Abstract
Objective: This study explored short-term healthcare costs of men managed with observation strategies (OBS) vs immediate treatment (IMT) for favorable risk prostate cancer (PCa) from the Geisinger Health System, a single integrated health system in Pennsylvania, as evidence from the community setting is limited.
Methods: A retrospective cohort study was conducted using electronic health records from men aged ≥40 years diagnosed with favorable risk PCa (T1 or 2, PSA ≤15 ng/mL, Gleason ≤7 [3 + 4]) between January 2005 and October 2013. Prostate-specific healthcare costs were compared between the OBS and IMT cohorts in men with ≥3 years of follow-up and available linked claims data. Sub-group analyses focused on those men with low-risk PCa (T1-2a, PSA ≤10 ng/mL, Gleason ≤6). Sensitivity analysis stratified the study sample in three cohorts: OBS, switched from OBS to definitive treatment (OBS switch), and IMT.
Results: A total of 352 patients were included (OBS = 70 and IMT = 282). Compared with IMT, OBS resulted in significantly lower cumulative PCa-related healthcare costs for the first 3 years ($15,785 vs $23,177; p-value <.001). The main cost drivers were outpatient procedures. The OBS cohort had the lowest incremental PCa-related healthcare costs in the first 3 years (OBS: $5,011 vs OBS switch: $26,040, net cost savings = $21,029, p < .001; OBS: $5,011 vs IMT: $24,064, net cost savings = $19,053, p < .001).
Conclusions: In favorable risk PCa, half of the patients who initially chose OBS eventually underwent treatment after their PCa diagnosis. As expected, OBS was associated with reduced disease management costs compared with IMT.
Keywords:
Introduction
Prostate cancer (PCa) is the second most common newly-diagnosed cancer in men, with more than 1.1 million new cases and 307,000 deaths globally in 2012Citation1. The widespread adoption of prostate-specific antigen (PSA)-based screening has led to a substantial increase in the detection of PCa. However, the use of PSA screening has resulted in considerable over-diagnosis and treatment of cancer that would have never caused symptomsCitation2,Citation3. Due to the high incidence of early, localized PCa, combined with the morbidity associated with its over-treatment, observation strategies (OBS) such as active surveillance (AS) and watchful waiting (WW) options have been introduced as alternatives to immediate treatment (IMT) with radical prostatectomy or radiotherapyCitation4,Citation5. AS involves close monitoring of men diagnosed with favorable risk PCa, which consists of low and intermediate risk PCa. The goal of AS is deferring curative treatment until evidence shows that patients have been wrongly graded at initial biopsy or more aggressive disease has developed or becomes apparent. In contrast, WW is commonly a relatively less intensive strategy, with palliative interventions triggered by symptomsCitation5–7.
The prevalent cost of PCa care in the US was estimated at ∼ $12 billion in 2010Citation4. Several economic models have shown the cost-effectiveness of using AS over IMT for low-risk PCa patients. Eldefrawy et al.Citation8 suggested that AS is the most cost-effective alternative for low-risk PC, and would incur $2,000–$10,000 cost savings in 10 years compared to other treatments. AS represents a cost-effective strategy for managing low-risk PCa. Compared with IMT, AS can result in net per-patient savings of $12,194 at 5 years and $4,329 at 10 yearsCitation9. Laviana et al.Citation10 used model estimations to indicate substantial cost variation during the first 5 years among different treatment strategies, with costs ranging from $7,298 for AS to $23,565 for intensity-modulated radiation therapy. Molinier et al.Citation11 also reported that, over the first 5 years, low-risk PCa patients managed with WW incurred the lowest cost of management compared to other types of interventional treatment.
To our knowledge, there is limited evidence of the healthcare costs of different management strategies for PCa patients from the community setting, as most of the evidence is from economic modeling or academic settings. The objective of the current study is to support short-term economic planning by quantifying and comparing the real-world healthcare costs of men with favorable risk PCa who underwent OBS vs those who received IMT within the Geisinger Health System (GHS), a US community-based healthcare system, using aggregate costs of the actual resource. The GHS includes the Geisinger Clinic, a hospital/provider network with more than 40 primary care clinics, a tertiary care teaching hospital, and 10 other hospitals, as well as an insurance provider (the Geisinger Health Plan [GHP]). Geisinger serves more than 4 million community residents throughout 45 counties in central, south-central, and northeast Pennsylvania, and is the dominant healthcare provider in this region. Geisinger employs ∼1,600 primary and specialty care physicians, and 30,000 total staff.
Methods
Data source
A retrospective analysis was performed using electronic medical records (EMRs), Geisinger oncology registry data, and enrollment information from the Geisinger Health System (GHS or Geisinger) database between January 2004 and April 2015. Beneficiaries of the GHP have administrative claims data. The database used for the current study was exempt from the Institutional Review Board (IRB), as data are linked by Honest Brokers using unique patient codes and are then de-identified prior to initiation of the analysis.
The EMR infrastructure contains longitudinal clinical patient data including patient demographics (age, gender, and race) and encounter details from inpatient, outpatient, and office-based settings such as diagnoses, medications orders, procedures, and laboratory results. The costs observed in the EMR encounter file are not reimbursement costs, but aggregate costs of the actual resources involved in the care, including facility, equipment, and provider; they incorporate the proportion of time of each resource involved and costs of each resource per unit time in the GHS.
The Geisinger oncology registry follows the Facility Oncology Registry Data Standards (FORDS) guidelines and included more than 34,000 patients as of January 2015. A few notable data elements available are the clinical and pathological stage, tumor size, grade, histology, laterality, regional lymph node examination, treatment (e.g. radiation, surgery, chemotherapy) and follow-up data.
Patient identification
Men aged ≥40 years and diagnosed with favorable risk PCa (International Classification of Diseases for Oncology [ICD-O3] site code C61.9 and morphology 81403, T1 or 2, prostate-specific antigen [PSA] ≤ 15 ng/mL, Gleason score ≤7 [3 + 4]) from January 2005 through October 2013 (identification period) were included in the study. The first PCa diagnosis date was defined as the index date. Patients were also required to be active in the GHS ≥12 months pre- index PCa diagnosis date. Patient data were assessed until the earliest of death or the end of the study period (April 2015). Key exclusion criteria were lymph node involvement, evidence of metastases, history of a previous cancer diagnosis ≤5 years prior to the initial PCa diagnosis (except for non-melanoma skin cancer), any PCa treatment (surgery, chemotherapy, radiation therapy, cryotherapy, and hormone therapy) before the index PCa diagnosis, or other cancer diagnosis within 6 months after the initial PCa diagnosis. Cancer diagnoses were extracted using ICD-O3 from the Geisinger oncology registry. Additional information was obtained from EMR data.
Claims data are only available for patients who were enrolled in GHP. Therefore, for this cost analysis, patients with GHP enrollment were considered and the eligible men with ≥3 years of follow-up and linked claims data were analyzed (). Three years was the longest follow-up time that yielded a large enough sample size to capture the healthcare costs associated with prostate cancer management.
Figure 1. Study sample collection criteria. PCa: Prostate Cancer; PSA: Prostate Specific Antigen; GHP: Geisinger Health Plan; OBS: Observation Strategies; IMT: Immediate Treatment.
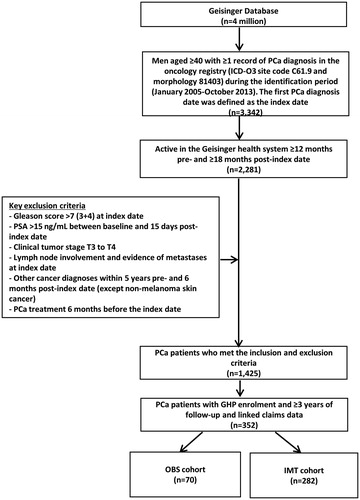
Cohorts were defined as receiving any PCa treatment (IMT) or no treatment within 6 months (OBS) after the index PCa diagnosis date. Treatment data was collected based on the presence of surgery, chemotherapy, radiation therapy, cryotherapy, and hormone therapy using Current Procedural Terminology (CPT), ICD-9 procedures, and Healthcare Common Procedure Coding System (HCPCS) codes.
Study variables
Outcome variables
The primary outcomes included PCa-related healthcare costs. PCa-related healthcare costs were calculated for inpatient and outpatient costs, and were further categorized as medical costs or medication costs. An encounter was considered as PCa-related if it was associated with a primary diagnosis of PCa, PCa treatment, or monitoring procedure: PSA tests, prostate biopsy, transurethral resection of the prostate, transperineal prostate biopsy, transrectal ultrasound, magnetic resonance imaging (MRI), computed tomography (CT) scanning, bone scan, or cystoscopy. The number of PCa-related medications ordered was calculated during inpatient and outpatient encounters. Only medications administered on-site in the Geisinger system were available for the cost analysis. All healthcare costs were adjusted to 2015 US dollars.
Covariates
Demographic and clinical characteristics during the 12 months prior to the index PCa diagnosis date (baseline period) were collected and assessed. Covariates included age, race, marital and insurance status, Charlson Comorbidity Index (CCI) score, body mass index (BMI), prevalence of individual comorbidities (identified using International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes), family history of PCa, and risk category (D’Amico classification). Comorbid conditions of interest included hypertension, diabetes, chronic pulmonary obstructive disorder, congestive heart failure, and benign prostatic hyperplasia. PCa characteristics associated with the index PCa diagnosis were also captured, including index PSA and index total Gleason score.
Statistical analysis
Statistical analyses were conducted with Statistical Analysis System (SAS) Version 9.3 (Cary, NC). All study variables were examined descriptively and compared between the OBS and IMT cohorts. Percentages and counts were calculated for categorical variables. Means and standard deviations were computed for continuous variables. P-values were calculated according to the Chi-square test for categorical variables; t-tests were used for continuous variables. The p-value level of significance was set at an α-level of 0.05.
A generalized linear model (GLM) with gamma distribution and log link function was applied to compare adjusted PCa-related healthcare costs between patients managed with OBS vs IMT. Based on model fitting and clinical rationale, independent variables controlled in the GLM model included the demographic, clinical, and PCa characteristics, as described above in the covariate section.
Sub-group and sensitivity analyses
A sub-group analysis was conducted among low-risk PCa patients. Low-risk patients were defined based on the D’Amico risk classificationCitation12 as patients with T1–T2a, PSA level ≤10 ng/mL, and Gleason score ≤6. All other patients who met the study inclusion criteria were considered intermediate risk. Sensitivity analysis was applied, and patients were further stratified into three cohorts: remained on OBS, switched from OBS to definitive treatment (OBS Switch), and received IMT. OBS switch was defined as patients who did not receive treatment within 6 months of the index prostate cancer diagnosis (index date), but received treatment any time during the follow-up period after 6 months post-diagnosis.
Results
A total of 3,342 men aged 40 and above had a diagnosis of PCa patients within the GHS during the identification period. We excluded patients with T3 or 4 disease, PSA >15 ng/mL, Gleason score >7 [3 + 4], lymph node involvement, evidence of metastases, or prior cancer diagnoses excluding non-melanoma skin cancer. A total of 352 men who had GHP continuous enrollment with ≥3 years of follow-up were included in the analysis of PCa-related healthcare costs. Of these 352 patients, 70 patients (19.9%) were from the OBS cohort and 282 (80.1%) from the IMT cohort ().
Patients managed by OBS strategies were older than the IMT cohort at index date (68.4 vs 64.6 years; p < .001). There were no significant differences between the OBS and IMT cohorts according to index PSA, tumor stage, and family history of cancer, BMI, CCI score, insurance status, index total Gleason score, risk category, or tobacco use ().
Table 1. Socio-demographic and clinical characteristics of prostate cancer patients at baseline in OBS vs IMT cohort (n = 352)
In descriptive analyses, OBS resulted in significantly lower cumulative PCa-related healthcare costs in the first 3 years ($17,628 vs $24,064, p = .0406), with a net cost saving of $6,436 compared to IMT, not adjusting for other covariates (). The OBS cohort incurred higher disease management costs in the first 3 years ($2,192 vs $1,624; p = .4984).
Figure 2. Prostate Cancer-related Health Care Costs among PCa Patients for the Main, Sensitivity, and Subgroup Analyses for the First 3 Years.
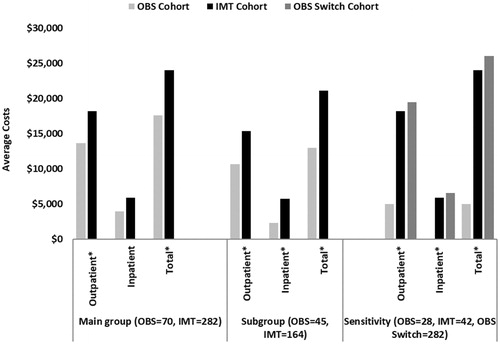
After adjusting for baseline demographic and clinical characteristics using GLM, OBS management strategies among 70 patients in the OBS cohort were associated with significantly lower cumulative PCa-related healthcare costs for the first year ($9,491 vs $19,943; p < .001), first 2 years ($14,111 vs $21,636; p < .001), and first 3 years ($15,785 vs $23,177; p < .001) compared to 282 patients in the IMT cohort. The main cost drivers were primarily outpatient costs (). A further examination of the outpatient costs showed that a major contributor was the initial definitive treatments, such as the prostatectomy or radiation therapy, rather than any longer-term subsequent care or management of treatment complications (data not shown).
Table 2. Adjusted PCa-related healthcare costs among PCa patients in OBS vs IMT using generalized linear models.Table Footnotea
Sub-group and sensitivity analyses
Similar trends in PCa-related healthcare costs were observed in sub-group and sensitivity analyses. Among the entire cohort, 209, 139, and four patients were classified as low-risk, intermediate risk, and unknown, respectively. Among the four patients with missing risk categories, three patients were without PSA information, and one lacked either Gleason score or tumor stage. Sub-group analysis focused on low-risk PCa patients with ≥3 years of GHP enrollment post-index date (OBS = 45 and IMT = 164). OBS resulted in lower cumulative PCa-related healthcare costs in 3 years ($12,954 vs $21,115, p < .0001), with a net cost savings of $8,161 compared to the IMT cohort ().
Sensitivity analysis () further stratified the study sample in three cohorts: remained in OBS (n = 28), switched from OBS to definitive treatment (n = 42), and received IMT (n = 282) in the 3-year follow-up period. Among the three cohorts, the OBS cohort had the lowest incremental PCa-related healthcare costs in the first 3 years (OBS: $5,011 vs OBS Switch: $26,040, net cost savings = $21,029, p < .001; OBS: $5,011 vs IMT: $24,064, net cost savings = $19,053, p < .001).
Discussion
This retrospective analysis assessed short-term PCa-related healthcare costs associated with OBS vs IMT for patients diagnosed with favorable risk PCa.
The study was intentionally not based upon a controlled clinical protocol, because it was intended to reflect the real-world implementation of routine PCa management. The inclusion/exclusion criteria were primarily based on the patient having lower risk, early stage of PCa at diagnosis. To achieve the observational nature of the study, no requirements or exclusions were made based upon management protocol.
The study findings demonstrated a significantly lower healthcare cost in patients managed with OBS strategies compared with IMT in a community setting. The present study reinforces the advantages of OBS in economic outcomes compared to IMT among patients diagnosed with favorable risk PCa. The current study supports previous research suggesting that low-risk PCa patients managed with immediate treatment incur higher costs than patients managed with OBSCitation11,Citation13,Citation14. Using the SEER-Medicare program data population, Aizer et al.Citation13 reported that over-treatment of low-risk PCa has an impact on quality-of-life and healthcare costs. Not treating 80% of men with low grade PCa, who are less likely to die from PCa, could yield national savings of $1.32 billion per yearCitation13.
WW and AS have increasingly been used as a means to manage favorable-risk PCa patients and prevent over-treatment, as well as complications that may arise from surgery and other interventions. In this study, we found patients who received OBS incurred lower healthcare costs in the first year compared to IMT patients. Although higher disease management costs were reported in the second and third year following their PCa diagnosis, incremental healthcare costs remained lower for men who continued OBS treatment strategies for the first year 3-years of the follow-up period. These findings were similar to Crawford et al.Citation14, which assessed the clinical and economic implications of WW and IMT in the management of PCa using the PharMetrics database. The average 2-year cost for WW was $24,809, compared to $59,286 for active treatmentCitation14. Similarly, a study by Corcoran et al.Citation15, examining the WW and AS management for low-risk PCa patients, showed a 43–79% reduction in cost when compared to men undergoing upfront radical prostatectomy. The majority of costs for favorable-risk PCa were due to outpatient care, which is similar to the results found by Wilson et al.Citation16 that observed outpatient costs as the main driver associated with WW and IMT in the follow-up period.
The sensitivity analysis using pure OBS management also further highlighted a decrease in the cost of management for low-risk PCa patients compared to the use of IMT or OBS for patients who eventually switched to treatment 6 months post-PCa diagnosis. The incremental differences of disease management costs between the OBS, OBS switch, and IMT cohorts ranged from $19,000–$21,000 in the first 3 years, indicating that substantial cost savings could be generated for men with favorable risk PCa when OBS are appropriate treatment optionsCitation11,Citation13.
However, the study has limitations. First, study cohorts were defined as receiving any PCa treatment (IMT) or no treatment (OBS) within 6 months after an index PCa diagnosis. Therefore, the OBS cohort likely included a mix of patients managed by AS or WW, since the expectant management strategies (e.g. AS or WW) were not prospectively collected or always clearly noted in the patient records. We also might have misclassified OBS patients who planned to receive definitive treatment but who took longer than 6 months to get the procedure completed.
Second, healthcare costs might be under-estimated. Although we endeavored to include complete information for each patient using multiple sources of data, some healthcare utilization may not have been captured if healthcare services were received outside of the Geisinger system. In addition, medication costs were only available for medications administered on-site (injections, infusions, orals). Retail and mail-order pharmacy costs were not accessible in this analysis.
Third, the costs reported in this study will be different than the reimbursed amounts, which are more commonly reported in the literature. This study reported the “true costs” of managing PCa patients from the provider perspective. Geisinger’s negotiated rates are considered proprietary and are, thus, not provided externally.
Fourth, healthcare costs were solely assessed for patients who received care within the Geisinger health system. Since the Geisinger health system serves patients in Pennsylvania specifically, the study results may not be generalizable to the entire US PCa population. PCa-related costs were analyzed for patients with ≥3 years of follow-up, which further excluded patients from both cohorts. Three years is a short time to assess the long-term costs of the different management for PCa. However, there is limited evidence of healthcare costs from even 3 years of follow-up for the different management strategies of PCa in a community setting. To fill this gap, data from the Geisinger Health System (GHS), a community-based health services organization, was used. The GHS has integrated EMR and claims data, but requiring both data sources limits the number of qualifying patients. Using follow-up periods of longer than 3 years would have further reduced the sample size; therefore, we believe 3 years is a reasonable balance.
Furthermore, healthcare costs were assessed during the first 3 years after PCa diagnosis. It is possible that, over time, the costs for OBS might exceed the costs for IMT. In fact, in the current analysis, men who switched from OBS to definitive treatment incurred higher costs; further switches post 3 years could lead to higher expenditures per-capita. Therefore, the short follow-up period of 3 years might not give enough time to evaluate the true difference in costs between the OBS and the IMT cohorts.
Finally, almost all the men that met the inclusion criteria and were, thereby, included in the analysis were white, which may also limit the generalizability of the study.
The findings should be interpreted considering the objectives of current analysis. Other economic costs associated with PCa, such as time lost from work for patient and caregivers, were not captured in the GHS data and are beyond the scope of this analysis. Potential savings of any treatment option should be considered in relation to costs associated with disability and mortality (DALY) that are not investigated in this paper and will need further investigation. The authors will report in a separate manuscript a comparison of the clinical outcomes in the two management cohorts.
While no single source provides comprehensive data for most US patients, the major strength of this study is the combination use of real-world EMRs, claims, and oncology registry data from a community setting to ensure that complete data was captured for each patient. Each of the three data sources provided complementary information.
Conclusion
Among favorable risk PCa patients, OBS management strategies were associated with lower disease management costs compared with definitive treatment in the 3 years of the follow-up period. This analysis suggests that substantial short-term cost savings could be generated for men with favorable risk PCa when OBS are appropriate treatment options. With the rapid changes in healthcare expenditures, it is important to find a more effective and cost-saving way to manage favorable risk PCa. The potential savings that can be obtained from managing favorable-risk PCa patients with OBS are very important. The current analysis also provides information on PCa management costs for managed care organizations or payers for budget planning. This data could provide insights to healthcare payers and assist the decision-making process and allocation of resources for short-term and early prostate cancer management. More educational activities may be needed to improve guideline awareness and increase the adoption of OBS in the community setting.
Transparency
Declaration of funding
Research funding for this study was provided by Roche Molecular Systems.
Declaration of financial/other relationships
FK, YW, and LX are employees of STATinMED Research, a paid consultant to Roche, Inc. IC was previously an employee of Roche Diagnostics. LW is an employee of Genetech, Inc. and stockholder of Roche, Inc. DM and IA are employees of Roche Diagnostics. EM was an employee of Geisinger Health System, a paid consultant to Roche, Inc., at the time of this study, and is currently an employee and stockholder of Eli Lilly and Company. JD is an employee of Geisinger Health System, a paid consultant to Roche, Inc. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Statistical analysis support and editorial support were provided by Adesuwa Ogbomo of STATinMED Research.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86
- NCI dictionary of Cancer Terms. National Cancer Institute; National Institute of Health. Bethesda, Maryland. 2017. https://www.cancer.org/publications/dictionaries/cancer-terms. Accessed April 5, 2017
- Esserman LJ, Thompson IM, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA 2013;310:797-8
- Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63:597-603
- Yabroff KR, Lund J, Kepka D, et al. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev 2011;20:2006-14
- Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007;177:2106-31
- Dahabreh IJ, Chung M, Balk EM, et al. Active surveillance in men with localized prostate cancer: a systematic review. Ann Intern Med 2012;156:582-90
- Eldefrawy A, Katkoori D, Abramowitz M, et al. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol 2013;31:576-80
- Dall’Era MA, Evans CP. The economics of active surveillance for prostate cancer. In: Klotz L, editor. Active surveillance for localized prostate cancer: a new paradigm for localized prostate cancer. 1st ed. New York, NY: Humana Press; 2012. p 179-85
- Laviana AA, Ilg AM, Veruttipong D, et al. Utilizing time-driven activity-based costing to understand the short- and long-term costs of treating localized, low-risk prostate cancer. Cancer 2016;122:447-55
- Molinier L, Castelli C, Bauvin E, et al. Cost study of the clinical management of prostate cancer in France: results on the basis of population-based data. Eur J Health Econ 2011;12:363-71
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74
- Aizer AA, Gu X, Chen MH, et al. Cost implications and complications of overtreatment of low-risk prostate cancer in the United States. J Natl Compr Canc Netw 2015;13:61-8
- Crawford ED, Black L, Eaddy M, et al. A retrospective analysis illustrating the substantial clinical and economic burden of prostate cancer. Prostate Cancer Prostatic Dis 2010;13:162-7
- Corcoran AT, Peele PB, Benoit RM. Cost comparison between watchful waiting with active surveillance and active treatment of clinically localized prostate cancer. Urology 2010;76:703-7
- Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer 2007;109:518-27
