Abstract
Aims: This study compared the risk for major bleeding (MB) and healthcare economic outcomes of patients with non-valvular atrial fibrillation (NVAF) after initiating treatment with apixaban vs rivaroxaban, dabigatran, or warfarin.
Methods: NVAF patients who initiated apixaban, rivaroxaban, dabigatran, or warfarin were identified from the IMS Pharmetrics Plus database (January 1, 2013–September 30, 2015). Propensity score matching (PSM) was used to balance differences in patient characteristics between study cohorts: patients treated with apixaban vs rivaroxaban, apixaban vs dabigatran, and apixaban vs warfarin. Risk of hospitalization and healthcare costs (all-cause and MB-related) were compared between matched cohorts during the follow-up.
Results: During the follow-up, risks for all-cause (hazard ratio [HR] = 1.44, 95% confidence interval [CI] = 1.2–1.7) and MB-related (HR = 1.57, 95% CI = 1.0–2.4) hospitalizations were significantly greater for patients treated with rivaroxaban vs apixaban. Adjusted total all-cause healthcare costs were significantly lower for patients treated with apixaban vs rivaroxaban ($3,950 vs $4,333 per patient per month [PPPM], p = .002) and MB-related medical costs were not statistically significantly different ($100 vs $233 PPPM, p = .096). Risk for all-cause hospitalization (HR = 1.98, 95% CI = 1.6–2.4) was significantly greater for patients treated with dabigatran vs apixaban, although total all-cause healthcare costs were not statistically different. Risks for all-cause (HR = 2.22, 95% CI = 1.9–2.5) and MB-related (HR = 2.05, 95% CI = 1.4–3.0) hospitalizations were significantly greater for patients treated with warfarin vs apixaban. Total all-cause healthcare costs ($3,919 vs $4,177 PPPM, p = .025) and MB-related medical costs ($96 vs $212 PPPM, p = .026) were significantly lower for patients treated with apixaban vs warfarin.
Limitations: This retrospective database analysis does not establish causation.
Conclusions: In the real-world setting, compared with rivaroxaban and warfarin, apixaban is associated with reduced risk of hospitalization and lower healthcare costs. Compared with dabigatran, apixaban is associated with lower risk of hospitalizations.
Introduction
Atrial fibrillation (AF) is a common medical condition characterized by sustained cardiac arrhythmia, and is most often non-valvular (NVAF) in natureCitation1. Persons with NVAF have significantly higher risk for stroke, and ∼20% of all strokes in the US are attributed to AFCitation2,Citation3. In 2010, it was estimated that AF affected over 5 million persons in the USCitation4. Based on trends in the incidence rate of AF and the growing number of elderly persons among the US population, the projected estimate of AF affected persons is to be 12.1 million by 2030Citation4.
Warfarin has been the predominant anti-coagulation therapy used for stroke risk reduction among NVAF patients for decadesCitation5. However, warfarin therapy has several disadvantages, including a limited therapeutic index, potential for drug–drug and drug–food interactions, and high bleeding riskCitation5,Citation6. These limitations, among others, have led to an under-prescribing and sub-optimal management of warfarin therapy among many patients with NVAF, especially those who are elderlyCitation6. New oral anticoagulants (NOACs), including dabigatran, rivaroxaban, and apixaban, have become available in the US since 2010 for stroke prevention among NVAF patients. The NOACs do not have many of the limitations of warfarin therapy, as they do not require regular monitoring and have few drug and food interactionsCitation7. In large phase III clinical trials that compared the efficacy and safety of apixaban, rivaroxaban, or dabigatran with warfarin, all three NOACs demonstrated equivalent or superior reduction in stroke risk and also similar or lower rates of bleedingCitation8–10.
Since many patient characteristics and clinical practice may differ in the real-world setting as compared with the clinical trial setting, it is important to evaluate the safety and effectiveness of NOACs in the routine clinical practice setting. A few recent studies have compared the safety of apixaban, rivaroxaban, and dabigatran in the real-world settings, but further confirmation of their findings is warrantedCitation11–14. Furthermore, no direct comparison of the different NOACs on healthcare resource use and costs has been conducted. Therefore, in this study we compared the risk for major bleeding (MB) and healthcare economic outcomes of NVAF patients after initiating treatment with apixaban vs rivaroxaban, dabigatran, or warfarin in a routine clinical practice setting.
Methods
Study design
The study was a retrospective cohort analysis using the IMS Pharmetrics Plus database.
Study population
AF patients (≥18 years of age) who initiated an oral anticoagulant (OAC): apixaban, rivaroxaban, dabigatran, or warfarin, between January 1, 2013 and September 30, 2015 were identified from the IMS Pharmetrics Plus database. The date of the study start was just after the FDA approval date of apixaban for stroke prevention in AF patients (December 28, 2012). The end of the study period represents the most recent data availability from the data source at the time this study was conducted. The IMS PharMetrics Plus database is an integrated source of managed care medical and pharmacy claims that includes enrollees of all age groups and encompasses more than 150 million US patients primarily with commercial health plans. Three component files of this database were used: medical, pharmacy, and eligibility. The medical file contains data on diagnostic and therapeutic services provided in both inpatient and outpatient settings. The diagnoses and procedures for these services are coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, Current Procedural Terminology (CPT), and Healthcare Common Procedure Coding System (HCPCS) codes. The pharmacy file contains data on prescription drugs dispensed (retail and mail-order) with accompanying information on the characteristics of the drug dispensed, such as the National Drug Code (NDC), quantity, and days of supply. For both medical and pharmacy files, the dates of service are recorded, as well as the amounts allowed (for maximum payment) for the service/drug. The eligibility file also contains data on demographic characteristics and periods of eligibility for each patient.
The date of the first OAC pharmacy claim to occur (index event) between January 1, 2013 and September 30, 2015 was defined as the index date. Patients were required to have 12 months of continuous insurance coverage prior to the index date (baseline period) and to have at least one inpatient or outpatient AF diagnosis (ICD-9-CM diagnosis code 427.31) before or on the index date. To ensure patients were OAC treatment naïve, patients were excluded if they had a pharmacy claim for any of the OACs during the baseline period or had >1 OAC pharmacy claim on the index date. Patients were also excluded if they had evidence of rheumatic mitral valvular heart disease, mitral valve stenosis, heart valve replacement/transplant, dialysis, kidney transplant, end-stage chronic kidney disease, diagnosis of venous thromboembolism 12 months prior to or on the index date, hip or knee replacement surgery within 6 weeks prior to the index date, reversible AF, and pregnancy during the study period, as identified by ICD-9-CM codes.
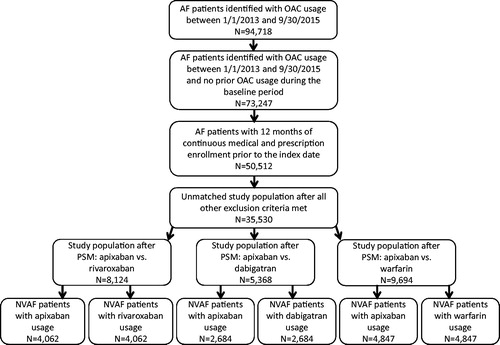
Eligible patients were grouped to the apixaban, rivaroxaban, dabigatran, or warfarin cohorts. shows the process of selection of study cohorts. Patients were matched as described below. Patients were followed for a variable period until the earliest of the following dates: treatment discontinuation date, the date that the patient switched from the index OAC to another OAC, health plan disenrollment date, patient death, or the end of the study period (September 30, 2015). OAC discontinuation was defined as the first day of a gap of at least 30 days (grace period) in which no drug days of supply for the index OAC was detected. A switch among OACs was defined as a prescription filled for non-index anticoagulant. Time to switch was defined as the last days of supply for the index OAC prescription or the switch date to a new non-index anticoagulant, whichever was earlier.
Measurement of MB outcomes
During the follow-up, MB events, including gastrointestinal MB, intracranial hemorrhage, and other MB, were evaluated among study cohorts based on having the first listed ICD-9-CM diagnosis or procedure code on hospital claims.
Measurement of healthcare resource use and costs
Healthcare resource use and costs during the baseline and follow-up periods were evaluated for propensity score matched study cohorts. Healthcare resources evaluated included number of hospitalizations, total hospital length of stay, number of outpatient medical service claims (including outpatient hospital visits, ambulatory services, lab, imaging, physician office visits, and other outpatient medical services), and number of outpatient pharmacy claims for all causes. Use of MB-related medical services was also evaluated and identified by ICD-9-CM codes for a primary or secondary diagnosis on either inpatient or outpatient healthcare encounters. Healthcare costs were measured for all evaluated healthcare resource use categories as described above. Total healthcare costs included those for inpatient admissions, outpatient medical services, and outpatient pharmacy claims. Total medical costs included those for inpatient and outpatient medical service claims. All costs were inflation adjusted to 2015 US dollars using the Consumer Price Index medical care component and reported as per patient per month (PPPM) costsCitation15.
Statistical analyses
Propensity score matching (PSM) was used to balance baseline patient characteristics. A multivariable logistic regression model was used to compute propensity scores. Independent variables included in the logistic regression model were age, gender, US geographic region, Charlson Comorbidity Index (CCI) score, CHA2DS2-VASc score, HAS-BLED score, duration of follow-up, baseline bleeding status, baseline stroke status, baseline all-cause medical service costs, baseline stroke-related medical service costs, baseline bleeding-related medical service costs, baseline all-cause total healthcare costs, baseline comorbidities (thrombocytopenia, congestive heart failure, diabetes, hypertension, renal disease, myocardial infarction, dyspepsia/stomach discomfort, peripheral vascular disease, transient ischemic attack, coronary artery disease), and baseline co-medication usage status (ACE inhibitor, amiodarone, angiotensin receptor blocker, beta blocker, H2-receptor antagonist, proton pump inhibitor, statin, anti-platelet). Based on propensity score, dabigatran, rivaroxaban, and warfarin treated patients were 1:1 matched to apixaban treated patients, separately. Thus, three matched cohorts were generated: apixaban vs rivaroxaban, apixaban vs dabigatran, and apixaban vs warfarin. Matching was conducted by using the nearest neighbor algorithm. Matched patient cohorts were inspected to ensure cohorts were well balanced, with key patient characteristics statistically similar (p > .05).
Bivariate comparisons of baseline characteristics and unadjusted healthcare resource use and costs were conducted using t-tests or Chi-square tests, as appropriate. The annual rates for MB events were evaluated based on Kaplan-Meier (KM) analyses, and p-values calculated from overall log-rank tests. After PSM, Cox proportional hazards regression models with only the OAC drugs as independent variables were used to assess the association between OACs (each OAC vs apixaban) on likelihood of first all-cause hospitalization and first hospitalization due to MB-related conditions during the follow-up. Sensitivity analyses were also conducted, in which baseline all-cause hospitalizations or NOAC dosage levels (low dose vs standard dose) were included as independent variables in the Cox regression models.
After the PSM, generalized linear models (GLMs) with log transformation and gamma distribution and with only the OAC drugs as independent variables were used to evaluate the impact of the different OAC treatments relative to apixaban treatment on the costs of total all-cause healthcare (inpatient, outpatient medical services, and outpatient pharmacy services), all-cause medical healthcare (inpatient and outpatient medical services), inpatient medical services, outpatient medical services, and outpatient pharmacy services. Least-squares means (LSMs) were estimated by analysis of covariance in the GLMs. Two-part regression analyses were used to evaluate the impact of the different OAC treatments relative to apixaban on MB-related healthcare costs and all-cause inpatient healthcare costs. In the two-part regression analysis for the MB-related costs, the first part was multivariable Cox regression with only the OAC drugs as independent variables, which was used to evaluate the impact of OAC treatment on the risk for MB. The second part involved the use of a multivariable GLM with log transformation and gamma distribution and with only the OAC drugs as independent variables, which was applied to the MB-related cost data to evaluate the incremental cost of MB events among patients with such events. The hazard ratio (HR) estimated from the first part was combined with the incremental cost of MB events estimated from the second part to estimate the incremental cost of MB events among all NVAF patients. The two-part calculations were further carried out in 1,000 cycles of random bootstrapping re-sampling to generate 1,000 such estimates. Univariate statistics of the 1,000 incremental MB-related costs among all patients were used to evaluate the MB costs. The 2.5- and 97.5-percentiles of the incremental MB-related costs estimated from the 1,000 cycles of bootstrapping was used to represent the lower and upper 95% confidence levels (CI), respectively. Such a corresponding two-part regression approach was also used to evaluate the impact of the different OAC treatments relative to apixaban treatment on all-cause inpatient healthcare costs. A p-value of <.05 was used to establish statistical significance. All data analyses were executed using statistical software SAS version 9.4.
Results
Study population
A total of 94,718 AF patients with OAC usage were identified between January 1, 2013 and September 30, 2015 from the IMS Pharmetrics Plus database (). After inclusion criteria were met and exclusion criteria applied, the unmatched NVAF study population with OAC usage was comprised of 35,530 patients ().
Patient demographics and clinical characteristics
shows the baseline demographics and clinical characteristics of study cohorts treated with apixaban vs rivaroxaban, apixaban vs dabigatran, and apixaban vs warfarin after PSM.
Table 1. Baseline demographics and clinical characteristics of study cohorts treated with oral anticoagulants after propensity score matching.
Apixaban vs rivaroxaban
After implementing PSM, 8,124 patients were matched with 4,062 patients in each study cohort. Among matched cohorts treated with apixaban vs rivaroxaban, mean ages (62.0 vs 62.0 years, p = .840), CCI scores (1.2 vs 1.2, p = .287), and stroke and bleeding risks, based on mean CHADS2 scores (1.3 vs 1.3, p = .640), CHA2DS2-VASc scores (2.1 vs 2.1, p = .908), and HAS-BLED scores (2.2 vs 2.2, p = .598), were similar (no statistically significant differences). The proportions of patients with prior bleeding diagnoses during the baseline period were also similar among NVAF patients treated with apixaban vs rivaroxaban (10.4% vs 10.2%, p = .827), as were the proportions with the evaluated comorbidities. Total all-cause healthcare costs ($1,425 vs $1,444 PPPM, p = .705) and costs for MB-related medical services ($94 vs $77 PPPM, p = .198) during the baseline period were not significantly different for matched NVAF patients treated with apixaban vs rivaroxaban. The mean duration of follow-up was 4.5 months for both cohorts (p = .506).
Apixaban vs dabigatran
After implementing PSM, 5,368 patients were matched with 2,684 patients in each study cohort. Among matched cohorts treated with apixaban vs dabigatran, mean ages (63.0 vs 63.0 years, p = .985), CCI scores (1.2 vs 1.2, p = .319), and stroke and bleeding risks, based on mean CHADS2 scores (1.4 vs 1.4, p = .758), CHA2DS2-VASc scores (2.1 vs 2.1, p = .964), and HAS-BLED scores (2.1 vs 2.1, p = .785), were similar (no statistically significant differences). The proportions of patients with prior bleeding diagnoses during the baseline period were also similar among NVAF patients treated with apixaban vs dabigatran (8.4% vs 8.5%, p = .883), as were the proportions with the evaluated comorbidities. Total all-cause healthcare costs ($1,123 vs $1,052 PPPM, p = .116) and costs for MB-related medical services ($41 vs $51 PPPM, p = .399) during the baseline period were not significantly different for matched NVAF patients treated with apixaban vs dabigatran. The mean duration of follow-up was 5.2 months among patients treated with apixaban, and 5.0 months among patients treated with dabigatran (p = .183).
Apixaban vs warfarin
After implementing PSM, 9,694 patients were matched with 4,847 patients in each study cohort. Among matched cohorts treated with apixaban vs warfarin, mean ages (63.9 vs 64.0 years, p = .788), CCI scores (1.4 vs 1.4, p = .301), and stroke and bleeding risks, based on CHADS2 score (1.5 vs 1.5, p = .128), CHA2DS2-VASc score (2.3 vs 2.3, p = .105), and HAS-BLED score (2.2 vs 2.2, p = .611), were similar (no statistically significant differences). The proportions of patients with prior bleeding diagnoses during the baseline period were also similar among NVAF patients treated with apixaban vs warfarin (10.9% vs 10.6%, p = .718), as were the proportions with the evaluated comorbidities. Total all-cause healthcare costs ($1,511 vs $1,474 PPPM, p = .566) and costs for MB-related medical services ($120 vs $127 PPPM, p = .685) during the baseline period were not significantly different for matched NVAF patients treated with apixaban vs warfarin. The mean duration of follow-up was 4.9 months among patients treated with apixaban, and 4.8 months among patients treated with warfarin (p = .310).
Unadjusted MB outcomes of matched cohorts
After PSM, but prior to additional regression analyses, MB outcomes were the following.
Apixaban vs rivaroxaban
The annual rate of MB estimated from KM analysis, which took into account the censoring nature of the variable follow-up period, was significantly lower for patients treated with apixaban, in comparison to that of patients treated with rivaroxaban (2.7% vs 3.5%, p = .033).
Apixaban vs dabigatran
The annual rate of MB estimated from KM analysis was numerically lower for patients treated with apixaban in comparison to that of patients treated with dabigatran, but the difference did not reach statistical significance (1.9% vs 2.3%, p = .303).
Apixaban vs warfarin
The annual rate of MB estimated from KM analysis was significantly lower for patients treated with apixaban in comparison to that of patients treated with warfarin (2.2% vs 3.7%, p < .001).
Unadjusted healthcare resource use and associated costs of matched cohorts
shows the unadjusted healthcare resource use and associated costs PPPM during the follow-up of study cohorts treated with apixaban vs rivaroxaban, apixaban vs dabigatran, and apixaban vs warfarin.
Table 2. Comparison of unadjusted healthcare resource use and associated costs per patient per month during follow-up.
Apixaban vs rivaroxaban
During the follow-up, the mean number of all-cause hospitalizations (0.04 vs 0.06 PPPM, p = .009) and inpatient costs ($1,112 vs $1,502 PPPM, p = .042) were significantly lower for patients treated with apixaban vs rivaroxaban. Apixaban vs rivaroxaban treatment was also associated with a significantly lower mean number of outpatient medical service claims for all causes (2.4 vs 2.6 PPPM, p = .003), but total mean costs for all-cause outpatient medical services were not statistically significant. Mean costs for pharmacy services ($532 vs $511 PPPM, p = .244), total medical costs ($3,294 vs $3,691 PPPM, p = .090), and total healthcare costs ($3,826 vs $4,202 PPPM, p = .110) for all causes were not statistically different among patients treated with apixaban vs rivaroxaban. However, MB-related medical costs were significantly lower for patients treated with apixaban vs rivaroxaban ($285 vs $584 PPPM, p = .049).
Apixaban vs dabigatran
During the follow-up, the mean number of all-cause hospitalizations (0.04 vs 0.05 PPPM, p = .107) and inpatient costs ($1,047 vs $1,230 PPPM, p = .340) were not statistically significantly different among study cohorts. Mean costs for pharmacy services ($523 vs $527 PPPM, p = .848), total medical costs ($2,951 vs $3,078 PPPM, p = .591), and total healthcare costs ($3,474 vs $3,606, p = .581) for all causes PPPM were also not statistically significantly different among patients treated with apixaban vs dabigatran; neither were costs for MB-related medical services ($227 vs $392 PPPM, p = .228).
Apixaban s warfarin
During the follow-up, the mean number of all-cause hospitalizations (0.04 vs 0.06 PPPM, p < .001), hospital length of stay (0.2 vs 0.5 days PPPM, p < .001), and inpatient costs ($1,180 vs $1,724 PPPM, p = .009) were significantly lower for patients treated with apixaban vs warfarin. Apixaban vs warfarin treatment was also associated with a lower mean number of outpatient claims for all causes (2.5 vs 3.8 PPPM, p < .001), but total mean costs for all-cause outpatient medical services were similar. Mean costs for pharmacy services for all causes were significantly higher for patients treated with apixaban, in comparison to that of patients treated with warfarin ($519 vs $383 PPPM, p < .001), but mean total medical costs ($3,281 vs $3,738 PPPM, p = .060) and total healthcare costs ($3,800 vs $4,121 PPPM, p = .188) for all causes were not statistically significantly different.
Adjusted all-cause and MB hospitalization risk and healthcare economic outcomes of matched cohorts
Apixaban vs rivaroxaban
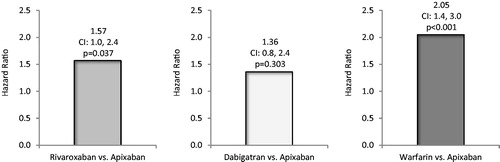
After Cox regression analysis, the risk of first all-cause hospitalization during the follow-up was significantly greater for matched patients treated with rivaroxaban vs apixaban (HR = 1.44, 95% CI = 1.2–1.7, p < .001, ), as also was the risk of MB-related hospitalization (HR = 1.57, 95% CI = 1.0–2.4, p = .037, ).
Figure 2. Likelihood of all-cause hospitalization during the follow-up among non-valvular atrial fibrillation patients treated with rivaroxaban, dabigatran, and warfarin vs apixaban.
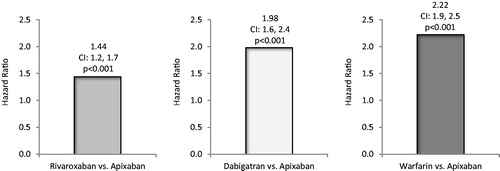
Figure 3. Likelihood of major bleeding-related hospitalization during follow-up among non-valvular atrial fibrillation patients treated with rivaroxaban, dabigatran, and warfarin vs apixaban.
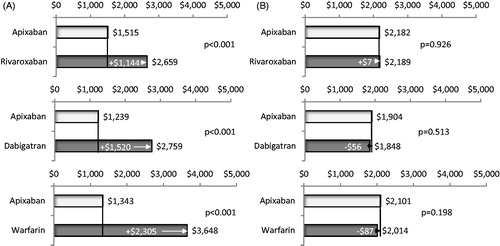
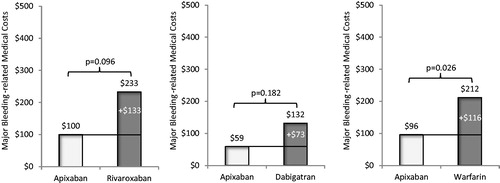
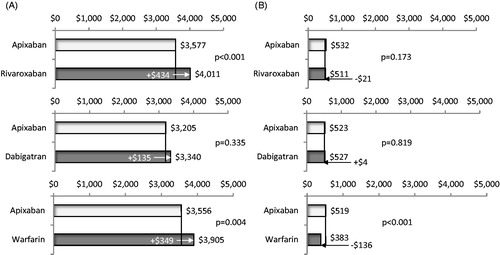
Adjusted mean total all-cause healthcare costs for patients treated with apixaban in comparison to that of patients treated with rivaroxaban were significantly lower ($3,950 vs $4,333 PPPM, p = .002; ), as were mean total all-cause medical costs ($3,577 vs $4,011 PPPM, p < .001, ). Mean monthly pharmacy costs were similar for patients treated with apixaban vs rivaroxaban (). Inpatient costs were significantly lower for patients treated with apixaban vs rivaroxaban ($1,515 vs $2,659 PPPM, p < .001), but outpatient medical service costs were similar ($2,182 vs $2,189 PPPM, p = .926) (). Mean total MB-related medical costs trended towards being lower for patients treated with apixaban vs rivaroxaban, but were not statistically significantly different among study cohorts. ($100 vs $233 PPPM, p = .096, ).
Figure 4. Comparison of adjusted total all-cause healthcare costs per patient per month during follow-up for cohorts treated with apixaban vs other oral anticoagulants. Total all-cause healthcare costs included all costs associated with any medical and pharmacy services.
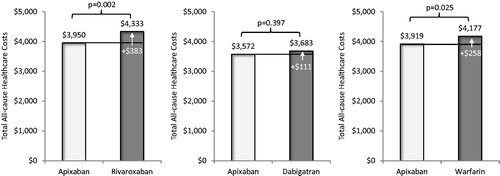
Figure 5. Comparison of adjusted total all-cause medical costs (a) and pharmacy costs (b) per patient per month during follow-up for cohorts treated with apixaban vs other oral anticoagulants. Medical costs included costs of inpatient and outpatient medical services.
Apixaban vs dabigatran
After Cox regression analysis, the risk of first all-cause hospitalization during the follow-up was significantly greater for matched patients treated with dabigatran vs apixaban (HR = 1.98, 95% CI = 1.6–2.4, p < .001, ). The risk of MB-related hospitalization was not statistically significantly different for patients treated with dabigatran vs apixaban (HR = 1.36, 95% CI = 0.8–2.4, p = .303, ).
Adjusted mean total all-cause healthcare costs for patients treated with apixaban in comparison to that of patients treated with dabigatran were not statistically different ($3,572 vs $3,683 PPPM, p = .397; ), and neither were mean total all-cause medical costs or pharmacy costs (). Inpatient costs were significantly lower for patients treated with apixaban vs dabigatran ($1,239 vs $2,759 PPPM, p < .001), but outpatient medical service costs were similar ($1,904 vs $1,848 PPPM, p = .513) (). Mean total MB-related medical costs trended towards being lower for patients treated with apixaban vs dabigatran ($59 vs $132 PPPM, p = .182, ); however, the difference did not reach statistical significance.
Apixaban vs warfarin
After Cox regression analysis, the risk of first all-cause hospitalization during the follow-up was significantly greater for matched patients treated with warfarin vs apixaban (HR = 2.22, 95% CI = 1.9–2.5, p < .001, ), as also was the risk of MB-related hospitalization (HR = 2.05, 95% CI = 1.4–3.0, p < .001, ).
Adjusted mean total all-cause healthcare costs for patients treated with apixaban in comparison to that of patients treated with warfarin were significantly lower ($3,919 vs $4,177 PPPM, p = .025; ), as were mean total all-cause medical costs ($3,556 vs $3,905 PPPM, p = .004, ). Mean monthly pharmacy costs were higher for patients treated with apixaban vs warfarin ($519 vs $383 PPPM, p < .001, ). Inpatient costs were significantly lower for patients treated with apixaban vs warfarin ($1,343 vs $3,648 PPPM, p < .001), but outpatient medical service costs were similar ($2,101 vs $2,014 PPPM, p = .198) (). Mean total MB-related medical costs were significantly lower for patients treated with apixaban vs warfarin ($96 vs $212 PPPM, p = .026, ).
For all healthcare cost comparisons between the different OACs and apixaban, the regressions results for the total medical costs, inpatient costs, and outpatient medical costs were obtained from different regression analyses and, therefore, the inpatient and outpatient medical cost difference regression results may not add up to the cost difference of the total medical costs.
Sensitivity analyses
In the sensitivity analyses of the comparisons of risk for MB associated with treatment with each OAC vs apixaban, in which baseline all-cause hospitalizations or NOAC dosage levels (low dose vs standard dose) were included as independent variables in the Cox regression models, the results were consistent with the default analyses.
Discussion
After balancing key significant differences in patient characteristics with PSM, the results of this claims database analysis showed that apixaban treatment was associated with lower risks for hospitalization for all causes and MB, as well as lower total all-cause healthcare costs vs rivaroxaban and warfarin treatment. The majority of the cost savings associated with apixaban vs rivaroxaban was attributed to lower hospitalization-related cost. Additionally, the findings of this study were that, although healthcare costs were not significantly different, NVAF patients were 2-times less likely to be hospitalized for any cause after initiating apixaban vs dabigatran treatment. The results of this study were directionally consistent among matched cohorts prior to and after further adjustment with regression analyses. As an observational study, unobserved confounders may exist for which the analysis did not control, such as drug adherence, time in therapeutic range for warfarin, and other patient characteristics not captured in the data source.
With regards to MB risk, the results of this study are also directionally consistent with the findings of other recent retrospective cohort studies, in which the safety of NOACs was comparedCitation12–14. However, due to some underlying differences in patient populations and variations in methodology, the degree of differences in MB outcomes between NVAF patients treated with the different NOACs varies to some extent across studiesCitation12–14. Lip et al.Citation12 also used PSM to balance differences among NVAF patients treated with apixaban or rivaroxaban or dabigatran identified from the MarketScan Commercial and Medicare databases. Among their study population, the risk for MB was nearly 2-fold less for NVAF patients who received apixaban vs rivaroxaban, and also apixaban was associated with numerically fewer MB events than dabigatran, although the difference was not statistically significantCitation12. Also consistent with our study, patients treated with apixaban had approximately half the risk for MB in comparison to those who received warfarin therapyCitation12. In another early evaluation of NVAF patients treated with the different NOACs, use of rivaroxaban, compared to apixaban, was associated with significantly greater risk of MB-related hospital re-admissions across two different database analysesCitation13. Additionally, in a recent study of NVAF patients identified from the Optum database that evaluated both efficacy and safety outcomes of patients treated with apixaban, rivaroxaban, and dabigatran, the risk for MB was also found to be significantly lower for patients treated with apixaban vs rivaroxaban (HR = 0.39, p < .001)Citation14. Furthermore, in this last study patients treated with apixaban vs dabigatran also had a significantly lower risk for MB (HR = 0.50, p < .001)Citation14.
We also conducted sensitivity analyses of the comparisons of risk for MB associated with treatment with each OAC vs apixaban, in which baseline all-cause hospitalizations or NOAC dosage levels (low dose vs standard dose) were included as independent variables in the Cox regression models, and the results were consistent with the default analyses. For the comparison of warfarin vs apixaban, our findings further confirm the results of the clinical trial, ARISTOTLE, which showed treatment with apixaban was associated with significantly less risk for MB than warfarinCitation8. The results of the current study complement those of the clinical trials, in which each OAC was compared with warfarin, with that of real-world findings of direct comparisons of the different OACsCitation8–10.
To our knowledge, the current study is the first comprehensive analysis to compare all types (inpatient, outpatient, and pharmacy) of healthcare resource use and associated costs between apixaban and other OAC treatments among NVAF patients, especially related to the MB hospitalizations. In a real-world study that evaluated hospital resource use of hospitalized NVAF patients, apixaban vs warfarin treatment was also associated with lower hospital costsCitation16. To better optimize the treatment strategies of NVAF patients, it is important to understand the clinical and economic outcomes of NVAF patients treated with different OACs in routine clinical practice. Our early findings of a relatively large study population provide potentially useful clinical and economic information about OAC therapy for payers, providers, and patients with NVAF. Future additional studies with larger sample sizes, longer time frames, and in other study populations may be needed to validate the results of this real-world comparative study of the safety and economic outcomes of treatment with the different NOACs.
Limitations
This was not a cost-effectiveness evaluation based on a head-to-head, comparative, randomized clinical trial with efficacy or safety data, since there are no head-to-head clinical trials comparing the efficacy and safety of any of the NOACs among patients with NVAF. This cost analysis does not imply comparable efficacy, safety, or product interchangeability. For this study, each cohort was independently propensity score matched against apixaban. Therefore, comparisons cannot be made among the three separate arms of the analysis. The follow-up period for patients was not uniform nor consistent with that of times in the clinical trials. Edoxaban was excluded from the analysis, since edoxaban patients were too few to include for meaningful statistical analysis. Clinical observations of patients, such as ejection factor among patients with heart diseases, as well as lab values, are not consistently captured in the IMS Pharmetrics database, and, therefore, could not be reliably assessed. This study did not compare stroke-related hospitalization rates among NVAF patients treated with OACs, since, according to the initial power calculation, the current sample size was not large enough to compare these rates. As usage of NOACs for stroke prevention among NVAF patients is increasing, and this was an early comparative study of outcomes, future studies with larger sample sizes and longer follow-up are needed for evaluation of stroke and bleeding rates, and also may provide useful information of sub-populations of AF patients with certain comorbidities. Additionally, costs related to complications were not specifically evaluated, and they could be higher among patients treated with warfarin; however, the all-cause costs did include all such potential complication costs. Administrative claims data are collected for purposes other than research, and the analysis is constrained by the codes, which may contain coding errors and missing data. Also, the database does not uniformly capture over-the-counter medications, such as aspirin, and could affect the treatment patterns of the anticoagulants being studied. As an observational study, unobserved confounders may exist for which the analysis did not control, such as drug adherence, time in therapeutic range for warfarin, and other patient characteristics not captured in the data source. This database contains information from administrative claims databases across the US, and may not be generalizable to the entire US population of NVAF patients. Furthermore, the claims from this source predominantly represent commercially-insured patients, and may not generalize appropriately to individuals covered by other types of health insurance, such as Medicare and Medicaid. Lastly, no causal relationship can be concluded based on the retrospective claims database analysis.
Conclusion
In this retrospective claims database analysis, our findings suggest that NVAF patients treated with apixaban are less likely to be hospitalized for any cause or MB, and also incur lower healthcare costs in comparison to patients treated with rivaroxaban or warfarin. Additionally, NVAF patients treated with apixaban vs dabigatran have a lower likelihood of hospitalization for any cause.
Transparency
Declaration of funding
This study was sponsored by Pfizer and Bristol-Myers Squibb.
Declaration of financial/other interests
JL, MLS, and BM are employees of Novosys Health, which has received research funds from Pfizer and Bristol-Myers Squibb in connection with conducting this study and development of this manuscript. JT and JM are employees of Pfizer and own stock in the company. KG, MY, and AN are employees of Bristol-Myers Squibb and own stock in the company. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
References
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8
- Bogousslavsky J, Cachin C, Regli F, et al. Cardiac sources of embolism and cerebral infarction-clinical consequences and vascular concomitants: the Lausanne Stroke Registry. Neurology 1991;41:855-9
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142-7
- American College of Cardiology Foundation, American Heart Association, European Society of Cardiology, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 2013;127:1916-26
- Mercaldi CJ, Ciarametaro M, Hahn B, et al. Cost efficiency of anticoagulation with warfarin to prevent stroke in Medicare beneficiaries with nonvalvular atrial fibrillation. Stroke 2011;42:112-18
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51
- Lip GY, Pan X, Kamble S, et al. Major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban or warfarin: a “real-world” observational study in the United States. Int J Clin Pract 2016;70:752-63
- Lip GYH, Keshishian A, Kamble S, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb Haemost 2016;116:975-86
- Deitelzweig S, Bruno A, Trocio J, et al. An early evaluation of bleeding-related hospital readmissions among hospitalized patients with nonvalvular atrial fibrillation treated with direct oral anticoagulants. Curr Med Res Opin 2016;32:573-82
- Noseworthy PA, Yao X, Abraham NS, et al. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest 2016;150:1302-12
- Consumer Price Index. USDL-14-0037. Washington DC: Bureau of Labor Statistics U.S. Department of Labor. February 2015. http://www.bls.gov/cpi/. Accessed March 15, 2017
- Xie L, Vo L, Keshishian A, et al. Comparison of hospital length of stay and hospitalization costs among patients with non-valvular atrial fibrillation treated with apixaban or warfarin: An early view. J Med Econ 2016;19:769-76