis results are reported in Supplementary Table 8.
Table 5. Number needed to treat results.
Discussion
This analysis, based on clinical data from the GetGoal Duo-2 trial, calculated a cost per responder for the composite end-points of HbA1c threshold of ≤7.5% AND no weight gain, and HbA1c threshold of ≤7.5% AND no weight gain AND no symptomatic hypoglycemia. Based on clinical data from GetGoal Duo-2, patients uncontrolled on insulin glargine on top of OADs treated with lixisenatide had a higher proportion of responders compared to IG QD and TID for the composite end-points. This higher responder rate made up for the higher treatment cost for lixisenatide, resulting in a lower cost per responder across the UK, Spanish, and Italian setting over a 52-week period. Although insulin glulisine is associated with good glycemic control, it is not as effective as lixisenatide when incorporating other outcomes such as less weight gain and reduced hypoglycemia. The sensitivity analyses showed that lixisenatide continued to have a statistically significant higher proportion of responders, and was consistently the intervention with the lowest cost per responder for the end-points that included a weight-related component. As the HbA1c threshold increased, the difference in proportion of responders between lixisenatide and the insulin regimens was amplified, and, therefore, the difference in cost per responder increased. Lixisenatide was consistently the treatment with the higher proportion of responders in all composite end-points with the HbA1c threshold of ≤7.0%, ≤7.5%, ≤8.0%, and ≤8.5%.
A limitation of the clinical inputs is the lack of 52-week trial data from GetGoal Duo-2, which led to the assumption of a maintained treatment effect from week 26 to 52. The long-term evidence from other lixisenatide trials showed no significant difference in the number of responders between week 24 and 52, but a limitation is the number of patients evaluable at week 52 was reduced from that at week 24 in all studies, and it is unknown whether these dropouts would still be responders. However, this assumption was based on long-term evidence from other lixisenatide trials, which provided justification for this assumption in lieu of randomized-controlled data.
As in previous cost per responder analyses, it is acknowledged this is a simple analysis that incorporates the clinical benefit of reaching a composite end-point and the costs associated with drug acquisition and resource useCitation32,Citation33. The benefit of this simplistic approach is that it results in a transparent and understandable analysis to support the evaluation of drug performance for healthcare payers. It is important to note this analysis does not incorporate other factors from the treatment and management of diabetes in clinical practice, such as side-effect management. However, it is likely that the increased proportion of controlled patients on lixisenatide would lead to lower costs in terms of reduced long-term complications, which would mean this cost per responder analysis is based on a conservative assumption for lixisenatide. This limitation has been acknowledged in previous cost per responder analyses for a GLP-1RACitation32. To incorporate medium- or long-term costs and complications (such as the cost of hypoglycemia for example) in a robust manner, a cost-effectiveness model would need to be used to evaluate lixisenatide treatment vs IG; however, this added complexity would take away from the simple, transparent nature of this analysis. A further limitation is the confidence intervals calculated around the cost per responder results are based on the uncertainty in the efficacy with the costs remaining fixed.
This analysis is the first cost per responder study performed for lixisenatide. Cost per responder analyses have been performed for another GLP-1RA, liraglutide, in the Canadian, Italian, and US settings using the composite end-point of HbA1c threshold <7.0%, with no weight gain and no hypoglycemic eventsCitation32–34. These analyses were based on an insulin-naïve population on top of metformin, which limits the comparability to this lixisenatide analysis. However, the results are comparable in terms of showing the additional cost of treating with a GLP-1RA is absorbed by the higher efficacy when considering a composite end-point, compared to other treatment options such as sitagliptin or pioglitazone, resulting in a lower cost per responder across multiple country settings.
The proportion of responders between the arms based on the GetGoal Duo-2 trial show that reductions in HbA1c for patients on an insulin intensification regimen on top of insulin glargine and metformin can occur at the cost of increased weight and hypoglycemic eventsCitation7. The importance of a multi-faceted approach to diabetes management, incorporating weight gain and hypoglycemia with glycemic control, is shown in guidelines for treatment in the UK, Italy, and SpainCitation3,Citation5,Citation6. It is when these additional factors for treatment success are included in a composite end-point that the cost benefit of lixisenatide can be seen with this analysis. In addition, the treatment burden on a patient is increased with greater treatment complexity when put on IG (up to three injections per day for IG TID) rather than lixisenatide (one injection per day).
This analysis used Lantus as the insulin glargine component to be consistent with the trial design of GetGoal Duo-2. However, further research could investigate the impact on cost per responder of using different options for this component of the analysis. For example, NPH insulin could be considered due to national and local recommendations in some European countries for the prescription of NPH if a basal insulin becomes a therapeutic option. In addition, further research could be of interest investigating the impact on cost per responder of an alternative, lower price for Lantus due to the upcoming biosimilar for basal insulin.
This analysis suggests lixisenatide is associated with a lower cost per responder than insulin glulisine QD and TID for patients who are uncontrolled on OADs and insulin glargine in the UK, Italian, and Spanish country settings. This result was driven by the significantly higher proportion of responders for lixisenatide for the composite end-points incorporating an HbA1c threshold, no weight gain, and no hypoglycemia, which make up for the higher treatment cost.
Transparency
Declaration of funding
The study was funded by Sanofi. Marion Afonso and Elisheva Lew are employees of Sanofi. Fay Ryan and Ashley Pitcher are employed by QuintilesIMS, and have provided consultancy services funded by Sanofi.
Declaration of financial/other relationships
The authors have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Brice Kitio-Dschassi for his invaluable assistance in the success of this work.
References
- International Diabetes Federation. IDF Diabetes Atlas. Brussels, Belgium: IDF; 2015 (7th Edition). http://www.diabetesatlas.org/resources/2015-atlas.html. Accessed June 1, 2016
- Inzucchi S, Bergenstal R, Buse J, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Diabetes Care 2015;38:140-9
- Nottingham Area Prescribing Committee. Treatment algorithm for the management of Type 2 diabetes. Nottingham, UK: NHS; 2016. www.nuh.nhs.uk/handlers/downloads.ashx?id = 62268. Accessed June 1, 2016
- Associazione Medici Diabetologi. Standard italiani per la cura del diabete mellito 2014. Italy: Associazione Medici Diabetologi; 2014. p 92. www.standarditaliani.it. Accessed June 1, 2016
- Franco J, Borrás J, Fernández R, et al. Guía para la prescripción y visado de antidiabéticos. Valencia, Spain: Instituto medico valenciano; 2016. http://www.imeval.org/pdf/Antidiabeticos_version_abril_2016.pdf. Accessed June 1, 2016
- Torre E, Tejedor F, Menéndez S, Núñez-Cortés J, García A. Recomendaciones para el tratamiento farmacológico de la hiperglucemia en la dibabetes tipo 2. Atención Primaria 2011;43:202.e1-202.e9
- Rosensotck J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 trial. Diabetes Care;39:1318-28
- Horton E, Silberman C, Davis K, et al. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabets receiving incretin therapies or insulin in a large cohort database. Diabetes Care 2010;33:1759-65
- Clinicaltrials.gov. GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes for glycemic control and safety evaluation, on top of metformin (GETGOAL-F1). U.S.: ClinicalTrials gov; 2016. https://clinicaltrials.gov/ct2/show/NCT00763451?term=Getgoal+F1&rank = 1. Accessed June 1, 2016
- Clinicaltrials.gov. GLP-1 receptor agonist lixisenatide (morning or evening) in patients with type 2 diabetes for glycemic control and safety evaluation, on top of metformin (GETGOAL-M). U.S.: ClinicalTrials gov; 2016. https://clinicaltrials.gov/ct2/show/NCT00712673?term=Getgoal+M&rank = 1. Accessed June 1, 2016
- Clinicaltrials.gov. GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes for glycemic control and safety evaluation, on top of basal insulin (GETGOAL-L). U.S.: ClinicalTrials gov; 2016. https://clinicaltrials.gov/ct2/show/NCT00715624?term=getgoal+L&rank = 2. Accessed June 1, 2016
- Clinicaltrials.gov. GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes for glycemic control and safety evaluation, on top of pioglitazone (GETGOAL-P). U.S.: ClinicalTrials gov; 2016. https://clinicaltrials.gov/ct2/show/NCT00763815?term=getgoal+P&rank = 1. Accessed June 1, 2016
- Clinicaltrials.gov. GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes for glycemic control and safety evaluation, on top of sulfonylurea (GETGOAL-S). U.S.: ClinicalTrials gov; 2016. https://clinicaltrials.gov/ct2/show/NCT00713830?term=getgoal+S&rank = 1. Accessed June 1, 2016
- Clinicaltrials.gov. GLP-1 receptor agonist lixisenatide versus exenatide in patients with type 2 diabetes for glycemic control and safety evaluation, on top of metformin (GETGOAL-X). U.S.: ClinicalTrials gov; 2016. https://clinicaltrials.gov/ct2/show/NCT00707031?term=GetGoal+X&rank = 1. Accessed June 1, 2016
- Wallis S. Binomial confidence intervals and contingency tests: mathematical fundamentals and the evaluation of alternative methods. J Quant Linguist 2013;20:178-208
- Bank of England. Statistical Interactive Database - daily spot exchange rates against Sterling. BoE; 2016. http://www.bankofengland.co.uk/boeapps/iadb/Rates.asp. Accessed June 15, 2016
- British National Formulary. Lixisenatide. BNF; 2016. https://www.medicinescomplete.com/mc/bnf/current/DMD21941511000001102.htm?q=lyxumia&t=search&ss=text&tot = 3&p=1#DMD21941511000001102. Accessed May 1, 2016
- Agenzia Italiana Del Farmaco. Lyxumia; Determina 20 novembre 2013. Italy: Gazzetta Ufficiale; 2013. http://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta = 2013-11-28&atto.codiceRedazionale = 13A09598&elenco30giorni=false. Accessed June 1, 2016
- Consejo general de colegios oficiales de farmaceuticos. BotPlus 2.0. Spain: BotPlus; 2013. https://botplusweb.portalfarma.com/. Accessed June 1, 2016
- British National Formulary. Insulin Glargine. U.K.: BNF; 2016. https://www.medicinescomplete.com/mc/bnf/current/DMD11933011000001106.htm?q=lantus&t=search&ss=text&tot = 5&p=3#DMD11933011000001106. Accessed May 1, 2016
- Agenzia Italiana Del Farmaco. Insulina glargine; Determinazione 4 luglio 2007. Italy: Gazzetta Ufficiale; 2007. http://www.gazzettaufficiale.it/eli/id/2007/07/18/07A06229/sg. Accessed June 1, 2016
- British National Formulary. Insulin Glulisine. U.K.: BNF; 2016. https://www.medicinescomplete.com/mc/bnf/current/DMD9528311000001103.htm?q=apidra&t=search&ss=text&tot = 5&p=2#DMD9528311000001103. Accessed June 1, 2016
- Agenzia Italiana Del Farmaco. Insulina lispro; Determinazione 5 agosto 2005. Italy: Gazzetta Ufficiale; 2005. http://www.gazzettaufficiale.it/eli/id/2005/08/24/05A08323/sg;jsessionid=d9KzJ+bkLRv8Om3mFxZAGw__.ntc-as2-guri2b. Accessed June 1, 2016
- British National Formulary. Metformin Hydrochloride. U.K.: BNF; 2016. https://www.medicinescomplete.com/mc/bnf/current/PHP4161-metformin-hydrochloride.htm?q=metformin&t=search&ss=text&tot = 119&p=2#PHP58895-prescribing-and-dispensing-information. Accessed June 1, 2016
- Agenzia Italiana Del Farmaco. Classe A Principio Attivo. Italy: Agenzia Italiana Del Farmaco; 2017 http://www.agenziafarmaco.gov.it/it/content/tabelle-farmaci-di-classe-e-h-al-15122014-27012015
- NHS. NHS Electronic Drug Tariff; Part IXA Appliances. U.K.: NHS; 2016. http://www.drugtariff.nhsbsa.nhs.uk/#/00315892-DC/DC00315235/Part IXA-Appliances. Accessed May 1, 2016
- Admministrazione Transparente. Dispositivi primo semestre. Italy: Admministrazione Transparente; 2014. http://www.aogarbagnate.lombardia.it/SalviniWEB/AmministrazioneTrasparente/DisposizioniGenerali/Dispositivi-primo-semestre-2014.pdf. Accessed June 1, 2016
- MIMS. BD Micro-Fine + Pen Needles. U.K.: MIMS; 2016. http://www.mims.co.uk/drugs/diabetes/diagnostic-tests-and-appliances/bd-micro-fine-pen-needles. Accessed May 1, 2016
- MIMS. BGStar Test Strip. U.K.: MIMS; 2016. http://www.mims.co.uk/drugs/diabetes/diagnostic-tests-and-appliances/bgstar-test-strip. Accessed May 1, 2016
- Regione del Veneto. Prescrizione e dispensazione dispositivi per diabetic. Venezia, Italy: Regione del Veneto; 2016. https://www.regione.veneto.it/web/sanita/prescrizione-e-dispensazione-dispositivi-per-diabetici. Accessed June 1, 2016
- Bender R. Calculating confidence intervals for the number needed to treat. Contr Clin Trials 2016;22:102-10
- Ferrario M, Lizán L, Montagnoli R, et al. Liraglutide vs sitagliptin add-on to metformin treatment for type 2 diabetes mellitus: short-term cost-per-controlled patient in Italy. Primary Care Diabetes 2016;10:220-6
- Skovgaard R, Ploug U, Hunt B, et al. Evaluating the cost of bringing people with type 2 diabetes mellitus to multiple targets of treatment in Canada. Clin Ther 2015;37:1677-88
- Langer J, Hunt B, Valentine W. Evaluating the short-term cost-effectiveness of liraglutide versus sitagliptin in patients with type 2 diabetes failing metformin monotherapy in the United States. J Manag Care Spec Pharm 2013;19:237-46
