Abstract
Aims: To evaluate healthcare resource utilization and economic burden of patients with chronic myeloid leukemia (CML) progression to the blast phase.
Methods: Patients (≥ 18 years) with ≥1 inpatient or ≥2 outpatient CML diagnoses were identified from the MarketScan Commercial and Medicare databases (January 1, 2007–June 30, 2015). CML patients were grouped into two study cohorts, those with evidence of disease progression to the blast phase and those without. Patients were required to have continuous medical and prescription coverage during a 12-month baseline period, in which demographics and clinical characteristics were evaluated. All-cause healthcare resource utilization and costs were evaluated during the baseline period, and a variable follow-up period, lasting ≥1 day and up to 1 year. Generalized linear models (GLM) were used to compare the incremental costs of CML patients with vs without progression.
Results: Of the overall study population, 587 (7%) experienced disease progression and 7,504 (93%) did not. On the index date, of patients with progression, ∼ 31% were treated with allogeneic hematopoietic cell transplant and 69% with chemotherapy. During the baseline period, mean total healthcare costs, including costs for hospitalizations and outpatient costs, were significantly greater for CML patients with progression as compared to those without progression ($143,778 vs $53,143, p < .001). During the follow-up, mean total healthcare costs, costs for hospitalizations, and outpatient medical service costs were substantially greater for patients with progression as compared to those without progression; however, costs for outpatient prescriptions were less for patients who progressed. When patient characteristics were controlled for, mean incremental 1-year cost for CML patients with vs without progression was $270,925 (confidence interval = $235,290–$311,958, p < .001).
Conclusions: The healthcare burden, in terms of healthcare resource utilization and costs, of patients with CML progression is substantial. Healthcare providers and payers should consider various strategies to minimize the rate of CML progression.
Introduction
Chronic myeloid leukemia (CML) is a progressive cancer of bone marrow stem cells with an annual incidence of one to two cases per 100,000 adultsCitation1. Approximately 15% of newly-diagnosed adult leukemia cases are CML (median age = 67 years)Citation1,Citation2. CML is classified into three phases, based on disease severity: chronic phase, accelerated phase, and blast phase, the most severe phase of the diseaseCitation2,Citation3. If untreated, patients with chronic phase CML will eventually progress to advanced phases in 3–5 yearsCitation4,Citation5.
Resulting from a chromosomal translocation, the constitutively active tyrosine kinase BCR-ABL1 oncoprotein plays a key role in the onset and progression of CMLCitation1–3. Targeted tyrosine kinase inhibitor (TKI) therapies have become the main therapeutic strategy for patients with CML, and have been shown to prolong the chronic phase and improve patient survivalCitation6. In clinical trials, between 65–83% of CML patients achieve a complete cytogenetic response (CCyR) within 12 months, depending on the TKI therapy used for treatmentCitation7. Patients who achieve early CCyR have improved survival compared to patients who do notCitation2,Citation3.
However, initial treatments that are effective in the chronic phase often fail in the blast phase of the disease. Thus, CML progression still represents a significant challenge to both patients and the healthcare system. The National Comprehensive Cancer Network (NCCN) guidelines recommend that patients with CML progression to blast phase be enrolled in a clinical trial, or treated with TKI or with chemotherapy agents (i.e. acute myeloid leukemia (AML)-like or acute lymphoblastic leukemia (ALL)-like chemotherapy treatments) and/or allogeneic hematopoietic cell transplants (HSCT)Citation2. The choice of treatment is dependent on the progression phase, age, and comorbidities of patientsCitation2. The clinical management of patients with CML progression can be associated with extensive healthcare resource utilization. However, there is a paucity of evidence quantifying the real-world healthcare burden of patients with CML progression. To address this need, the objectives of this study were to evaluate healthcare resource utilization and the economic burden of patients with CML progression using real-world data.
Methods
Study population
Patients (≥ 18 years of age) with at least one inpatient or at least two outpatient (≥ 30 days apart) diagnoses of CML (International Classification and Diseases, 9th revision code: 205.1) were identified from the Truven Health Analytics MarketScan Commercial and Medicare healthcare claims databases from January 1, 2007 to June 30, 2015. This time frame was selected to provide the most recent data available, and to ensure a sufficient number of CML patients would be included in the analysis. The claims data from the MarketScan databases include inpatient and outpatient information reflecting real-world treatment patterns and costs. In compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA), the databases consist of fully de-identified data sets. Patient data were extracted from both the Commercial and Medicare Supplemental databases to maximize the representation of patients from different age groups.
Patients with disease progression were identified based on the presence of claims for NCCN guidelines recommended treatments for blast phase CML, which are AML-like or ALL-like chemotherapy treatments, or a HSCT. For patients with progression as defined in this study, the earliest date of a claim for either of such treatments related to progression was defined as the index date. For patients without evidence of progression, a random date after the first CML diagnosis was identified as the index date. In order to increase the accuracy of identifying patients with CML progression, patients with other cancers, including AML and ALL, documented prior to the first recorded diagnosis of CML, were excluded from the study. Due to the patient selection criteria, patients who were diagnosed with CML in the blast phase at their first CML diagnosis were not included in the study population. Patients included in the study population were also required to have continuous medical and prescription insurance coverage during the 12 months prior to the index date (baseline period). Patients were evaluated after the index date in a follow-up period with variable duration, lasting at least 1 day and up to 1 year. This variable follow-up period was used to minimize survival bias and to allow for patients who died shortly after disease progression to contribute data to the analysis.
Demographics and clinical characteristics
For each selected CML patient, demographics including age, gender, health plan type, and US geographic region of care, and clinical characteristics including Charlson Comorbidity Index (CCI), index treatment, and duration of follow-up were determined.
Healthcare resource utilization and associated costs
Healthcare resource utilization for all causes, including hospitalizations, outpatient medical services, and outpatient prescription drug usage, and the associated costs (total payment from health plans, patients, and/or third party payers) were captured during the 12-month baseline period and a variable follow-up period, lasting at least 1 day and up to 1 year. All healthcare resource utilization and costs measurements were normalized to the basis of per one patient year and compared among study cohorts. All costs were inflation adjusted to 2015 cost level using the Medical Care component of the Consumer Price Index.
Statistical analyses
Descriptive statistics were used to compare patient demographics, clinical characteristics, healthcare resource utilization, and costs for the study cohorts. T-tests and Chi-square tests were used for continuous and categorical variables, respectively. A generalized linear model (GLM) was used to further compare the incremental total all-cause healthcare costs of CML patients with vs without progression. Log transformation and gamma distribution were applied to the cost data in the GLM. Covariates were age, gender, US geographic region of care, health plan type, CCI, and follow-up duration. A second GLM was carried out as a sensitivity analysis that controlled total all-cause healthcare costs during the 12-month baseline period, in addition to the above covariates. Statistical software SAS version 9.4 (Cary, NC) was utilized to perform all data analyses.
Results
Demographics and clinical characteristics
The selection process of the study population is shown in . Patient demographics and clinical characteristics are presented in for the study population with and without CML progression. Of the overall identified CML study population, 587 (7%, mean age = 58.6 years) experienced disease progression, and 7,504 (93%, mean age = 58.4 years) did not. A greater percentage of male than female patients had evidence of disease progression, and CML patients with progression had more comorbidities, as measured by CCI, than those without progression (mean CCI = 3.7 vs 3.1, p < .001). On the index date, among patients with CML progression, ∼ 31% were treated with HSCT, most of which were allogeneic, and 69% were treated with chemotherapy. During the follow-up period, among patients with CML progression to the blast phase, an additional 8% (n = 45) received HSCT after the index date.
Figure 1. Selection of study population. CML, Chronic myeloid leukemia; AML, Acute myeloid leukemia-like; ALL, Acute lymphoblastic leukemia-like.
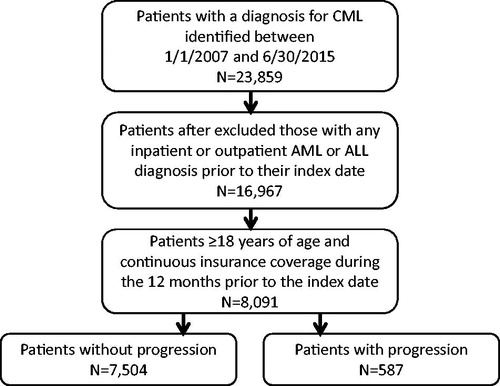
Table 1. Demographics and clinical characteristics of study cohorts.
Descriptive analysis
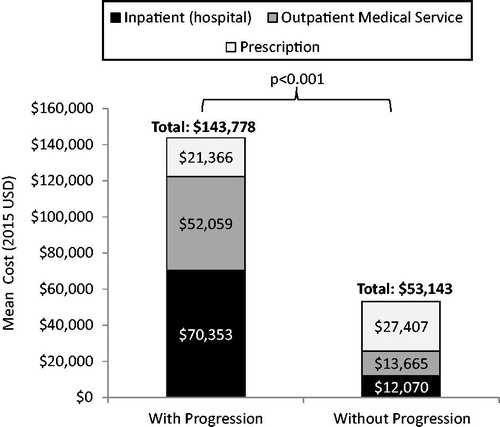
During the baseline period, mean total all-cause healthcare costs, including costs for hospitalizations and outpatient medical service costs were significantly greater for CML patients with progression as compared to those without progression (). However, mean outpatient prescription costs were lower for patients who progressed ().
Figure 2. Total all-cause medical and outpatient prescription costs per patient year in the baseline period.
All-cause healthcare resource utilization and associated costs during the follow-up are shown in and . During the follow-up, patients with evidence of progression had a significantly greater mean number of hospitalizations (1.7 vs 0.3 per patient year, p < .001), longer mean length of hospital stay (25.5 vs 1.7 days per patient year, p < .001), more outpatient medical service claims (397.0 vs 82.8 per patient year, p < .001), and more prescription claims (36.0 vs 21.7 per patient year, p < .001) than those without progression. This greater healthcare resource use was reflected in higher mean total healthcare costs per patient year, costs for hospitalizations, and costs for outpatient medical services for patients with progression, in comparison to those without progression. However, costs for outpatient prescriptions were lower for patients with progression.
Figure 3. Total all-cause medical and outpatient prescription costs per patient year in the follow-up.
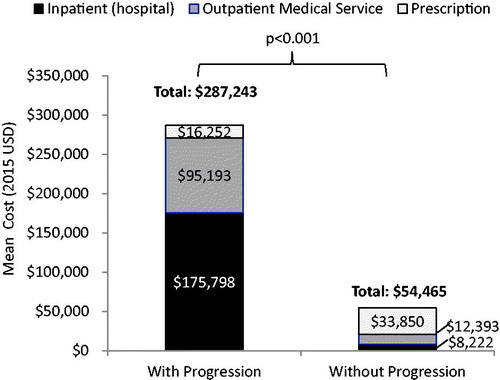
Table 2. All-cause healthcare resource utilization and associated costs per patient year of study cohorts during the follow-up.
Multivariable regression analysis
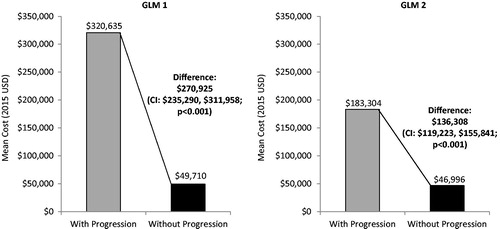
When patient characteristics were taken into consideration in the multivariable regression analysis, the mean incremental 1-year total all-cause healthcare cost for CML patients with vs without progression was $270,925 (). When an additional GLM was used in which the baseline healthcare costs of CML patients were controlled for, the mean incremental 1-year total all-cause healthcare cost for patients with vs without progression was $136,308 ().
Figure 4. Adjusted comparison of total all-cause healthcare costs per patient year in the follow-up. GLM 1: Covariates were age, gender, US geographic region, health plan type, Charlson Comorbidity Index, and follow-up duration. GLM 2: Additionally included total healthcare costs per patient during the baseline period. GLM, Generalized linear model; CI, 95% confidence interval.
Discussion
Based on this analysis of 8,091 patients with CML in the real world, 7% had the evidence of disease progression to the blast phase. Total healthcare costs were 2.7-fold and over 5-fold higher 12 months prior to and after initiating treatment for disease progression, respectively, among patients with CML progression relative to those without progression. In this analysis, the date of disease progression onset was considered to be the date when the first claim for an NCCN recommended treatment for the blast phase of CML was captured in the data source. This could have been after the actual clinical evidence of progression onset. This is a typical limitation of using healthcare claims to draw inferences about the timing of onset of a condition or event, as there are no ways of verifying the true start date of that event. Thus, appreciable costs and resource utilization prior to the index date in patients with evidence of disease progression were likely related to progression, and might have increased the estimate of baseline healthcare cost. When patient characteristics were taken into consideration with a GLM, the mean incremental 1-year cost for CML patients with vs without progression was $270,925 and when, additionally, the differences in baseline healthcare costs were controlled for, the incremental cost was $136,308. It is important to look at both adjusted incremental 1-year costs, as it is likely that the two GLM results may represent the higher and lower estimates of the incremental economic burden of CML progression.
This study demonstrated that the economic burden of CML progression to the blast phase was driven by extensive hospital resource use, as well as use of outpatient medical services. Outpatient prescription costs represented only 5.7% of the total healthcare costs for patients with CML progression and 62.2% of the total costs of patients without progression during the follow-up. This may have resulted from less use of costly targeted therapies to treat CML. The substantial cost burden of patients with CML progression in comparison to those without suggests that it is imperative to optimally monitor patients during the chronic phase of the disease for treatment responsiveness to prevent or delay transformation to the blast phase and, thereby, reduce costs. A recent study conducted on 1,431 patients with CML showed that greater than one-third of patients received no monitoring in the first year of TKI therapyCitation7. Furthermore, in this study, monitoring according to NCCN guidelines and increased adherence to TKI treatment were associated with a reduction in healthcare resource utilization and costsCitation7. Specifically, each monitoring test (estimated cost: $223) was associated with a cost savings of $2,918 per patient per yearCitation7. Insufficient monitoring of patients with CML may have a negative impact on clinical outcomes, lead to omission of treatment resistance, and consequently increase healthcare costs.
The results of this study are aligned with that reported by Knopf et al.Citation8, who assessed the economic burden of TKI treatment failure among patients with CML identified between 2008–2011 from the IMS PharMetrics claims database. Among 547 treatment failures matched to 547 non-failures, Knopf et al.Citation8 reported lower pharmacy costs, but significantly higher medical costs for treatment failures during a 1-year follow-up. Furthermore, in a follow-up to this study, the healthcare economic burden associated with TKI treatment failure increased with each sequential line of TKI therapyCitation9. Some of the differences in the actual estimated costs for CML patients between our study and that of Knopf et al.Citation8 could potentially be related to whether to require patients to have received TKI treatment and the different types of healthcare cost data contained within the corresponding data sources.
Limitations
This study has several limitations that are important to note. Patients with progression were identified through patterns of healthcare resource use, as there were no specific ICD-9 diagnosis codes for CML progression. However, within the confines of what could be detected through claims, the pattern of healthcare resource use that served as a proxy for disease progression was consistent with NCCN guideline recommendations for treatment of the blast phase of CML. Since such evidence was based on insurance claims and not from patient clinical records, such determination may not fully reflect CML progression and treatment in its entirety in the real-world setting. For instance, this methodology does not allow for capturing patients that have progressed to the blast phase, but are treated with TKIs without chemotherapy, or patients unfit for chemotherapy or HSCT. This study may have incorrectly included patients who did not have CML disease progression, but might have received chemotherapy or HSCT for reasons other than CML progression. Furthermore, since TKI therapy may be used for all phases of CML, it was not used as a criteria to determine disease progression. This might have resulted in an under-estimation of the full cost of CML progression. Mortality information is not reliably captured in the data source and, thus, this outcome was not evaluated in this study. However, the variable follow-up period was used to include patients who might have died and, thus, disenrolled from the health plan. In addition, there have been other limitations that are inherent to any claims analysis. Healthcare claims are submitted by healthcare providers to insurance companies for reimbursement and not for research purposes. Such claims are subject to possible coding errors, coding for a rule-out of a diagnosis rather than actual disease, and under-coding, without the possibility of verifying reported diagnoses. The MarketScan databases contain data primarily from large employers, while medium and small firms may not be represented. Finally, the data source was skewed towards younger patients, and the study population may not accurately reflect the age distribution of the CML population in the US. Future studies are warranted to evaluate how age may affect the healthcare and economic burden of CML patients with disease progression.
Conclusions
In conclusion, the healthcare and economic burden, in terms of healthcare resource utilization and costs, of patients with CML progression is substantial. Healthcare providers and payers should consider various strategies to minimize the rate of CML progression, including proper treatment selection, as well as regular monitoring for treatment response.
Transparency
Declaration of funding
This study was funded by Bristol-Myers Squibb.
Declaration of financial/other relationships
EJ is a consultant to Bristol-Myers Squibb. JL, MLS, and BM are employees of Novosys Health, which has received research funds from Bristol-Myers Squibb in connection with conducting this study and development of this poster. LRS is an employee of Bristol-Myers Squibb. DM is an employee of Bristol-Myers Squibb and owns stock in the company. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Some aspects of this study were presented at the 58th American Society of Hematology Annual Meeting, December 3–6, 2016, in San Diego, CA.
References
- Jabbour EJ, Kantarjian H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am J Hematol 2016;91:252-65
- National Comprehensive Cancer Network Guidelines. Chronic myeloid leukemia, v. I, 2016. Fort Washington, PA: NCCN
- Jabbour EJ, Hughes TP, Cortés JE, et al. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia. Leuk Lymphoma 2014;55:1451-62
- Alvarez RH, Kantarjian H, Cortes JE. The biology of chronic myelogenous leukemia: implications for imatinib therapy. Semin Hematol 2007;44:S4-S14
- Jabbour EJ, Cortés J, Kantarjian H. Treatment selection after imatinib resistance in chronic myeloid leukemia. Target Oncol 2009;4:3-10
- Jabbour EJ, Cortés JE, Kantarjian H. Long-term outcomes in the second-line treatment of chronic myeloid leukemia. Cancer 2011;117:897-906
- Latremouille-Viau D, Guerin A, Gagnon-Sanschagrin P, et al. Health care resource utilization and costs in patients with chronic myeloid leukemia with better adherence to tyrosine kinase inhibitors and increased molecular monitoring frequency. J Manag Care Spec Pharm 2017;23:214-24
- Knopf KB, Divino V, McGarry L, et al. Economic burden of tyrosine kinase inhibitor treatment failure in chronic myeloid leukemia. Clin Lymphoma Meyeloma Leuk 2015;15:e163-71
- McGarry LJ, Chen YJ, Divino V, et al. Increasing economic burden of tyrosine kinase inhibitor treatment failure by line of therapy in chronic myeloid leukemia. Curr Med Res Opin 2016;32:289-99
