Abstract
Aim: To estimate health resource utilization (HRU) associated with the management of pacemaker complications in various healthcare systems.
Methods: Electrophysiologists (EPs) from four geographical regions (Western Europe, Australia, Japan, and North America) were invited to participate. Survey questions focused on HRU in the management of three chronic pacemaker complications (i.e. pacemaker infections requiring extraction, lead fractures/insulation breaches requiring replacement, and upper extremity deep venous thrombosis [DVT]). Panelists completed a maximum of two web-based surveys (iterative rounds). Mean, median values, and interquartile ranges were calculated and used to establish consensus.
Results: Overall, 32 and 29 panelists participated in the first and second rounds of the Delphi panel, respectively. Consensus was reached on treatment and HRU associated with a typical pacemaker implantation and complications. HRU was similar across regions, except for Japan, where panelists reported the longest duration of hospital stay in all scenarios. Infections were the most resource-intensive complications and were characterized by intravenous antibiotics days of 9.6?13.5 days and 21.3?29.2 days for pocket and lead infections respectively; laboratory and diagnostic tests, and system extraction and replacement procedures. DVT, on the other hand, was the least resource intensive complication.
Limitations: The results of the panel represent the views of the respondents who participated and may not be generalizable outside of this panel. The surveys were limited in scope and, therefore, did not include questions on management of acute complications (e.g. hematoma, pneumothorax).
Conclusions: The Delphi technique provided a reliable and efficient approach to estimating resource utilization associated with chronic pacemaker complications. Estimates from the Delphi panel can be used to generate costs of pacemaker complications in various regions.
Introduction
Permanent cardiac pacing is the only therapy that effectively treats symptomatic bradycardia. An estimated 1 million patients worldwide receive pacing therapy each yearCitation1–3. Conventional pacemakers consist of a generator, typically implanted in a subcutaneous pocket in the chest wall, and one or two leads threaded through the subclavian or cephalic vein into the heart. Despite being an established and frequently used therapy, complications related to the lead and pocket of conventional pacemakers occur in up to 10% of patientsCitation4.
Pacemaker complications are a burden to healthcare systems, as treatment will usually involve surgical intervention or hospitalizationCitation4. Most complications, i.e. pneumothorax, hematoma, perforations, and lead dislodgements, are acute, procedural events that occur at the time of implant. However, complications such as pocket and lead infections, lead fractures, and upper extremity deep venous thrombosis (DVT) are chronic events that could occur multiple times over the lifetime of the device. These chronic complications are potentially an economic burden to the healthcare system, as they could lead to multiple hospital admissions and surgical procedures for individual patients. Estimates of rates of chronic complications vary widely in published literature. Reported rates of infection range from 0.1–1.9%Citation5,Citation6; lead fracture rates range from 0.2–1.0% per yearCitation7, and reported rates of DVT range between 0.4–5.2%Citation8,Citation9, depending on the definition used for the complication (e.g. with or without pacemaker or lead extraction) and duration of follow-up.
The burden of pacemaker complications on healthcare systems is not well understood. For one, several countries do not have large electronic databases with outcomes and HRU data to enable the analysis of the economic burden of complications. Second, diagnosis codes specific to chronic complications including device-related infections or lead fractures are not available, making it difficult to assess the costs of these complications in claims data. The need to understand the economic burden of chronic pacemaker complications is made even more relevant following the introduction of next generation ‘leadless’ pacemaker systems that eliminate the lead and pocket, thus potentially eliminating chronic pacemaker complicationsCitation10. The costs of chronic pacemaker complications could, therefore, provide important benchmark data for policymakers to use when assessing the economic benefits of leadless pacing.
In the absence of empirical data on the costs of complications, we applied a Delphi panel approach to generate estimates of health resource utilization (HRU) in the management of chronic pacemaker complications in various countries.
Methods
The Delphi method is a structured approach to generating consensus on a particular topic from a group of experts. The Delphi process takes place iteratively in a series of rounds. The first round typically begins with administering a round of open-ended questions to solicit relevant information on a topic. The information collected is then converted into a structured questionnaire with relevant study items. Survey responses are aggregated and summarized into group-wide responses in the form of means and/or medians and presented back to study participants. Participants in subsequent rounds are asked to compare their individual responses with the group-wide responses, and may elect to change their responses to match the group response. This process is repeated until there is convergence on opinion among the group. Typically, two or three rounds of surveys are considered sufficient for reaching consensusCitation11.
Our study used a modified Delphi approachCitation12 in which survey items were developed through a literature review and consultation with two electrophysiologists (EPs). Pre-defining the survey items using a modified Delphi approach helped to reduce the rounds of surveys required, thereby reducing the cognitive burden and maximizing the response rate. The survey questions were piloted on a team of Medtronic’s health economics experts and the two EPs involved in survey development, and assessed for comprehensiveness, correctness of terminology, clarity, and presentation.
Study panelists
EPs with experience implanting pacemakers and managing pacemaker-related complications were targeted for participation. Panelists were implanting physicians known to Medtronic or recommended by other experts (snowball sampling), and were enrolled from Western Europe (i.e. Austria, Belgium, England, France, Germany, Netherlands, Portugal, and Spain), Australia, Japan, and North America (i.e. the US and Canada). Study participants were blinded to one another to avoid direct interaction. Non-English speaking EPs and the two EPs involved in survey development and testing were not allowed to participate. Ethical approval was not required, as patient-specific data was not collected. However, physicians were allowed to consult medical records to assist recall. Panelists received honoraria for each survey completed.
Questionnaire design
The first survey included 60 questions and used a combination of open-ended questions, multiple choice questions, and free-response comment boxes (Supplementary Materials). Survey content covered three pacemaker-related scenarios (1) the management of pacemaker infections requiring system extractions; (2) the management of lead fractures or insulation breaches resulting in lead replacement; and (3) the management of upper extremity DVT. For each scenario, the data parameters collected included: number of pre-procedural physician visits; oral and parenteral therapies administered; length of inpatient stays; surgical procedures performed and personnel involved; critical or intensive care; laboratory and diagnostic tests administered; and number of complication-related follow-up visits.
Furthermore, to avoid confounding, panelists were instructed to consider only pacemaker-related complications and procedures in their responses, and not complications related to other cardiac implantable devices. Study questions were related to pacemakers in general and not manufacturer-specific. Responses from the first round were collated and analyzed for convergence of opinion. Survey items that failed to achieve consensus were re-tested in a second round. Separate surveys were developed for each geographic region, targeting questions that failed to reach consensus among panelists from a specific region (Supplementary Materials). Panelists were given 4 months to complete the first-round survey and 2 months to complete the second survey. All surveys were conducted in English, and were programmed and fielded by YouGov.
Analysis
Descriptive statistics (i.e. mean, median, and range) were used to present the collective judgments of the panelistsCitation13, and summarized by geographic region. Evaluation of the median and interquartile range (IQR) was used to determine consensus. Previous Delphi panels have described consensus as being achieved if the IQR is no greater than 2 points on a 10-point Likert scaleCitation14. Similarly, we considered consensus to be achieved on a specific item if the IQR of the response was no greater than 20% of the highest recorded value.
For the analysis, categories of antibiotic use were generated as follows: cephalosporins (i.e. cefazolin, cefuroxime, cephalexin, ceftriaxone, cefamezin); vancomycin (including teicoplanin); beta lactams (i.e. flucloxacillin, amoxicillin, ampicillin, cloxacillin); gentamycin; and other (i.e. daptomycin, ciprofloxacin, clindamycin, and levofloxacin). Categories of anticoagulant use were defined as follows: coumadins (i.e. warfarin, acenocoumarol, phenprocoumon, fluindione); novel oral anticoagulants, NOAC (i.e. dabigatran, rivaroxaban, apixaban, edoxaban); and other (i.e. urokinase).
In addition, pacemaker and/or lead extraction methods were grouped as follows, based on the Heart Rhythm Society (HRS) lead extraction guidelinesCitation15:
Traction methods;
Mechanical sheath (including polypropylene sheaths, Cook Medical Evolution, Spectranetics Tightrail);
Laser sheath;
Electrosurgical sheath (includes electrocautery and PEAK PlasmaBlade); and
Rotating Threaded Tip Sheath.
Alternative routes were, e.g., femoral extraction and open extraction via sternotomy. Final results included a combination of first survey results and second survey revisions, in the case the panelist had modified their answers during the second round. If a panelist did not participate in the second survey, first survey responses were retained for calculation of the final regional estimates. All analyses were conducted using SAS version 9.4.
Results
Description of Delphi panelists
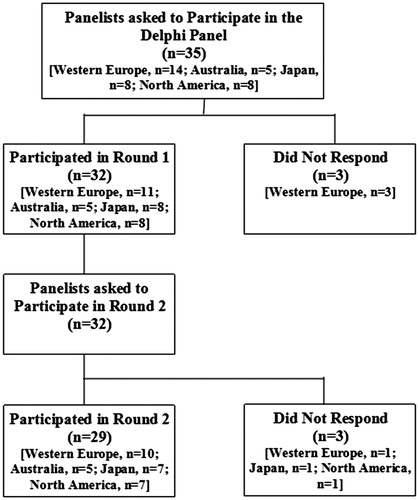
The study was conducted between April 2015 and January 2016. Thirty-five EPs were invited to participate, of whom 32 panelists participated in the first survey (response rate: 91%), and 29 panelists participated in the second survey (response rate: 91% of first-survey panelists, ). Panelists who participated in the first survey were located in Western Europe (34.4%, n = 11), Australia (15.7%, n = 5), Japan (25.0%, n = 8), and North America (25.0%, n = 8). On average, each had been a certified EP for at least 12 years, with North American panelists having been certified for the longest period of time, i.e. 20.8 years on average ().
Table 1. Description of panelists’ clinical experience.
Consensus
Consensus was reached on most survey items after the first round, as evidenced by IQRs that fell below the threshold of 20%. The exception was the question on duration of antibiotic therapy for treatment of infections where there was a high level of disagreement among participants from the same region. For this reason, the second survey was modified to include questions on differences in antibiotic prescribing, based on whether an infection was pocket or lead-related. The second survey demonstrated a growing consensus among the questions revisited, with almost all changes reducing the variability within the response (i.e. reducing the IQR) and meeting the requirements for consensus.
Scenario 1: Management of pacemaker infections requiring system extraction
Panelists estimated that, on average, patients with a pacemaker infection made 1.5–2.4 EP visits before a system extraction (). Patients were initiated on IV antibiotics; with cephalosporins and vancomycin the most commonly prescribed antibiotics (Supplementary Table 1). Panelists estimated that less than half of patients (16.9% to 41.8%) were prescribed oral antibiotics in addition to parenteral antibiotics, with North American panelists reporting the lowest proportion compared to other regions (). Beta lactam antibiotics and cephalosporins (21.8%, n = 7) were the most commonly prescribed oral antibiotics prior to a system extraction (Supplementary Table 1). Blood cultures, transthoracic echocardiograms, blood typing/cross matching (57.7–97.4%), and transesophageal echocardiograms were performed on the majority of patients prior to an infections-related system extraction ().
Table 2. Scenario 1: Management of pacemaker infections requiring system extractions.
Panelists used electrosurgical sheaths, laser sheaths, and mechanical sheaths in infection-related pacemaker extractions (Supplementary Table 1). Electrosurgical sheath was the most commonly applied extraction method, reported by 75–100% of panelists. Laser sheath extraction was commonly used by North American and Australian panelists (63% and 80%, respectively) and less commonly by Japanese and Western European panelists (13% and 18%, respectively). Mechanical sheath was not commonly used for infections-related extractions according to panelists (0–13% of panelists). Panelists reported that, in addition to the EP, other personnel commonly present during an infection-related system extraction were: a theater nurse; anesthetist, scrub nurse, and cardiac surgeon (Supplementary Table 1). A majority of pacemaker extractions reportedly occurred in an operating theater (61.8–97.1%) and were estimated to take an average of 78.2–131.3 min (). In addition, panelists estimated 18.2–30.0% of patients undergoing an infection-related system extraction would require monitoring in intensive care or critical care units. The average length of stay in intensive care ranged from 1.8–4.2 days. Following an extraction, an estimated 48.0–81.4% of pacemaker system replacements were performed as inpatients.
A majority of panelists (65.6%, n = 21) reported that the duration of antibiotic days varied by the type of infection (). The mean estimated total number of IV antibiotic days for a pocket infection ranged between 9.6–13.5 days, compared to 21.3–29.2 days for a lead infection. Furthermore, the mean estimated duration of oral antibiotics for pocket and lead infections ranged from 6.3–14.5 days, and 2.5–10.2 days, respectively. For panelists who reported no difference in antibiotic prescribing by type of infection, the estimated duration of IV and oral antibiotic therapy was 14.6–24.5 days, and 7–8.3 days, respectively.
Table 3. Scenario 1: Duration of antibiotics by infection type.
Panelists estimated that 48.8–86.3% of patients with pacemaker infections received their full course of IV antibiotics as inpatients. Of note, North American panelists reported that less than half of patients received their full course of antibiotics as inpatients compared to 50–86.3% in other regions (). Vancomycin and cephalosporins were the most commonly prescribed antibiotics following a system extraction (Supplementary Table 1). In addition, patients who were discharged before completing their full course of IV antibiotics were released 3.5–5.6 days after initiating antibiotic therapy. Finally, the mean number of additional follow-up visits in patients treated for infection requiring a system extraction was 2.0–2.5 over a 1-year period.
Scenario 2: Management of lead fracture or insulation breach requiring lead replacement
Panelists reported that, on average, patients with lead fractures made 1.5–3.0 EP visits; with North American panelists reporting the highest number of visits (). Panelists estimated that fewer than half of fractured leads would require an extraction (13.8–43.2%). Traction, mechanical sheath, and laser sheath were commonly used methods of fracture-related lead extractions, according to panelists (Supplementary Table 2).
Table 4. Scenario 2: Management of lead fractures or insulation breaches resulting in lead replacement.
Aside from the EP, an EP trainee/assistant, theater, and scrub nurse were the most commonly present personnel during a lead extraction procedure (Supplementary Table 2). The majority of extractions (85.0–100.0%) were reported to be performed as inpatients; mostly in an operating theatre (58.3–99.4%), and took between 85.5–116.3 min (). The average length of hospital stay was estimated to be between 2.0–9.5 days, and was longest for Japan (9.5 days) compared to other regions (2.3–2.7 days). Patients who underwent lead extractions were estimated to make an additional 1.5–2.8 visits) over a 1-year period.
Scenario 3: Treatment of upper extremity DVT
Panelists estimated that few patients (1.0–2.4%) with symptomatic upper extremity DVT received a lead extraction (). Australian panelists reported that the majority of patients (62.0%) with upper extremity DVT would be treated as inpatients compared to 5.5–23.4% of patients in other regions. The estimated mean length of inpatient stay ranged from 1.9–8.8 days, and was highest for Japan (8.8 days), compared to 1.9–4.2 days in other regions. The most common IV anticoagulation therapy prescribed was heparin (Supplementary Table 3) for an average duration of 4.3–7.8 days (). The most common oral anticoagulation therapy prescribed was warfarin (Supplementary Table 3) for an average duration of 95.0–182.2 days (). Finally, the estimated mean number of additional follow-up visits following symptomatic upper extremity DVT was 1.9–3.3 visits over a 1-year period.
Table 5. Scenario 3: Treatment of upper extremity DVT.
Clinician confidence in responses
Most panelists were highly confident in the answers they provided for each scenario, with the majority rating their level of confidence as at least 70%.
Discussion
We used a Delphi panel approach to generate consensus on the management of infections, lead fractures, and DVTs in four regions (i.e. North America, Western Europe, Japan, and Australia). The impetus for a Delphi panel approach was to obtain estimates of HRU related to pacemaker complications in the absence of real-world economic data. The Delphi panel provides region-specific data that would enable countries to estimate the costs of complications within their healthcare contexts.
Consensus on most survey items was achieved after the first round results, suggesting general within-region agreement on resource use for management of pacemaker complications. In addition, the mean estimates were generally similar across regions, suggesting low inter-region variability. The latter finding was surprising, as we had postulated that HRU would differ due to variable healthcare systems across regions. Notably, Japan panelists reported longer durations of hospital stay for patients with lead fractures and DVTs than other regions. More research is needed to discern whether the longer durations of hospital stay reported for Japan reflect typical clinical practice in that country or are due to possible differences in pacemaker patient characteristics compared to other regions. Of the three complications, infections were the most resource-intensive to manage and were characterized by prolonged parenteral antibiotic therapy and hospital stays, laboratory and diagnostic procedures, system extractions and replacements, and critical or intensive care for some patients. DVTs on the other hand were the least resource-intensive and were predominantly treated as outpatients with oral anticoagulation therapy; with less than 2% of cases requiring surgical intervention. Lead fractures were moderately resource intensive to treat, as a third of fractured leads reportedly resulted in an extraction in addition to a replacement.
We found panelist responses on management of pacemaker infections and lead extractions to be consistent with the 2015 British Society for Antimicrobial Chemotherapy (BSAC) guidelines for diagnosis and management of cardiac implantable device (CIED) infectionsCitation16, and the 2009 HRS lead extraction guidelinesCitation15, respectively. In the first round of surveys, there was substantial variability in responses provided on duration of antibiotic use during an infection. However, variability was reduced when panelists were asked whether treatment differed by the type of infection, to which most panelists affirmed. The BSAC guidelines provide recommendations for antimicrobial therapy for CIED-related infections, depending on whether an infection is considered pocket or lead-related. For pocket infections, the guidelines recommend parenteral antibiotics for 14 days, and 42 days for lead infections.
However, the guidelines also highlight that the duration of antibiotic use is contingent on the severity of infection and whether or not a system replacement is planned. These nuances were not captured in our survey questions, and might explain why some panelists stated that the duration of antibiotic therapy was the same regardless of the type of infection.
Both HRS and BSAC guidelines recommend complete system extractions, where infections involving cardiac implantable devices are evidentCitation15,Citation16. A majority of panelists stated that electrosurgical sheath, a method commonly used to remove chronically implanted devices, was the preferred extraction method for infected pacemaker systemsCitation17. Although pacemaker infections can occur acutely in the first few months post-implant, the risk is elevated during a pacemaker replacement following battery end of life, ∼12 years post-implantCitation10. Electrosurgical methods using electrocautery are used in generator removal to remove fibrotic tissue that encapsulates the generator over time, and to prevent bleedingCitation17. In addition, electrosurgical sheaths use their energy source to vaporize scar tissue, allowing chronically implanted leads to be removed with less force. There appeared to be regional differences in the use of laser sheath extraction methods, with more North American and Australian panelists using laser sheath extraction compared to panelists from Japan and Western Europe. It is not clear whether the observed regional differences reflected a true difference in clinical practice or a study artifact, and more research is needed. Nevertheless, lead extraction using laser sheath has been shown to have high success rates and low complication ratesCitation18, and is, therefore, a good adjunct to electrosurgical sheath extraction.
According to the HRS guidelines, there is no clear consensus on how lead complications that are not infection-related should be managedCitation15. This is because, unlike infections, abandoned non-infected leads do not carry an increased morbidity and mortality risk to the patient. Therefore, the decision to extract the lead is a risk-benefit trade-off contingent on the health status of the patient, complication risk, and operator experience. This might explain why study panelists reported that less than half of fractured leads were extracted. In patients for whom a lead extraction was indicated, traction, mechanical sheath, and laser sheath were the predominate extraction methods applied. Generally, the extraction method chosen for a non-infected lead will depend on the time from implantation. Traction and mechanical sheaths are effective in removing leads that have been in situ for short periods of time (<1 year from implantation), whereas laser extraction will be attempted where there is considerable lead fibrosis or where extraction using traction or mechanical sheaths failsCitation19.
Survey panelists reported that 1–2.4% of leads that resulted in symptomatic upper extremity DVT were extracted. The HRS guidelines recommend that lead extraction should be considered when symptomatic lead-related thrombotic events occur, or when venous occlusions impede pacemaker implantationCitation15. However, the same guidelines also state that other approaches, such as allowing collaterals to develop, limb elevation, and anticoagulation, can alleviate symptoms, and should be considered prior to lead extraction. Studies have shown that most cases of lead-related venous thrombosis present during the first 3–12 months post-implant, and respond well to anticoagulation therapyCitation8,Citation9,Citation20,Citation21. On the other hand, anticoagulation is not effective in treating thrombotic events occurring in chronically implanted leads, and extraction is warranted insteadCitation15. Symptomatic thrombotic events resulting from chronic occlusions tend to be uncommon (2–6%)Citation22, which might explain why survey panelists estimated that only a small proportion of leads with thrombotic events were extracted.
Strengths and limitations
The main strength of the Delphi panel method is that it provided a structured framework for examining a complex problem with a diverse group of panellists, with a goal of achieving consensus of opinion. We intentionally targeted experienced EPs who regularly perform pacemaker implants and extractions to maximize validity of the survey results. Given a paucity of large databases collecting economic data pertaining to pacemaker complications, an alternative approach would have been to conduct medical chart reviews or initiate prospective cohort studies to track outcomes in pacemaker patients, which would have been both time- and resource-intensive undertakings. The Delphi panel, therefore, provided an avenue for collecting valid estimates of HRU in a timely and less costly fashion.
The Delphi method is not without limitations. The consensus reached in a Delphi may not be a true consensus, and may represent a compromised position or a middle-of-the-road consensus due to a tendency to eliminate extreme positionsCitation23. In addition, the results of the panel represent the views of the respondents who participated, and may not be generalizable outside of this panel. Furthermore, in order to prevent respondent fatigue, the surveys were limited in scope and, therefore, did not include questions on management of acute complications (e.g. hematoma, pneumothorax). Although panelists were explicitly instructed to consider only resource use related to pacemaker complications in their responses, it is possible that physicians may have also been considering resource use pertaining to other cardiac implantable devices, thus confounding the results. We did not parse out how physicians might manage the same complication if it occurred shortly after a pacemaker implant vs after a considerable amount of time post-implant. Several studies have categorized complications into early and late complications, time cut-offs that vary from 2–3 monthsCitation4,Citation24,Citation25. This has resulted in variability in the types and rates of complications reported as early and late complications across studies. For example, whereas lead dislodgements were categorized as both early (<2 months post-implant) and late complications in the FOLLOWPACE studyCitation4, lead dislodgments were early complications only in studies where a 3-month cut-off was appliedCitation24,Citation25. It is even less clear how physicians define early and late complications in clinical practice when managing pacemaker complications. We, therefore, elected not to include time cut-offs in our survey questions, and focused instead on understanding how physicians typically managed pacemaker complications in clinical practice. Finally, data on patient characteristics and patient outcomes were not collected in the panel. Therefore, differences across regions, as well as underlying comorbidities or advanced age, were not accounted for in these surveys.
Conclusions
This study provided insights into the practice patterns of EPs in four key global regions. The Delphi technique proved to be a feasible and efficient method for implementation across these different regions. It also generated a reasonably high level of agreement across responses of the participating country-level panels, and enabled the estimation of complication costs.
Transparency
Declaration of funding
This work was supported by Medtronic.
Declaration of financial/other relationships
CWW, SE, and CW are employees of Medtronic. DJW has received honoraria from Boston Scientific, Medtronic, and St Jude Medical, and a research grant from Boston Scientific for an investigator initiated study. KWW and AS are employees of Evidera, who were paid consultants by Medtronic in connection with this study and development of this manuscript. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Supplemental_Material.docx
Download MS Word (58.4 KB)Acknowledgments
The authors thank Dr Yaariv Khaykin, Southlake Regional Health Center (Canada), for assisting with survey development and validation. Formatting support for this manuscript was provided by Fritz Hamme from Evidera. The authors also thank all 32 electrophysiologists who participated in the Delphi panel.
References
- Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281-329
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;51:e1-62.3
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol 2011;34:1013-27
- Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012;9:728-35
- Johansen JB, Jorgensen OD, Moller M, et al. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J 2011;32:991-8
- Pakarinen S, Oikarinen L, Toivonen L. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace 2010;12:103-8
- Bennet MT, Yee R, Gula LJ, et al. Management of lead systems for implantable devices: techniques and interventions. In: Saksena S, Camm AJ, editors. Electrophysiological Disorders of the Heart: Expert Consult. St Louis, USA: Elsevier Saunders; 2011;1253-64
- Da Costa SS, Scalabrini Neto A, Costa R, et al. Incidence and risk factors of upper extremity deep vein lesions after permanent transvenous pacemaker implant: a 6-month follow-up prospective study. Pacing Clin Electrophysiol 2002;25:1301-6
- Mandal S, Pande A, Mandal D, et al. Permanent pacemaker-related upper extremity deep vein thrombosis: a series of 20 cases. Pacing Clin Electrophysiol 2012;35:1194-8
- Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016;374:533-41
- Brooks K. Delphi technique: Expanding applications. N Cent Assoc Q 1979;54:377-85
- Hsu, Chia-Chien & Sandford, Brian A. The Delphi Technique: Making Sense of Consensus. Practical Assessment Research & Evaluation, 2007. 12(10). http://pareonline.net/getvn.asp?v=12&n=10 (Accessed 6 March 2017)
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nursing 2000;32:1008-15
- Linestone HA, Turoff M, editors. The Delphi Method: Techniques and applications. 2002. http://www.is.njit.edu/pubs/delphibook/delphibook.pdf. [Last accessed 6 March 2017]
- Wilkoff, BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009;6:1085-104
- Sandoe JA, Barlow G, Chambers JB, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother 2015;70:325-59
- Kypta A, Blessberger H, Saleh K, et al. An electrical plasma surgery tool for device replacement—Retrospective evaluation of complications and economic evaluation of cost and resource use. PACE 2015;38:28-34
- Tarakji KG, Ellis CR, Defaye P, et al. Cardiac implantable electronic device infection in patients at risk. Arrhythm Electrophysiol Rev 2016;5:65-71
- Oto A, Aytemir K, Yorgun H, et al. Percutaneous extraction of cardiac pacemaker and implantable cardioverter defibrillator leads with evolution mechanical dilator sheath: a single-centre experience. Europace 2011;13:543-7
- Lelakowski J, Domagala TB, Ciesla-Dul M, et al. Association between selected risk factors and the incidence of venous obstruction after pacemaker implantation: demographic and clinical factors. Kardiol Pol 2011;69:1033-40
- van Rooden CJ, Molhoek SG, Rosendaal FR, et al., Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. J Cardiovasc Electrophysiol 2004;15:1258-62
- Boczar K, Zabek A, Haberka K, et al. Venous stenosis and occlusion in the presence of endocardial leads. Adv Clin Exp Med 2016;25:83-91
- Mitroff II, Turoff M. Philosophical and methodological foundations of Delphi. In: Linstone HA, Turoff M, editors. The Delphi Method: Techniques and applications. Boston (MA): Addison-Wesley; 1975. p 17-35
- Eberhardt F, Bode F, Bonnemeier H, et al. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart 2005;91:500-6
- Wiegand UK, Bode F, Bonnemeier H, et al. Long-term complication rates in ventricular, single lead VDD, and dual chamber pacing. Pacing Clin Electrophysiol 2003;26:1961-9