Abstract
Aims: To evaluate the cost-effectiveness of antimicrobial stewardship (AS) program implementation focused on critical care units based on assumptions for the Spanish setting.
Materials and methods: A decision model comparing costs and outcomes of sepsis, community-acquired pneumonia, and nosocomial infections (including catheter-related bacteremia, urinary tract infection, and ventilator-associated pneumonia) in critical care units with or without an AS was designed. Model variables and costs, along with their distributions, were obtained from the literature. The study was performed from the Spanish National Health System (NHS) perspective, including only direct costs. The Incremental Cost-Effectiveness Ratio (ICER) was analysed regarding the ability of the program to reduce multi-drug resistant bacteria. Uncertainty in ICERs was evaluated with probabilistic sensitivity analyses.
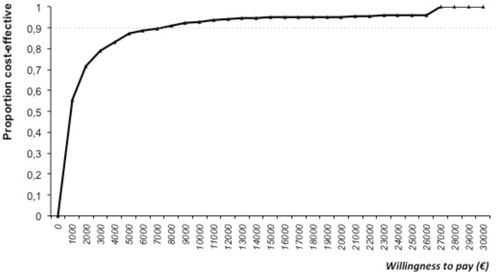
Results: In the short-term, implementing an AS reduces the consumption of antimicrobials with a net benefit of €71,738. In the long-term, the maintenance of the program involves an additional cost to the system of €107,569. Cost per avoided resistance was €7,342, and cost-per-life-years gained (LYG) was €9,788. Results from the probabilistic sensitivity analysis showed that there was a more than 90% likelihood that an AS would be cost-effective at a level of €8,000 per LYG.
Limitations: Wide variability of economic results obtained from the implementation of this type of AS program and short information on their impact on patient evolution and any resistance avoided.
Conclusions: Implementing an AS focusing on critical care patients is a long-term cost-effective tool. Implementation costs are amortized by reducing antimicrobial consumption to prevent infection by multidrug-resistant pathogens.
Introduction
Over the two last decades, there has been an exponential increase in bacterial resistance globally, making it one of the most important threats to public health around the world todayCitation1. The excessive and inappropriate use of antimicrobial agents, together with a lack of prevention and control measures, has contributed to the expansion of multi-drug resistant bacterial (MDRB) species. This phenomenon is particularly important in the hospital setting, where it has been estimated that up to 50% of antimicrobial prescriptions are inappropriateCitation2,Citation3. The annual expense generated by these multi-resistant species is thought to be close to 1.5 billion euros in the European Union, and they are responsible for ∼25,000 deaths each yearCitation4.
Fast and appropriate initiation of antimicrobial treatment is considered essential to improve the prognosis of patients with serious infectionsCitation5. However, this pretext is employed on many occasions as an excuse for an over-use of broad-spectrum antibiotics, favoring the selection of multi-resistant strains, and having a direct impact on the morbidity of the patient and the costs associated to treatmentCitation6. Various studies have demonstrated the fact that a high antibiotic pressure due to excessive use is directly related to an elevated resistance rate, as well as an increase in the morbidity and mortality of patientsCitation7. This problem receives special interest in critical patient units, where the empirical use of broad-spectrum antimicrobial agents is a common practice, and infections by MDRB are one of the greatest threats to the patientsCitation8.
Reduced consumption of antimicrobials has been identified as one of the most effective measures for reducing the selection of bacterial resistanceCitation9. Among the different strategies published, the implementation of antimicrobial stewardship programs (AS), based on prospective prescription audits, has proved to be the strategy that has the greatest impact on the use of antimicrobialsCitation10.
The term AS includes a set of multidisciplinary interventions destined to improve antimicrobials use, with the aim of limiting the appearance of resistance in these organisms. The implementation of this type of program has been promoted by various national and international bodies, and has proved to be an essential element in controlling the use of antimicrobialsCitation11.
The cost effectiveness analysis is a very useful tool for guiding the selection of efficient strategies for healthcare institutions, considering the impact of the diverse interventions both in the short- and long-term. Given the increased number of studies demonstrating the effectiveness of AS programs in diverse centres, the present study is aimed at assessing the impact of an AS implementation, focusing on the use of antimicrobials in critical patients.
Materials and methods
In this study, we analysed the cost-effectiveness of implementing an AS for controlling the use of antimicrobials in critical care patients.
A model was constructed that evaluates the effects of implementing the program, both short- (1 year) and long-term (3 years), in a hospital with a total of 50 critical patient beds, considering an average stay of 5.9 days in the unitCitation12 at an 80% occupation rateCitation13, making a total number of 2,474 patients per year. The characteristics of the patients and the costs considered in the model are shown in .
Table 1. Costs, infection rate, and mortality considered in the model.
The model is approached from the perspective of Spain’s National Health System, and includes only direct medical costs. The AS program included the following interventions: antimicrobial restriction (including antibiotics and antifungals), formal consultation, implementation of protocols for de-escalation and guidelines for antibiotic prophylaxis or treatment, formal re-assessment of antimicrobials, and implementation of computer-assisted decision supportCitation14. The costs considered for the AS team include the salaries of a specialist in infectious diseases, a microbiologist, and a pharmacist, all full-time dedicated to the program.
Short-term model
According to the results published in the literature, in the short-term, implementing this type of program in critical care units can reduce patient consumption of antimicrobialsCitation14, as well as the incidence of Clostridium difficile infections (CDI)Citation15. Due to the limited evidence available so far, the model does not take into account the possible reduction of other antimicrobial adverse events, hospital stay, and morbidity of the patient after the AS program.
Benefits of AS were obtained from the review of Kaki et al.Citation14. The daily cost of antimicrobial treatment was taken as the average of the values included in the study (€34.94), and the percentage short-term cost decrease after implementation was considered to be the average reduction achieved in the studies included in the review (–42.8%). In order to estimate the impact of an AS on reducing CDI, the meta-analysis of Feazel et al.Citation15 was employed, considering an incidence reduction of 48.0% (95% CI = 38.0–62.0) from an initial incidence of 8.7 episodes each 10,000 days of hospital stayCitation16.
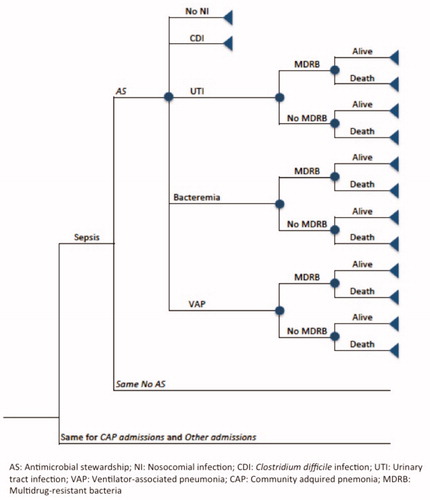
To simulate the evolution of the patients in the critical units, with and without an AS, a decision tree was designed (). Given the complexity of including all the infections suffered by the critical patients, for the short-term, the model considered the effects of implementing the program on patients with sepsis, community-acquired pneumonia (CAP), and the three most common hospital-acquired (nosocomial) infections in this group of patients: ventilator-associated pneumonia (VAP), catheter-related bacteremia, and urinary tract infection (UTI). Therefore, a patient could be admitted to a critical care unit due to sepsis, CAP, or other cause. During the critical care unit stay, the patient could suffer a nosocomial infection. In those units with an AS program, antimicrobial consumption could be reduced in any of these infection processes. According to available data published for SpainCitation17, the model considers that 10% of patients admitted to the unit are suffering from sepsis (following the 2001 International Sepsis Definitions Conference)Citation18, and 25% enter because of community-acquired pneumonia. Information about nosocomial infection incidence was obtained from the national register of nosocomial infections in intensive care unitsCitation19, considering an incidence of 2.8% for UTI, 2.0% for catheter-related infection, and 3.0% for VAP. In order to simplify the model, the infections are considered mutually exclusive.
Long-term model
For the long-term model, the evolution of patients entering the critical care units was simulated by means of a Markov chain model (), assuming that the implementation of the program achieves a 20% reduction in the incidence of infection by MDRBCitation20, considering MDRB as bacteria resistant to at least three or more antimicrobial classes, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci, Pseudomonas aeruginosa, Acinetobacter baumannii, and extendedspectrum β-lactamase (ESBL)-producing or carbapenemase-producing gram-negative bacilli.
Patients are included as in the short-term model, but differences in MDRB infection prevalence in a critical care unit with and without an AS program are considered. The economic impact of resistance reduction is considered for all nosocomial infections in critical care units, considering that MDRB cause 40% of nosocomial infectionsCitation21, and taking into account a further cost due to infection by MDRB of €5,500Citation22, with an additional mortality rate of 40%Citation23. After AS program implementation, a moderate reduction on antimicrobial consumption has been reported after the short-term impactCitation24. For the long-term model (second and third year), we considered a 10% annual reduction in antimicrobial treatment cost over the previous year. An annual discount rate of 3% for antimicrobial costs was applied in line with Spanish health economic evaluation recommendationsCitation25.
Economic evaluation
For the short-term model, the change in antimicrobial spending between the AS program and no intervention was calculated.
For the long-term model, the Incremental Cost-Effectiveness Ratio (ICER) was calculated regarding the ability of the program to reduce bacterial resistance and its mortality. The ICER was calculated based on the cost of implementing the AS program and standard care, dividing the difference in calculated cost between the two scenarios, with the difference in calculated effectiveness of the two scenarios. Cost per life of years gained (LYG) avoiding infection by MDRB was calculated considering the average age of patients admitted to the critical care unit to be 59.1 yearsCitation26, and a life expectancy of 20 years after critical care unit dischargeCitation27,Citation28.
Sensitivity analysis
The final results of the model were calculated using Microsoft Excel software. A univariate sensitivity analysis was carried out to establish the robustness of the short-term model in the presence of uncertainty variables, including reason for admittance to critical care (20%), incidence of nosocomial infection (20%), reduction in the incidence of CDI (50%), and the AS’s impact on the average cost of treatment (30%). Sensitivity analyses were performed, modifying the value of one base case parameter at a time, and recording the corresponding antimicrobial cost.
Probabilistic sensitivity analysis was undertaken to analyse the long-term cost of avoided resistance and LYG. The variables included in the analysis were the short-term AS benefit (30%), the incidence of nosocomial infections (20%), the percentage of multi-resistant species present in the unit (20%), and the impact of the AS on the prevalence of infection by multi-resistant bacteria, considering a null impact as the worst-case scenario and a best-case of a 40% reduction. The variation in additional mortality due to multi-resistant strains was considered to be 20%. The analysis was carried out using a Monte-Carlo simulation on the uncertainty variables considered, simulating a 1,000-patient cohort admitted to the unit either following the implementation of an AS, or without this program, with point estimates of the ICER and cost per LYG. Each point estimate contains a random selection of values from within the range considered. All distributions of variables were assumed to represent beta distributions. Beta distributions were estimated from the mean and standard deviation for the variables in .
Table 2. Probability values and fixed applied during probabilistic sensitivity analysis.
Results
In the short-term, for the proposed model, implementing an AS to control antimicrobials in critical care units reduces the consumption of antimicrobials with respect to the intervention group [€391,598 (95% CI = 254,291–420,017) vs €463,337 (324,050–623,576)], with a net benefit of €71,738 (95%CI = 12,156–144,766), making it a dominant option.
The results of the univariate analysis of short-term model are shown in . Reduction in daily treatment cost was the variable that most influenced the model, being the threshold value a reduction of 26.8% in daily treatment cost to obtain benefits after the implementation of AS program..
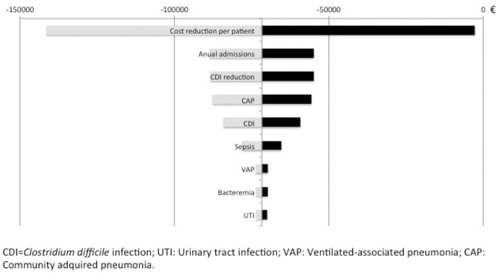
Figure 2. Results of short-term univariate analysis. Each horizontal line represents how the variation of the variables affects the final result obtained in the model (–€71,738). The variations considered were 20% for the reason of admission in the critical care unit (Sepsis and NAC) and in the incidence of nosocomial infection, 50% for the reduction in the incidence of CDI, 30% for the reduction of the average cost of antimicrobial treatment, and 10% for the number of patients admitted in the critical care unit.
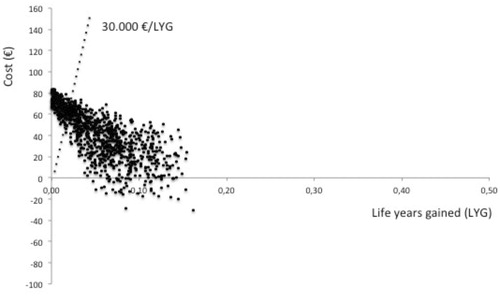
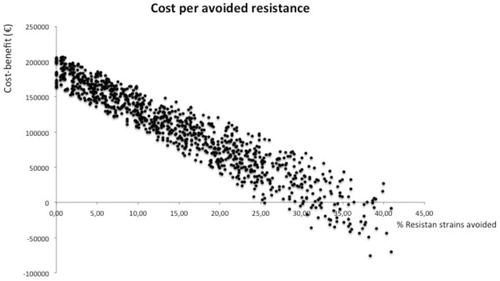
In the long-term, the maintenance of the program involves an additional cost to the system of €107,569 (95% CI = −26,312–216,527). With respect to the baseline situation, supposing an average reduction of 20% in the incidence of infection by MDRBCitation20, cost per avoided MDRB nosocomial infection would be €7,342 (95% CI = −308–28,348). Considering a mortality rate attributable to infection by MDRB strains of above 40%Citation22, the cost per LYG with the implementation of this program would be €9,788 (95% CI = −95–22,094).
The results of the probabilistic sensitive analysis model are shown in (Cost per LYG) and (Cost per resistance avoided). The acceptability curve showed a greater than 90% probability that the model would be cost-effective for €8,000 ().
Discussion
According to the model proposed, implementing an AS to control antimicrobials in critical care units is, in the short-term, a cost-effective tool. In the long-term, the maintenance of the program has an impact on patient survival of less than €30,000 per LYG.
Antimicrobials are a key element in the managing of critical patients. However, diverse studies have shown that their use is, in many cases, excessiveCitation29. On the other hand, the role of antimicrobials in the selection of MDRB is well known. During the last years, AS programs have emerged as an effective tool to control antimicrobial use and reduce healthcare cost in hospital general wards and in critical care unitsCitation14,Citation30.
Like any healthcare intervention, the implementation of an AS requires an economic evaluation. However, we have found no studies to date that include pharmacoeconomic models for this type of intervention. Scheetz et al.Citation31 published a study on the economic impact of an AS on patients with bacteremia, demonstrating that its implementation is cost-effective, with a cost per QALY of $2,367. However, this study considers implementation costs to include the cost of an electronic support system to aid clinical decisions and not the maintenance of staff members dedicated to this activity.
It should be noted that the potential benefits of implementing such programs include not only a reduction in antimicrobial consumption, but also an improvement in infection management. This fact should result in improved treatment response, with a reduction in the hospital stay and the mortality associated with the infection, which also contribute to a significant cost reduction in the infection process. However, to date there are few studies that evaluate the impact of an AS on the clinical benefit to patients, so this phenomenon has not been considered in the model proposed. Moreover, we have not considered other indirect expenses that are expected to decrease after AS program implementation, such as from drug side-effects. Opportunity costs associated with antibiotic administration optimization, such as continuous infusion administration for b-lactams or vancomycin, have also not been considered, which could lead to additional savings in the cost of treatmentsCitation32,Citation33. It should also be born in mind that AS interventions and infection control measures go hand in hand, and improvements in infection control may lead to additional cost savings.
The short-term economic benefit of implementing this type of program has been demonstrated in many published experiencesCitation14,Citation34. However, a recent review has established that the way of measuring the economic impact of this intervention in the various studies is very heterogeneous, since they include not only costs associated to reduced treatment, but also derivatives like hospital stay, re-admissions, and so onCitation35.
In the present study, the savings in antimicrobial consumption considered were higher in the first year (50%), becoming less in subsequent years. We consider that an AS has a greater economic impact during the first phase, where prescriptions are the most irregular and prone to optimization. The educational work of this type of program leads to an improvement in the prescription of antimicrobials, with cost normalization in the long-term.
For long-term model, we have based the value of the cost of dealing with multi-resistant species on the lowest value obtained in the study by CosgroveCitation22. We must take into account the fact that other published works recorded a greater cost associated to infection by MDRBCitation34,Citation36, meaning, therefore, that the economic benefit may be greater than that considered by our model. However, for a proper assessment, it is still necessary to continue analysing the true economic impact of such programs on the prevention of bacterial resistance.
Our study reveals several limitations. First, there is a wide variability of economic results obtained from the implementation of this type of program, as well as little information on their impact on patient evolution and any resistance avoidedCitation37. Due to the lack of information available in our country regarding the antimicrobial cost per patient in critical care units, we have been forced to use averaged cost from the review of Kaki et al.Citation14, which represent antimicrobial cost in a range of countries. In this review, cost savings due to this kind of program ranged from –4.6 to –72.3 US$/patient day, which means a great variability between different centers. On the other hand, studies included in this revision are heterogeneous with respect to the interventions attempted in the critical care units. Therefore, it is difficult to predict the impact of an AS program in a particular hospital, since there are several influential factors, not only for antimicrobial use at the baseline, but also for the personnel that integrate this type of program. To take into account this variability, we have tried to cover a wide range of situations in the sensitivity analysis, considering a variation in the reduction of daily treatment cost of 30%. With this variance, we have included a range of values in which the majority of the studies are found.
On the other hand, the phenomenon of bacterial resistance is complex, and is affected by several factors. Little information is available on the impact of AS intervention in the incidence of MDRB infectionCitation37. We have considered a wide range of variability to consider different scenarios. According to this model, the worse the initial situation, the more likely the program is cost-effective. Moreover, the impact of MDRB on patient’s outcomes has been considered from a study relating a specific pathogen and populationCitation34, which could not reflect the effect of multi-resistance on other types of bacteria. Both assumptions could reduce the strength of our results. Given the scarce information available, the possible effect of this type of program on length of stay in the critical care unit, as well as on the reduction of adverse events associated with the use of antimicrobials, has not been considered, in order to not introduce more uncertainty into the model.
Due to the Spanish cost perspective, and considering the large variability in drug costs and salaries between countries, the results of this CEA cannot be directly extrapolated to other settings. It should also be considered that our model has included a tertiary hospital, with a high number of beds allocated to critical patients. Therefore, its extrapolation to small hospitals could also be unsuitable.
The phenomenon of bacterial resistance constitutes one of the greatest public health threats across the globe, leading to increased healthcare costs. However, evidence for the cost-effectiveness of interventions is poor and there are important methodological limitations. It is necessary to propose an economic evaluation of different interventions to determine the most cost-effective combination of strategies to minimize bacterial resistance. The results of our study firmly support the implementation of an AS aimed at controlling antimicrobials, with periodic monitoring of antimicrobial consumption and bacterial resistance.
Conclusion
In summary, implementing an AS dedicated to controlling antimicrobial treatment in critical care units is a cost-effective tool, although it is subject to a high variability. Further high-quality studies are necessary to evaluate the impact of this type of program on the clinical evolution of patients and the reduction of resistance, in order to confirm these results.
Transparency
Declaration of funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of financial/other interests
None of the authors have any potential financial conflict of interest. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowlodgment
None.
References
- Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect 2009;59:S4-S16
- Hecker MT, Aron DC, Patel NP, et al. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003;163:972-8
- Willemsen I, Groenhuijzen A, Bogaers D, et al. Appropriateness of antimicrobial therapy measured by repeatedprevalence surveys, Antimicrob Agents Chemother 2007;51:864-7
- The bacterial challenge: time to react. A call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. Stockholm: European Centre for Disease Prevention and Control; 2009. http://ecdc.europa.eu/en/publications/. Accessed March 2015
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228
- Paruk F, Richards G, Scribante J, et al. Antibiotic prescription practices and their relationship to outcome in South Africa: findings of the prevalence of infection in South African intensive care units (PISA) study. S Afr Med J 2012;102:613-6
- Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159-77
- Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care 2011;1:47
- Grau S, Bou G, Fondevilla E, et al. How to measure and monitor antimicrobial consumption and resistance. Enfermedades Infecc Microbiol Clínica 2013;31:S16-24
- Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013;4:CD003543
- Allerberger F, Lechner A, Wechsler-Fördös A, et al. Optimization of antibiotic use in hospitals—antimicrobial stewardship and the EU project ABS international. Chemotherapy 2008;54:260-7
- Santana-Cabrera L, Lorenzo-Torrent R, Sánchez-Palacios M, et al. [Analysis of stay and mortality in an intensive care unit]. Rev Calid Asist 2014;29:121-3
- Colmenero M. [El ritual de la falta de camas]. Med Intensiva 2011; 35:139-42
- Kaki R, Elligsen M, Walker S, et al. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother 2011;66:1223-30
- Feazel LM, Malhotra A, Perencevich EN, et al. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother 2014;69:1748-54
- Bouza E, Rodríguez-Créixems M, Alcalá L, et al. Is Clostridium difficile infection an increasingly common severe disease in adult intensive care units? A 10-year experience. J Crit Care 2015;30:543-9
- Azkárate I, Sebastián R, Cabarcos E, et al. [Registro observacional y prospectivo de sepsis grave/shock séptico en un hospital terciario de la provincia de Guipúzcoa]. Med Intensiva 2012;36:250-6
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESCIM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6
- Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias (GTEIS-SEMICYUC). [Estudio Nacional de Vigilancia de Infección Nosocomial en UCI (ENVIN-HELICS)]. 2013. Retrieved from http://hws.vhebron.net/envin-helics. Accessed April 2015
- Ma X, Xie J, Yang Y, et al. Antimicrobial stewardship of Chinese ministry of health reduces multidrug-resistant organism isolates in critically ill patients: a pre-post study from a single center. BMC Infect Dis 2016;16:704
- López-Pueyo MJ, Barcenilla-Gaite F, Amaya-Villar R, et al. [Multirresistencia antibiótica en unidades de críticos]. Med Intensiva 2011;35:41-53
- Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006;42:S82-S9
- Pop-Vicas A, Opal SM. The clinical impact of multidrug-resistant gram-negative bacilli in the management of septic shock. Virulence 2014;5:206-12
- Peto Z, Benko R, Matuz M, et al. Results of a local antibiotic management program on antibiotic use in a tertiary intensive care unir in Hungary. Infection 2008;36:560-4
- López Bastida J, Oliva J, Antoñanzas F, et al. [Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias]. Gac Sanit 2010;24:154-70
- Rebert C. [Estudio epidemiológicode la infección nosocomial en el Servicio de UCI del Hospital Universitario de Canarias]. Thesis doctoral. Universidad de la Laguna; 2005
- Lindemark F, Haaland A, Kvåle R, et al. Age, risk, and life expectancy in Norwegian intensive care: a registry-based population modelling study. PLoS One 2015;10:e0125907
- Brinkman S, de Jonge E, Abu-Hanna A, et al. Mortality after hospital discharge in ICU patients. Crit Care Med 2013;41:1229-36
- Meyer E, Schwab F, Gastmeier P, et al. Surveillance of antimicrobial use and antimicrobial resistance in German intensive care units (SARI): a summary of the data from 2001 through 2004. Infection 2006;34:303-9
- Micek ST, Ward S, Fraser VJ, et al. A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator-associated pneumonia. Chest 2004;125:1791-9
- Scheetz M, Bolon MK, Postelnick M, et al. Cost-effectiveness analysis of an antimicrobial stewardship team on bloodstream infections: a probabilistic analysis. J Antimicrobial Chemother 2009;63:816-25
- Hong LT, Goolsby TA, Sherman DS, et al. Continuous infusion vs intermittent vancomycin in neurosurgical intensive care unit patien. J Crit Care 2015;30:1153.e1-6
- Bao H, Lv Y, Wang D, et al. Clinical outcomes of extended versus intermittent administration of piperacillin/tazobactam for the treatment of hospital-acquired pneumonia: a randomized controlled trial. Eur J Clin Microbiol Infect Dis 2017;36:459-66
- Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009;49:1175-84
- Dik J-WH, Vemer P, Friedrich AW, et al. Financial evaluations of antibiotic stewardship programs-a systematic review. Front Microbiol 2015;6:317
- Lautenbach E, Weiner MG, Nachamkin I, et al. Imipenem resistance among pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect Control Hosp Epidemiol 2006;27:893-900
- Karanika S, Paudel S, Grigoras C, et al. Clinical and economic outcomes from the implementation of hospital based antimicrobial stewardship programs: a systematic review and meta-analysis. Antimicrob Agents Chemother. 2016;60:4840-52
- Asensio Á, Bouza E, Grau S, et al. [Cost of Clostridium difficile associated diarrhea in Spain]. Rev Esp Salud Pública 2013;87:25-33
- Generalitat Valenciana. [Retribuciones del personal sanitario 2015]. 2016. Retrived from: http://www.san.gva.es/web/dgrhs/retribuciones-personal-iiss. Accessed on May 2016
- Bantar C, Sartori B, Vesco E, et al. A hospital wide intervention program to optimize the quality of antibiotic use: impact on prescribing practice, antibiotic consumption, cost savings, and bacterial resistance. Clin Infect Dis 2003;37:180-6