Abstract
Aims: To develop a budget impact model (BIM) for estimating the financial impact of formulary adoption and uptake of calcipotriene and betamethasone dipropionate (C/BD) foam (0.005%/0.064%) on the costs of biologics for treating moderate-to-severe psoriasis vulgaris in a hypothetical US healthcare plan with 1 million members.
Methods: This BIM incorporated epidemiologic data, market uptake assumptions, and drug utilization costs, simulating the treatment mix for patients who are candidates for biologics before (Scenario #1) and after (Scenario #2) the introduction of C/BD foam. Predicted outcomes were expressed in terms of the annual cost of treatment (COT) and the COT per member per month (PMPM).
Results: At year 1, C/BD foam had the lowest per-patient cost ($9,913) necessary to achieve a Psoriasis Area and Severity Index (PASI)-75 response compared with etanercept ($73,773), adalimumab ($92,871), infliximab ($34,048), ustekinumab ($83,975), secukinumab ($113,858), apremilast ($47,960), and ixekizumab ($62,707). Following addition of C/BD foam to the formulary, the annual COT for moderate-to-severe psoriasis would decrease by $36,112,572 (17.91%, from $201,621,219 to $165,508,647). The COT PMPM is expected to decrease by $3.00 (17.86%, from $16.80 to $13.80).
Limitations: Drug costs were based on Medi-Span reference pricing (January 21, 2016); differences in treatment costs for drug administration, laboratory monitoring, or adverse events were not accounted for. Potentially confounding were the definition of “moderate-to-severe” and the heterogeneous efficacy data. The per-patient cost for PASI-75 response at year 1 was estimated from short-term efficacy data for C/BD foam and apremilast only.
Conclusions: The introduction of C/BD foam is expected to decrease the annual COT for moderate-to-severe psoriasis treatable with biologics by $36,112,572 for a hypothetical US healthcare plan with 1 million plan members, and to lower the COT PMPM by $3.00.
Introduction
As new therapeutic agents enter the marketplace, payers are interested in investigating the value of these treatment options when making formulary and reimbursement decisions. In the absence of head-to-head studies that can demonstrate the comparative cost-effectiveness of treatments, indirect analyses may be used to aid in treatment decision-making. Budget impact models (BIMs) can be particularly helpful in this regard.
Psoriasis vulgaris, a chronic inflammatory and incurable condition associated with a wide variety of comorbiditiesCitation1, requires ongoing treatment for most people affected, and presents a significant economic and social burden for both patients and societyCitation2. Plaque psoriasis, the most common form of the disease, accounts for at least 80% of the 7.5 million cases of psoriasis in the USCitation3,Citation4. In 2015, calcipotriene and betamethasone dipropionate foam (C/BD foam; Enstilar Foam [calcipotriene 0.005% plus betamethasone dipropionate 0.064%]) fixed combination received US Food and Drug Administration approval for the topical treatment of plaque psoriasis in adults aged 18 years and olderCitation5. C/BD foam has a well-tolerated safety profile, and demonstrates superior efficacy relative to other topical anti-psoriatic medicationsCitation5–8, having been studied in comparison with BD or calcipotriene monotherapy and Taclonex (calcipotriene plus BD) ointment. Lesion severity improves rapidly, and ∼50% of patients with up to 30% body surface area (BSA) involvement achieve controlled disease within 4 weeks with C/BD foamCitation6–10.
For the treatment of moderate-to-severe plaque psoriasis, oral systemic agents or biologics are indicated. Treatment guidelines published by the American Academy of Dermatology (AAD)Citation11,Citation12 and the National Psoriasis Foundation (NPF)Citation13 state that topicals and topical combination therapy should be used as first-line treatment in patients with limited diseaseCitation11,Citation13 and in conjunction with oral systemics and biologicals in patients with more extensive or moderate-to-severe psoriasisCitation11–13. BSA involvement in patients with moderate psoriasis ranges between 5–10%Citation11. C/BD foam, indicated for the treatment of adult patients with plaque psoriasisCitation5, was used to treat patients with BSAs of up to 30% in one of the clinical trialsCitation7 leading to its approval. It should be considered that adherence issues and safety concerns may be more common in those patients who need to treat a more extensive BSA with topicals, e.g. >20%Citation12. For example, systemic absorption leading to side-effects or toxicity have been observed, albeit rarely, with the use of topical salicylate for psoriasis BSAs of >20%Citation12.
With the addition of C/BD foam to the treatment options for psoriasis, C/BD foam uptake, formulary adoption, and reimbursements will be informed by cost comparisons with these other therapies. A BIM for C/BD foam may, thus, be of value for healthcare plan decision-makers. The objective of this study was to develop a BIM for C/BD foam that would estimate initial and long-term treatment costs associated with formulary adoption and uptake for patients with moderate-to-severe psoriasis vulgaris who might otherwise be treated with a biologic agent. Direct comparison to biologics was chosen because biosimilars are not available in the US, and conventional systemics have safety concerns associated with long-term use and are a step-through requirement for all biologics. This study was designed from the perspective of the decision-maker in terms of the accuracy and applicability of the results for use in formulary decisions or decisions regarding drug access via public payors (Medicaid/Medicare, Department of Defense, Indian Health Service, Veterans Administration) and private payors (commercial health insurance plans) for healthcare in the US.
Methods
Patient population
The model population consisted of patients with moderate-to-severe psoriasis in a US healthcare plan with 1 million plan lives ().
Table 1. Psoriasis population estimates in a hypothetical US healthcare plan.Table Footnotea
Model structure
The BIM was used to illustrate quantified cost variations associated with two scenarios of relative market shares of psoriasis therapies in the moderate-to-severe sub-population who are candidates for biologic treatment (). This is a hypothetical analysis. Scenario #1 was based on recent market data. Scenario #1 assessed the treatment mix before the introduction of C/BD foam, while Scenario #2 assessed it after the introduction of C/BD foam to 20% of patients in Scenario #1. This structure allowed for comparisons of user-adaptable scenarios defined by the real-world shares of comparator products for psoriasis in the US managed care clinical practice setting. The 20% number in Scenario #2 was a conservative assumption representing the estimated percentage of patients with moderate-to-severe psoriasis who would qualify for topical therapy. The hypothetical treatment mixes analyzed in Scenario #1 and Scenario #2 were intended to reflect the relative market shares before and after the introduction of C/BD foam. Several factors influenced the market share distribution in Scenario #2, which was meant to reflect the percent of patients after the introduction of C/BD. We assumed that use of etanercept, the most widely used biologic, would likely decrease disproportionately, and use of ixekizumab, as a new product, would increase over time. It is important to note that market shares in real-world settings may differ depending on actual market uptake for each treatment, and the model did not assess the impact on the cost of treating patients with milder disease. Outcomes predicted by the model were expressed in terms of the annual cost of treatment (COT), the costs per responder, and the COT per member per month (PMPM). Annual cost per responder is calculated as the cost for a patient who responded to the treatment during the trial period and continued on the same treatment for the remainder of the year using the recommend dosage from the prescribing information. A non-responder after the trial period was assumed to discontinue treatment. It is assumed that all patients would receive a similar treatment after failing their first treatment, and that this would not affect cost savings overall.
Table 2. Treatment mix scenarios.Table Footnotea
Model inputs
Efficacy
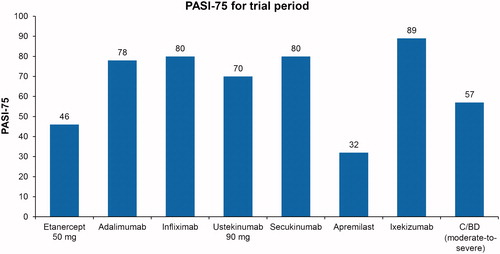
Treatment efficacy in the model was based on each product’s prescribing information, except for C/BD foam. Treatment efficacy data for C/BD foam were obtained from a published sub-analysis of a sub-set of patients with moderate-to-severe psoriasis who had used the product for up to 12 weeksCitation14, a timeframe comparable to that for other systemics and biologics. Response was measured by the percentage of patients who achieved a 75% reduction in their Psoriasis Area and Severity Index PASI score (PASI-75). Of these patients, 57.1% achieved PASI-75 at 12 weeks (). Because of the lack of long-term efficacy data for PASI-75 responders on apremilast, C/BD foam, and ixekizumab, it is assumed that short-term response rates were maintained for the full year for these drugs.
Treatment costs
The treatment cost inputs included the unit dosage based on each product’s prescribing information (). Default data on the unit cost (the wholesale acquisition cost) were provided for the users and are derived from Medi-Span reference pricing (as of January 21, 2016). Hypothetical price discounts were applied to determine the actual unit cost. The number-needed-to-treat (NNT) relative to C/BD foam was calculated using the formula
Table 3. Drug costs.Table Footnotea
The incremental cost per responder (ICPR) was calculated using the formula
One-way sensitivity analysis
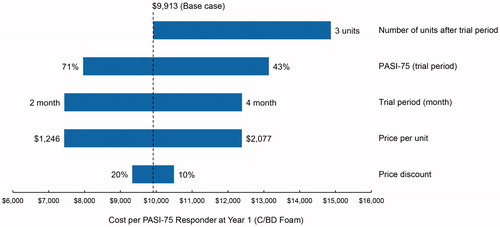
The sensitivity analysis was conducted for the parameters of the deterministic model, which included the unit price, the price discount, the trial period, the proportion of patients with PASI-75 (trial period), and the number of units after the trial period. We set the range of parameter variations at ±20% for PASI-75 proportion and unit price, ±33% for price discount, ±1 month for the trial period, and +2 units for the number of units after the trial period (see ). Regarding price discount, the 33% refers to 33% of the 20% discount set in . We examined the variation of outcome as the cost per PASI-75 responder at year 1, based on the above variation of key parameters.
Results
Using the efficacy data from the prescribing information, we determined the estimated drug costs based on the actual unit cost and the units needed for the trial period stated and the costs per PASI-75 responder for the trial period and after the trial period (). The total units of the drug needed and the associated cost per PASI-75 responder sum of the costs were calculated for year 1. C/BD foam had the lowest per-patient cost ($9,913) to achieve PASI-75 response, as compared with etanercept ($73,773), adalimumab ($92,871), infliximab ($34,048), ustekinumab ($83,975), secukinumab ($113,858), apremilast ($47,960), and ixekizumab ($62,707).
Table 4. Annual cost per PASI-75 responder (year 1).
A tornado diagram () summarizes the deterministic one-way sensitivity analyses. Bars of the diagram show the range of cost per PASI-75 responder at year 1. The cost per PASI-75 responder at year 1 was sensitive to changes in four parameters, specifically, the unit price, the trial period, the proportion of PASI-75 responders (trial period), and the number of units after the trial period.
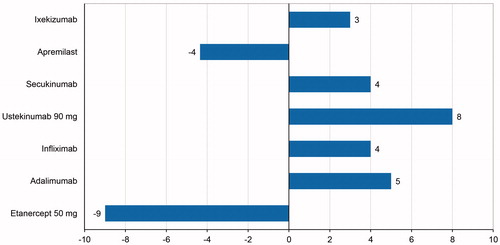
The NNT relative to C/BD foam represents the number of patients who would need to be treated with the comparator instead of C/BD foam to gain one additional PASI-75 responder. The estimated NNT ranged from –9 for etanercept to 8 for ustekinumab ().
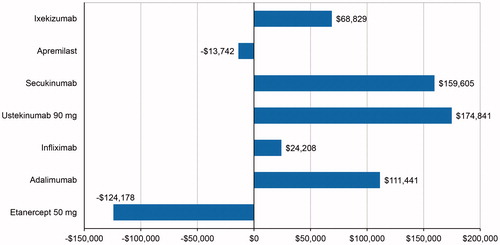
The ICPR relative to C/BD foam was estimated by the NNT, multiplied by the treatment cost difference between each biologic treatment, and C/BD foam to gain one additional PASI-75 responder (). The estimated ICPRs ranged from –$124,178 for etanercept to $174,841 for ustekinumab.
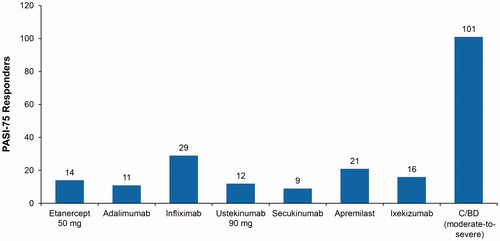
The annual budget needed to achieve 100 PASI-75 responders was derived from the cost per PASI-75 responder for year 1. The annual budget needed to achieve 100 PASI-75 responders ranged from $991,327 for C/BD foam to $11,385,766 for secukinumab. Additionally, based on the cost per PASI-75 responder for year 1, the number of responders for each drug was calculated given a hypothetical annual budget of $1,000,000. The number of PASI-75 responders with an annual budget of $1,000,000 ranged from nine PASI-75 responders for secukinumab to 101 PASI-75 responders for C/BD foam ().
The annual treatment cost per 1,000,000 healthcare plan lives, derived from the cost per PASI-75 responder for year 1, decreased by $36,112,572 (17.91%, from $201,621,219 without C/BD foam to $165,508,647 after its introduction) (). The COT PMPM was expected to decrease by $3.00 (17.9%, from $16.80 to $13.80).
Table 5. Estimated annual treatment cost for moderate-to-severe psoriasis.
Discussion
Use of the BIM developed in the current study indicated that addition of an effective topical agent such as C/BD foam for the treatment of moderate-to-severe psoriasis would result in a reduction in costs for systemic and biologic therapies. C/BD foam could, therefore, be considered as part of a step-wise approach to moderate-to-severe psoriasis management in which effective topical agents are used prior to more expensive therapeutics. Incorporating home phototherapy into such a stepwise approach has also been suggested as a potential means to help limit exposure to and cost of biologic treatments. It should be noted that this study is a BIM, and cost-effectiveness is not reported in the current study.
Models such as the one developed and used in the current study are particularly needed for policy-makers involved in formulary and reimbursement decisions pertaining to the treatment of patients with psoriasis. This BIM was developed using on-label data within known treatment periods and excluding additional costs. The lack of head-to-head studies demonstrating the comparative cost-effectiveness of treatments for psoriasis has necessitated a reliance on data from indirect analyses to aid in treatment decision-making; however, these indirect analyses have been limited mainly to systemic treatments. For example, one study used the cost-per-responder model to calculate the expected cost per responder of apremilast vs etanercept, adalimumab, and ustekinumab in patients with moderate-to-severe psoriasis by considering PASI-75 response rates. Compared with the other systemic agents, apremilast was associated with the lowest wholesale acquisition cost per PASI-75 responder at week 16 ($18,938) and year 1 ($34,472), as well as with the lowest cost to achieve 100 PASI-75 responders at week 16 ($1,893,761)Citation15.
Another study used a network meta-analysis to estimate the NNT for PASI-75 for all biologic treatments (adalimumab, etanercept, infliximab, ustekinumab, and secukinumab) relative to apremilast and to evaluate the incremental drug cost per PASI-75 responder for all biologic treatments relative to apremilast. Results indicated that the lowest incremental costs per PASI-75 responder relative to apremilast over the trial period were seen with adalimumab ($19,548) and infliximab ($12,361) for weeks 10–16 (depending on the study), and for year 1 ($44,651 and $21,617, respectively)Citation16.
Decision-makers should be aware of the limitations of the current analysis. Drug costs were based on wholesale acquisition cost prices exported from the Medi-Span reference pricing database on January 21, 2016. Any differences in treatment costs for drug administration, laboratory monitoring, or adverse events among treatments are not accounted for in the BIM. Biologic therapies are associated with additional costs related to administration and monitoring, as well as adverse events, as compared with traditional therapies. In addition, by simulating market share based on previous real world data, it should be noted that different input of hypothetical market share data will influence the results. For example, if all patients are put on the least expensive biologics (Infliximab), the total cost for 1 year is $108,952,533 in Scenario 2. If they are put on the most expensive biologics (Secukinumab), the total cost is $364,344,521, although current real world data could help alleviate this concern. Furthermore, because there were no head-to-head efficacy data available, we used data derived from the two pivotal Phase 3 clinical trials, as described in the prescribing information. Since the efficacy data used in the analysis were derived from different clinical trials performed in different patient populations, the results may be confounded. Additional clinical trials providing direct comparison would be required to draw a clearer picture. It is also important to note that many of the input data used in the model, which were based on clinical trial results, may not reflect real-world treatment patterns and effectiveness. Only short-term efficacy data for apremilast and C/BD foam were used in the analysis to estimate the per-patient cost to achieve PASI-75 response for year 1; therefore, uncertainty remains regarding the long-term efficacy of comparator treatments. In the case of apremilast and C/BD foam, 1-year costs were based on the same PASI-75 efficacy values in each treatment cycle as in the initial trial period. The model did not examine impact of formulary adoption of C/BD foam on costs for patients with milder disease.
Conclusions
The BIM estimates that 4,000 individuals in a hypothetical US healthcare plan with 1 million members would receive either a biologic therapy or C/BD foam for the treatment of moderate-to-severe psoriasis. Adding C/BD foam to the healthcare plan formulary results in an expected savings of $36,112,572 in the annual COT and a decrease in the COT PMPM of $3.00 for this population. Use of this BIM suggests the potential benefit of a step-wise approach to moderate-to-severe psoriasis treatment, involving the use of topical agents as part of a comprehensive approach to limit exposure to risky and/or expensive systemic or biologic treatments for the sub-population with moderate-to-severe disease.
Transparency
Declaration of funding
This study was sponsored and funded by LEO Pharma, Inc.
Declaration of financial/other interests
CVA is an advisor for Novo Nordisk and Pacira, an investigator for Pacira, and a consultant for LEO Pharma and Takeda. MK has nothing to disclose. SRF is a consultant for Abbvie, Advance Medical, Caremark, Celgene, Galderma, Gerson Lehrman Group, Guidepoint Global, Janssen, Kikaku, LEO Pharma Inc., Lilly, Merck & Co. Inc., Mylan, Novartis Pharmaceuticals Corporation, Pfizer Inc., Qurient, Sanofi, Suncare Research, and Xenoport; a speaker for Abbvie, Celgene, Janssen, LEO Pharma Inc., Lilly, Novartis Pharmaceuticals Corporation, and Taro; a founder, stockholder, and chief technology officer for Causa Technologies; and a majority owner of stock in Medical Quality Enhancement Corporation. SRF also receives grant support from Abbvie, Celgene, Galderma, Janssen, Novartis Pharmaceuticals Corporation, Pfizer Inc., Qurient, Sanofi, and Taro and royalties from Informa, UpToDate, and Xlibris. PZ was an employee of LEO Pharma Inc. at the time of this study. ML is an employee of LEO Pharma Inc. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
Poster was presented at the Academy of Managed Care Pharmacy (AMCP) Nexus 2016; October 3–6, 2016; National Harbor, MD.
Acknowledgments
Writing assistance was provided by p-value communications and funded by LEO Pharma Inc.
References
- Machado-Pinto J, Diniz MS, Bavoso NC. Psoriasis: new comorbidities. An Bras Dermatol 2016;91:8-16
- Evans C. Managed care aspects of psoriasis and psoriatic arthritis. Am J Manag Care 2016;22:S238-S43
- Hazard EH, Cherry SB, Lalla D, et al. The clinical and economic burden of psoriasis. Manag Care Interface 2006;19:20-6
- National Psoriasis Foundation. Psoriasis Fact Sheet. https://www.psoriasis.org/publications/patient-education/fact-sheets.
- Accessed November 2, 2016
- Enstilar Foam [package insert]. Parsippany, NJ: LEO Pharma Inc.; 2015
- Leonardi C, Bagel J, Yamauchi P, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris—a randomized phase III study (PSO-FAST). J Drugs Dermatol 2015;14:1468-77
- Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone and dipropionate aerosol foam vs ointment in patients with psoriasis vulgaris: a randomized phase II study. J Dermatol Treat 2016;27:120-7
- Lebwohl M, Tyring S, Bukhalo M, et al. Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase 2 study. J Clin Aesthet Dermatol 2016;9:34-41
- Queille-Roussel C, Olesen M, Villumsen J, et al. Efficacy of an innovative aerosol foam formulation of fixed combination calcipotriol plus betamethasone dipropionate in patients with psoriasis vulgaris. Clin Drug Investig 2015;35:239-45
- Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol 2017;31:119-26
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol 2011;65:137-74
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009;60:643-59
- Van Voorhees AS, Feldman SR, Koo JYM, et al. The Psoriasis and Psoriatic Arthritis Pocket Guide: Treatment Algorithms and Management Options. 4th ed. Portland, OR: National Psoriasis Foundation; 2016. p 1-134
- Paul C, Leonardi C, Menter A, et al. Calcipotriol plus betamethasone dipropionate aerosol foam is effective in patients with moderate-to-severe psoriasis: post-hoc analysis of the PSO-ABLE study. Poster presented at the 25th EADV Congress, September 28–October 2, 2016; Vienna, Austria.
- Feldman SR, Tencer T, Clancy Z, et al. Cost per responder of apremilast vs etanercept, adalimumab, and ustekinumab in patients with moderate to severe psoriasis. Presented at the 73rd Annual Meeting of the American Academy of Dermatology, March 20–24, 2015; San Francisco, CA
- Armstrong AW, Betts KA, Li J, et al. Numbers needed to treat and costs per responder for novel treatments of moderate to severe psoriasis. Poster presented at the 74th Annual Meeting of the American Academy of Dermatology, March 4–8, 2016, Washington, DC
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008;58:826-50