Abstract
Aims: An increase in the prevalence of antimicrobial resistance among gram-negative pathogens has been noted recently. A challenge in empiric treatment of complicated intra-abdominal infection (cIAI) is identifying initial appropriate antibiotic therapy, which is associated with reduced length of stay and mortality compared with inappropriate therapy. The objective of this study was to assess the cost-effectiveness of ceftolozane/tazobactam + metronidazole compared with piperacillin/tazobactam (commonly used in this indication) in the treatment of patients with cIAI in UK hospitals.
Methods: A decision-analytic Monte Carlo simulation model was used to compare costs (antibiotic and hospitalization costs) and quality-adjusted life years (QALYs) of patients infected with gram-negative cIAI and treated empirically with either ceftolozane/tazobactam + metronidazole or piperacillin/tazobactam. Bacterial isolates were randomly drawn from the Program to Assess Ceftolozane/Tazobactam Susceptibility (PACTS) database, a surveillance database of non-duplicate bacterial isolates collected from patients in the UK infected with gram-negative pathogens. Susceptibility to initial empiric therapy was based on the measured susceptibilities reported in the PACTS database.
Results: Ceftolozane/tazobactam + metronidazole was cost-effective when compared with piperacillin/tazobactam, with an incremental cost-effectiveness ratio (ICER) of £4,350/QALY and 0.36 hospitalization days/patient saved. Costs in the ceftolozane/tazobactam + metronidazole arm were £2,576/patient, compared with £2,168/patient in the piperacillin/tazobactam arm. The ceftolozane/tazobactam + metronidazole arm experienced a greater number of QALYs than the piperacillin/tazobactam arm (14.31/patient vs 14.21/patient, respectively). Ceftolozane/tazobactam + metronidazole remained cost-effective in one-way sensitivity and probabilistic sensitivity analyses.
Conclusions: Economic models can help to identify the appropriate choice of empiric therapy for the treatment of cIAI. Results indicated that empiric use of ceftolozane/tazobactam + metronidazole is cost-effective vs piperacillin/tazobactam in UK patients with cIAI at risk of resistant infection. This will be valuable to commissioners and clinicians to aid decision-making on the targeting of resources for appropriate antibiotic therapy under the premise of antimicrobial stewardship.
Introduction
Intra-abdominal infections (IAIs) represent a wide variety of conditions involving lesions of the intra-abdominal organs. Complicated IAIs (cIAIs) arise when the infection progresses beyond a single organ, causing localized or diffuse peritonitisCitation1. Complicated intra-abdominal infections remain an important source of patient morbidity, and are very often associated with poor clinical prognoses, especially in high-risk patientsCitation2. Management of cIAIs involves both surgical and antibiotic therapy, with empiric antibiotic therapy being an important component of treatmentCitation3. Given the short, episodic nature of cIAIs, the risk of disease progression is amplified if the initial antibiotic therapy is inappropriateCitation4.
Gram-negative pathogens, including multi-drug resistant pathogens, account for 69.2% of cIAIs in EuropeCitation2. Patients with cIAIs due to resistant pathogens are more likely to receive initially inappropriate antibiotic therapy, defined as microbiological documentation of an infecting pathogen that was not effectively treated at the time of its identificationCitation5,Citation6. Therefore, if empiric therapy is needed, the antibacterial spectrum of the antibiotic agent should be appropriate and adequate, to include the most relevant pathogens prior to microbiological results becoming available. Appropriate and adequate agents are particularly important, as a significant number of critically ill patients who are at increased risk for resistant pathogens never receive culture results and treatment failure is only detected through inadequate clinical response. Inappropriate therapy in gram-negative infection is associated with a longer length of stay (LOS), higher hospital costs, and higher rates of mortalityCitation7,Citation8. This has been demonstrated in two observational studies, one in the US and another in ItalyCitation7,Citation8. In a US database study in over 6,000 patients with cIAI, the additional LOS for inappropriate therapy relative to appropriate therapy was 4.6 days (11.6 days vs 6.9 days total per patient), with additional hospital costs of $6,368 ($16,520 vs $10,152) and substantial excess mortality (9.5% vs 1.3%) in the study sample of patients admitted between April 1, 2003 and March 31, 2004Citation7. Similarly, in an Italian study, inappropriate therapy was associated with an additional 8.2 days of antibiotic therapy, an additional LOS of 11 days, and an additional €5,592 in hospitalization costs compared with appropriate therapyCitation8.
Piperacillin/tazobactam is commonly used for empiric therapy. Increasing resistance of Escherichia coli, Klebsiella pneumonia and Pseudomonas spp. to piperacillin/tazobactam has been observed between 2010–2014 in a recent Public Health England report, although the authors advise to interpret these findings with caution due to a change in laboratory testing standards over the same time periodCitation9. However, this trend has also been observed in the British Society of Antimicrobial Chemotherapy (BSAC) resistance surveillance projectCitation10.
Ceftolozane/tazobactam is a novel cephalosporin/β-lactamase inhibitor combination with activity against multi-drug resistant gram-negative pathogens, including extended-spectrum β-lactamase-producing Enterobacteriaceae and drug-resistant P. aeruginosa. The objective of this study was to evaluate the cost-effectiveness of ceftolozane/tazobactam + metronidazole compared with piperacillin/tazobactam (commonly used in this indication) in the treatment of patients in the UK hospitalized with cIAI at risk of infection with a resistant pathogen. The model reflects treatment algorithms that are routinely used to treat this condition.
Methods
Model structure
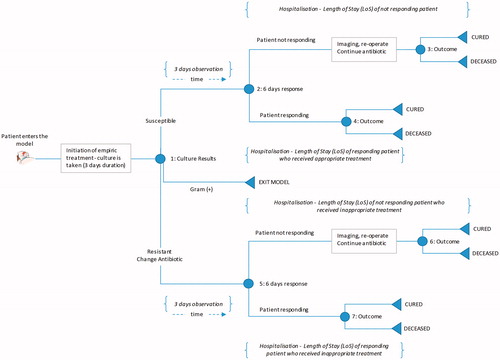
We developed a decision-analytic microsimulation model to estimate the quality-adjusted life expectancy and costs of patients admitted to a hospital, diagnosed with cIAI, and administered empiric antibiotic therapy. A graphical representation of the model structure is provided in .
Patients enter the model at the time of cIAI diagnosis by the treating clinician, which is assumed to be concurrent with the initiation of empiric antimicrobial therapy. Each patient receives empiric antibiotic treatment with either ceftolozane/tazobactam + metronidazole or piperacillin/tazobactam. At the point of cIAI diagnosis, a specimen is obtained for culture to determine the pathogen and its in vitro susceptibility pattern.
The pathogen distribution and in vitro susceptibility is based on randomly selected bacterial isolates from the Program to Assess Ceftolozane/Tazobactam Susceptibility (PACTS) surveillance dataset, with each isolate representing a single patient. Only UK isolates from the PACTS database (Supplementary Appendix 1) were included in the model and subsequent analyses.
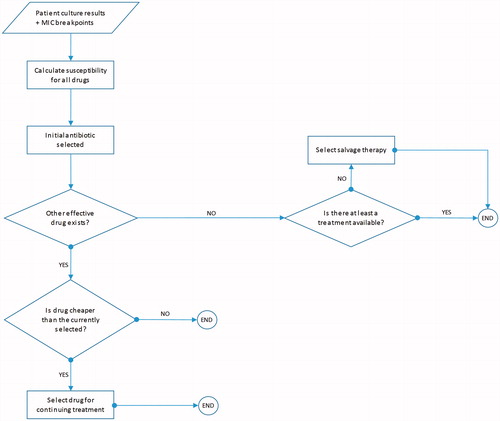
After a patient is selected, their treatment pathway and disease progression is estimated using a decision-tree, shown in . Patients continue empiric treatment until culture results are available. Once culture results are available, patients are switched to the least expensive therapy to which the causative pathogen is susceptible. If the pathogen is not susceptible to any of the modeled comparators, patients are switched to salvage therapy (meropenem plus colistin). While culture may not always be administered in clinical settings, the available PACTS database provides us with information regarding the underlying pathogen and the appropriateness of the therapy. Thus, the proportion of the time a therapy is appropriate or not will be accurately predicted by our method, irrespective of culture administration.
Patients with a gram-negative abdominal culture have two potential pathways (). Patients with gram-positive infection exit the model after 3 days of initial therapy, and only the costs for initial therapy are taken into consideration, as all subsequent outcomes and costs are assumed to be comparable for both arms.
Table 1. Treatment pathway for patients with a gram-negative abdominal culture.
Each patient enters the decision tree, and relevant costs along the treatment pathway are accumulated as they progress through the model. The appropriateness of initial antibiotic therapy determines each patient’s length of hospital stay and treatment outcome. The model assumes that patients are either cured or die at the end of treatment; inappropriate therapy is assumed to result in excess inpatient mortality and a longer hospital stay. Re-occurring infection and/or re-admission are not considered in the model.
Inputs
Susceptibility: customizing PACTS database to represent cIAI patients
The in vitro surveillance data from the PACTS database is the only available, patient level, real-world data reflecting IAI patients at risk of resistant infection in the UK, and includes isolates susceptibility to ceftolozane/tazobactam. Isolates obtained from UK sites between 2012–2013 were included in this analysis. The following organisms were included in line with the approved label for ceftolozane/tazobactam, Enterobacter cloacae, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumonia, Proteus mirabilis and Pseudomonas aeruginosa. These encompass the major pathogens implicated in cIAIsCitation11. The percentage of patients with gram-positive infections in the cohort was 29.1%Citation11.
Susceptibility breakpoints
Susceptibility is evaluated using European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpointsCitation12.
Drugs used for the model
The empiric treatments used in the model are ceftolozane/tazobactam + metronidazole and piperacillin/tazobactam, which are consistent with their respective marketing authorizations and international cIAI treatment guidelinesCitation3,Citation13. Metronidazole is co-administered with ceftolozane/tazobactam, due to the limited spectrum of activity against anaerobic bacteria, and also per the prescribing information for ceftolozane/tazobactamCitation14. Piperacillin/tazobactam is active against a broader range of anaerobic bacteria, and the addition of metronidazole is not recommended per treatment guidelinesCitation3, and also per the prescribing information for piperacillin/tazobactamCitation15. The following additional drugs were considered for switching upon pathogen confirmation: ceftriaxone, ciprofloxacin, colistin, imipenem, meropenem, and tigecycline.
Clinical inputs
The key clinical inputs are summarized in . Mortality rates and length of stay were based on Edelsberg et al.Citation7. The duration of empiric therapy was assumed to be 3 days. Actuarial life-tables from the Office of National Statistics were used for the prediction of life expectancy (cohort tables, according to gender)Citation16. The percentage of patients with cIAI requiring re-intervention has been reported at 8–9%Citation2,Citation17. While most studies examining the impact of inappropriate therapy on treatment outcomes in cIAI did not report re-intervention rates, there is evidence from at least one study that inappropriate therapy may increase the risk of re-intervention (relative risk ratio = 5.1; 95% CI = 1.7–15.4)Citation18. As the Krobot et al.Citation18 study was relatively small and conducted over a decade ago, this analysis conservatively assumed that there was no differential impact of inappropriate therapy on re-intervention rates and associated costs.
Table 2. Clinical and economic inputs.
Economic inputs
Hospitalization costs per day () were derived from NHS reference costs (2013–2014), based on the cost of an elective inpatient excess bed day for gastrointestinal infections with a single intervention (Complication and Comorbidity Score 5+)Citation19.
Daily drug costs () were calculated for the duration of hospitalization based on licensed dosesCitation20. Salvage therapy costs were based on combination therapy with meropenem and colistin.
A utility value of 0.85 was applied to cured patients for the remainder of their lives (). This was a conservative assumption based on a utility value of 0.9 proposed by Jansen et al.Citation21.
QALYs were discounted at a rate of 3.5% per annumCitation22,Citation23. No discounting was applied to costs, as all costs occur within the first year, given the acute nature of cIAI.
Analysis
A lifetime time horizon was applied to capture the utility of healthy survivors over their lifetime in accordance with ISPOR modeling guidelinesCitation24. The model adopted the perspective of the NHS and personal social servicesCitation23.
To compare the two treatment strategies the following outcomes were estimated from the model: proportion of patients appropriately and inappropriately treated (sensitive/resistant to empiric therapy with ceftolozane/tazobactam + metronidazole or piperacillin/tazobactam), cost per QALY saved, drug costs, hospitalization costs, proportion of cases by pathogen, total costs (undiscounted), and total QALYs (undiscounted and discounted). Differences in these outcomes of interest were estimated, along with the ICER based on total cost per QALY gained.
One-way sensitivity analyses (OWSA) and probabilistic sensitivity analysis (PSA) were performed to quantify the uncertainty in the model outcomes based on the uncertainty of the input parameters. The parameters identified in the OWSA with the greatest impact on the ICER were summarized with tornado diagrams.
For the PSA, 10,000 samples were taken from the defined distributions of clinical and economic inputs. Log-normal distributions were used for odds ratios, beta distributions for utilities, and gamma distributions for resource use and costs.
For each treatment strategy, the probability of being cost-effective at different willingness to pay (WTP) thresholds was expressed with cost-effectiveness acceptability curves. This was calculated as the proportion of the 10,000 iterations for which the net monetary benefit (NMB) was greatest for a given treatment strategy out of all strategies.
An amount of £20,000/QALY was used as a WTP threshold, which is within the maximum acceptable ICER range (£20,000–£30,000 per QALY gained) considered cost-effective by NICECitation23.
Risk factors associated with infection due to resistant pathogens (vs susceptible pathogens) have been identified in the literatureCitation25,Citation26. Information regarding some of these risk factors for cIAI was available for patients in the PACTS dataset, including (a) nosocomial infection, (b) age ≥65 years, and (c) patients situated in the intensive care unit (ICU) service.
Scenario analyses were performed, first using only nosocomial bacterial isolates and, second, using only nosocomial isolates from high risk patients aged ≥65 years or requiring an ICU stay.
Results
Base case results
The analysis was performed on a cohort of 1,000 patients with an average age of 63.1 years, ranging from 18–94 years.
The key results from the model are summarized in . Under the base case scenario, the ceftolozane/tazobactam + metronidazole arm resulted in higher total costs than the piperacillin/tazobactam arm (£2,576/patient vs £2,168/patient, respectively). However, the ceftolozane/tazobactam + metronidazole arm experienced a greater number of QALYs than the piperacillin/tazobactam arm (14.31/patient vs 14.21/patient, respectively). This resulted in an ICER of £4,350/QALY and an additional 0.36 hospitalization days/patient saved.
Table 3. Results.
In patients receiving ceftolozane/tazobactam + metronidazole as empiric therapy, 29.6% were resistant (i.e. received inappropriate empiric therapy) compared with 37.3% receiving piperacillin/tazobactam. There were 37.2 deaths (3.7%) in the ceftolozane/tazobactam + metronidazole arm, compared with 43.6 (4.4%) in the piperacillin/tazobactam arm, a difference of 0.7%. Amongst those who died, a larger proportion were resistant to initial therapy in the piperacillin/tazobactam arm than the ceftolozane/tazobactam + metronidazole arm. The ceftolozane/tazobactam + metronidazole arm generated a total of 94 more QALYs (discounted).
Hospital associated costs were the largest contributor to total costs in both treatment arms. The average hospital cost per patient in the ceftolozane/tazobactam + metronidazole arm was lower than in the piperacillin/tazobactam arm (£1,887/patient vs £2,005/patient, respectively). However, drug costs in the ceftolozane/tazobactam + metronidazole arm were higher than in the piperacillin/tazobactam arm (£689/patient vs £163/patient, respectively). The resultant total cost per patient was higher in the ceftolozane/tazobactam + metronidazole arm than in the piperacillin/tazobactam arm (£2,576 vs £2,168, respectively).
All of the patients in the ceftolozane/tazobactam arm who received appropriate therapy were able to be switched to a less expensive therapy after 3 days (following culture results). All patients in the piperacillin/tazobactam arm, who received appropriate therapy, remained on piperacillin/tazobactam, as this was the least expensive treatment available. No patients in either arm required salvage therapy.
One-way sensitivity analysis
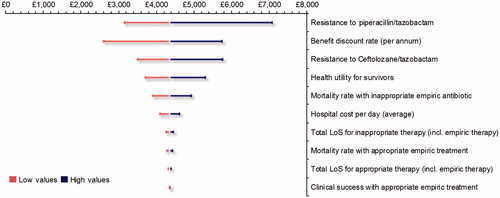
The results of the one-way sensitivity analysis are presented in a tornado graph (). Varying the resistance to piperacillin/tazobactam resulted in the largest impact on the resultant ICER. The other input parameters which impacted the ICER when varied were: discount rate (for benefits), resistance to ceftolozane/tazobactam, utility value applied to survivors, mortality rate with inappropriate therapy, cost per hospital day, total length of stay for inappropriate therapy, mortality rate for appropriate therapy, and the total length of stay for appropriate therapy. In all instances, the ICER ranged between £2,595/QALY and £7,063/QALY, below the maximum WTP threshold considered cost-effective per NICE guidance (£20,000–£30,000/QALY)Citation27.
Probabilistic sensitivity analysis
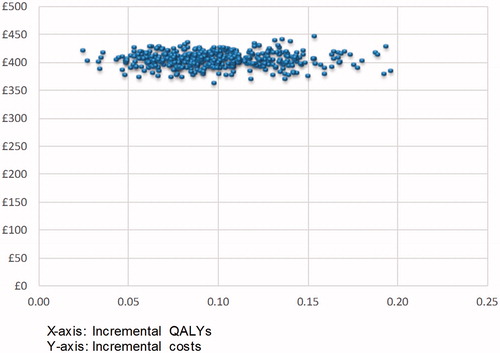
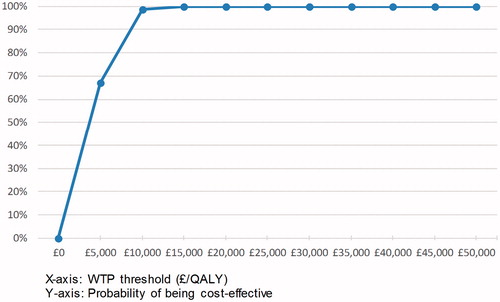
The distribution of ICER estimates from the PSA show that, in all instances, ceftolozane/tazobactam + metronidazole is more effective, but more costly than piperacillin/tazobactam (). Ceftolozane/tazobactam + metronidazole has a 67.0% probability of being cost-effective compared with piperacillin/tazobactam at a WTP of £5,000/QALY gained and a 98.6% probability of being cost-effective at a WTP of £10,000/QALY. At a WTP threshold of £20,000/QALY, the probability is 100% ().
Scenario analysis
Forty-eight per cent of patients from the UK PACTS dataset had bacterial isolates from nosocomial sources. Amongst these patients, 51% were aged ≥65 years or required an ICU stay, and were deemed at “high-risk” of having an infection due to resistant pathogens.
Results of the two scenarios are presented in . Overall, the cost-effectiveness favors ceftolozane/tazobactam + metronidazole vs piperacillin/tazobactam in both scenarios analyzed. The proportion of patients susceptible to empiric therapy with ceftolozane/tazobactam + metronidazole remained unchanged in both scenarios; however, the proportion of patients susceptible to empiric therapy with piperacillin/tazobactam reduced to 58.0% in the first scenario and 55.5% in the second scenario. Differences in total QALYs and the number of hospitalization days (not reported) are larger in both of the scenarios explored compared with the base case; however, the difference in total costs is lower.
Table 4. Scenario analysis results.
Discussion
The objective of our analysis was to evaluate the use of ceftolozane/tazobactam + metronidazole compared with piperacillin/tazobactam in the empiric treatment of UK patients with cIAI at risk of infection due to a resistant g ram-negative pathogen. In this analysis we used an approach similar to that employed by Zilberberg et al.Citation28 and subsequently replicated by othersCitation29–31 whereby data were culled from large multinational data sources on antimicrobial susceptibility to assess the effectiveness and cost impacts of alternative antimicrobial therapies. The attractiveness of this method is that it utilizes a large data source on pathogen prevalence and antimicrobial susceptibility, to estimate the effectiveness of ceftolozane/tazobactam in cIAI.
The ability of either treatment to provide appropriate empiric coverage is a key concept in the model and the source of economic differentiation between the two treatments. Ceftolozane/tazobactam + metronidazole provides a greater proportion of appropriate empiric coverage than piperacillin/tazobactam, as demonstrated by the PACTS data.
Our findings suggest that the use of ceftolozane/tazobactam + metronidazole as initial empiric treatment is cost-effective compared to piperacillin/tazobactam. Additionally, use of ceftolozane/tazobactam saves an average of 0.36 hospital days per patient.
The impact of inappropriate therapy as a key driver in the model is further emphasized in our two scenarios in high risk patients. In both scenarios, susceptibility rates to ceftolozane/tazobactam + metronidazole remain largely unchanged; however, susceptibility rates to piperacillin/tazobactam are lower compared to the base case. This translated to increased hospital costs in the piperacillin/tazobactam arm. The increase in drug costs in both arms can be attributed to an increase in the cost of drugs used post-culture.
Cost-effectiveness is a function of several model parameters, including duration of empiric therapy, susceptibility among comparators, and the increase in length of stay due to inappropriate therapy. In this study, differences in cost-effectiveness are derived solely from differences in antimicrobial activity between ceftolozane/tazobactam + metronidazole and piperacillin/tazobactam.
One of the limitations of the model is that it does not include differences in clinical sequelae beyond the length of stay and mortality associated with appropriate and inappropriate therapy. The model results need to be interpreted with caution when dealing with antimicrobial classes that are deemed to be high-risk for Clostridium difficile infection. Any savings in length of stay or mortality could be offset by increased costs and mortality associated with Clostridium difficile infection. The model does not account for Clostridium difficile infection as, at present, there is no evidence of an increased incidence of Clostridium difficile infection associated with ceftolozane/tazobactam + metronidazole use vs piperacillin/tazobactam.
We have demonstrated how national surveillance data can be used to guide the choice of empiric therapy. With clinical trials often conducted in a variety of different geographic locations/settings, the patients enrolled may not necessarily reflect the specific populations who will receive these treatments in real life. In clinical practice, patient outcomes can be improved through improvements in the collection of local surveillance data and the use of local antibiograms in decision-making and guideline development, thus supporting good antimicrobial stewardship of new antimicrobial therapies.
The cost-effectiveness reported in this analysis may be under-estimated, as patients were switched immediately to the least expensive susceptible drug (typically with a narrower spectrum of activity) once culture results were available. This may not be reflective of clinical practice.
An important limitation of the model is that it does not account for further treatment changes after any initial de-escalation/escalation, as patients are assumed to be fully cured or dead at the end of hospitalization. Additionally, the costs associated with recurrence and/or re-admission were not incorporated into the model.
Second, the model assumes that the duration of therapy, whilst shorter for appropriate therapy compared with inappropriate therapy, is not directly impacted by the different drugs used following culture results. In practice, some treatments may shorten/prolong the length of hospital stay.
As indicated in the methods section, the model does not include the sequelae for infections caused by gram-positive pathogens after culture. Both ceftolozane/tazobactam and piperacillin/tazobactam are not indicated for the treatment of gram-positive infections. We assumed that the disease progression with both drugs would be similar (i.e. no resolution of symptoms), and the same therapeutic option would be pursued after culture for both the arms. Subsequently, the costs and benefits for this alternative therapy would be the same for both treatment arms, and, from an incremental economic analysis perspective, these costs and benefits can be excluded from the analysis. Since the drug-acquisition costs of ceftolozane/tazobactam and piperacillin/tazobactam are different, we included the drug-acquisition costs only until culture results were known, as these drugs will likely be discontinued after the underlying causative pathogen is known. We assume that the proportion of gram-positive infections is 29.1%. A higher proportion of gram-positive pathogens is likely to make ceftolozane/tazobactam less cost-effective (increase the ICER), as the drug acquisition cost of ceftolozane/tazobactam is higher than that for piperacillin/tazobactam. Similar logic can be extended to anaerobes. We assume that the disease progression for anaerobes is the same for ceftolozane/tazobactam and metronidazole compared with piperacillin/tazobactam. As a result, the associated costs, other than the drug acquisition costs of empiric therapy, and QALYs for both arms are similar.
Also, within the PACTS database, only one isolate per patient infection was included in the surveillance, whereas, in clinical practice, more than one isolate per patient may be isolated. However, our study is a good first step towards understanding the economic impact of antimicrobial susceptibility and empiric therapy.
Additionally, dose adjustments were not considered, and bacterial resistance over time as well as costs of antibiotic preparation and administration, monitoring, and adverse events (which were assumed to be similar across treatments) were excluded from the model. Alternative assumptions and inclusion of these parameters can potentially change the results of the CEA results.
Finally, the model only considers gram-negative aerobes when, in practice, gram-positive aerobes and anaerobes (both gram-positive and gram-negative) are often implicated.
Conclusion
Economic models can help to identify the appropriate choice of empiric therapy for the treatment of cIAI. The results of this cost-utility analysis indicate that the empiric use of ceftolozane/tazobactam and metronidazole is cost-effective and results in lower patient mortality vs piperacillin/tazobactam in cIAI patients at risk of resistant infection in the UK. This finding will be valuable to decision-makers, such as health commissioners and clinicians, in targeting of resources for appropriate antibiotic therapy, under the premise of antimicrobial stewardship.
Transparency
Declaration of funding
Financial support for this study was provided by Merck & Co., Inc. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Declaration of financial/other interests
VP, HA, ES, and SM report other conflicts of interest from Merck & Co., Inc, during the conduct of the study. JF reports personal fees from Merck & Co., Inc, during the conduct of the study.
Appendix
Download MS Word (28.5 KB)Acknowledgments
Dimitris Kabranis was involved in the development of the original model.
References
- Menichetti F, Sganga G. Definition and classification of intra-abdominal infections. J Chemo 2009;21(Suppl1):3–4
- Sartelli M, Catena F, Ansaloni L, et al. Complicated intra-abdominal infections in Europe: a comprehensive review of the CIAO study. W J Emerg Surg 2012;7:36
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect 2010;11:79–109
- Davey PG, Marwick C. Appropriate vs. inappropriate antimicrobial therapy. Clin Microbiol Infect Offic Publ Eur Soc Clin Microbiol Infect Dis 2008;14(Suppl3):15–21
- Falagas ME, Barefoot L, Griffith J, et al. Risk factors leading to clinical failure in the treatment of intra-abdominal or skin/soft tissue infections. Eur J Clin Microbiol Infect Dis 1996;15:913–21
- Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999;115:462–74
- Edelsberg J, Berger A, Schell S, et al. Economic consequences of failure of initial antibiotic therapy in hospitalized adults with complicated intra-abdominal infections. Surg Infect 2008;9:335–47
- Dalfino L, Bruno F, Colizza S, et al. Cost of care and antibiotic prescribing attitudes for community-acquired complicated intra-abdominal infections in Italy: a retrospective study. W J Emerg Surg 2014;9:39
- Public Health England. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) 2010 to 2014. Report 2015. 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477962/ESPAUR_Report_2015.pdf 9 September 2016
- British Society for Antimicrobial Chemotherapy. BSAC Resistance Surveillance Project. 2015. http://www.bsacsurv.org/. [Last accessed 5 May 2017]
- Sartelli M, Catena F, Ansaloni L, et al. Complicated intra-abdominal infections worldwide: the definitive data of the CIAOW Study. W J Emerg Surg 2014;9:37
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial resistance surveillance in Europe 2011. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); Stockholm: ECDC 2012
- Eckmann C, Dryden M, Montravers P, et al. Antimicrobial treatment of “complicated” intra-abdominal infections and the new IDSA guidelines? A commentary and an alternative European approach according to clinical definitions. Eur J Med Res 2011;16:115–26
- Zerbaxa U.S. Prescribing Information. 2016. http://www.merck.com/product/usa/pi_circulars/z/zerbaxa/zerbaxa_pi.pdf. [Last accessed 8 December 2015]
- Zosyn® (Piperacillin and Tazobactam for Injection, USP) Prescribing Information. 2017. http://labeling.pfizer.com/showlabeling.aspx?id=416. [Last accessed 5 May 2017]
- Office for National Statistics. A1.1 2014-based expectation of life, 1981–2064, principal projection, United Kingdom; 2015. http://www.ons.gov.uk/ons/rel/lifetables/past-and-projected-data-from-the-period-and-cohort-life-tables/2014-based/rft-a1-1.xls 18 February 2016
- Sturkenboom MC, Goettsch WG, Picelli G, et al. Inappropriate initial treatment of secondary intra-abdominal infections leads to increased risk of clinical failure and costs. Br J Clin Pharmacol 2005;60:438–43
- Krobot K, Yin D, Zhang Q, et al. Effect of inappropriate initial empiric antibiotic therapy on outcome of patients with community-acquired intra-abdominal infections requiring surgery. Eur J Clin Microbiol Infect Dis 2004;23:682–7
- Department of Health. NHS reference costs 2013 to 2014. 2015. https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 18 February 2016
- Monthly Index of Medical Specialties. 2016. http://www.mims.co.uk/ 18 February 2016
- Jansen JP, Kumar R, Carmeli Y. Cost-effectiveness evaluation of ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections accounting for antibiotic resistance. Value Health J Int Soc Pharmacoecon Outcomes Res 2009;12:234–44
- Scottish Medicines Consortium. Guidance to Manufacturers for Completion of New Product Assessment Form (NPAF). October 2014. https://www.scottishmedicines.org.uk/files/submissionprocess/Guidance_on_NPAF_Final_October_2014.doc 18 February 2016
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. 2013. https://www.nice.org.uk/article/pmg9/chapter/foreword 18 February 2016
- Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–3. Value Health J Int Soc Pharmacoecon Outcomes Res 2012;15:812–20
- Marchaim D, Gottesman T, Schwartz O, et al. National multicenter study of predictors and outcomes of bacteremia upon hospital admission caused by Enterobacteriaceae producing extended-spectrum beta-lactamases. Antimicrob Agents Chemo 2010;54:5099–104
- Aloush V, Navon-Venezia S, Seigman-Igra Y, et al. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemo 2006;50:43–8
- National Institute for Health and Care Excellence. Judging whether pubilc health interventions offer value for money. Local government briefing [LGB10]. 2013. https://www.nice.org.uk/advice/lgb10/chapter/judging-the-cost-effectiveness-of-public-health-activities#nices-approach-to-assessing-public-health-interventions 5 May 2017
- Zilberberg MD, Mody SH, Chen J, et al. Cost-effectiveness model of empiric doripenem compared with imipenem-cilastatin in ventilator-associated pneumonia. Surg Infect 2010;11:409–17
- Kauf TL, Medic G, Dryden M, et al. Use of surveillance data to examine the cost-effectiveness of alternative approaches to empiric antibiotic therapy in gram-negative nosocomial pneumonia. Am J Respir Crit Care Med 2015;191:A5443
- Prabhu V, Sen S, Miller B, et al. Using an economic model to choose initial appropriate antibiotic therapy based on differences in in-vitro susceptibility to cftolozane/tazobactam and piperacillin/tazobactam. Value Health J Int Soc Pharmacoecon Outcomes Res 2015;18:A536
- Kauf TL, Prabhu VS, Medic G, et al. Cost-effectiveness of ceftolozane/tazobactam compared with piperacillin/tazobactam as empiric therapy based on the in-vitro surveillance of bacterial isolates in the United States for the treatment of complicated urinary tract infections. BMC Infect Dis 2017;17:314