Abstract
Aims: An economic evidence is a vital tool that can inform the decision to use costly insulin analogs. This study aimed to evaluate long-term cost-effectiveness of insulin detemir (IDet) compared with insulin glargine (IGlar) in type 2 diabetes (T2DM) from the Thai payer’s perspective.
Methods: Long-term costs and outcomes were projected using a validated IMS CORE Diabetes Model, version 8.5. Cohort characteristics, baseline risk factors, and costs of diabetes complications were derived from Thai data sources. Relative risk was derived from a systematic review and meta-analysis study. Costs and outcomes were discounted at 3% per annum. Incremental cost-effectiveness ratio (ICER) was presented in 2015 US Dollars (USD). A series of one-way and probabilistic sensitivity analyses were performed.
Results: IDet yielded slightly greater quality-adjusted life years (QALYs) (8.921 vs 8.908), but incurred higher costs than IGlar (90,417.63 USD vs 66,674.03 USD), resulting in an ICER of ∼1.7 million USD per QALY. The findings were very sensitive to the cost of IDet. With a 34% reduction in the IDet cost, treatment with IDet would become cost-effective according to the Thai threshold of 4,434.59 USD per QALY.
Conclusions: Treatment with IDet in patients with T2DM who had uncontrolled blood glucose with oral anti-diabetic agents was not a cost-effective strategy compared with IGlar treatment in the Thai context. These findings could be generalized to other countries with a similar socioeconomics level and healthcare systems.
Introduction
The advent of the aging society, increasingly unhealthy lifestyles with poor diets, and growing prevalence of excess body weight and obesity potentially influence the growth in the number of people with diabetes worldwide. This phenomenon has been observed in both developed and developing countriesCitation1,Citation2, including ThailandCitation3–5. According to the 4th Thai National Health Examination Survey (NHES IV), 7.5% of Thai adults aged 20 and older had diabetes; one-third of these groups were undiagnosedCitation6. In addition, data from diabetes clinics in 11 tertiary hospitals from different parts of Thailand reported that 94.6% were type 2 diabetes (T2DM), and 5.4% were type 1 diabetes (T1DM). Of those T2DM, only 30.7% had HbA1c below the optimal value of 7.0%Citation7.
As the number of people with diabetes grows, a proportion of national healthcare expenditure for persons with diabetes is expected to rise. Data from the Ministry of Public Health in Thailand indicate that the financial burden of diabetes was on the rise, and that the rate of diabetes-related hospitalization increased from 795.04 per 100,000 in the year 2000 to 1,081.25 per 100,000 in the year 2013Citation8. High costs of diabetes and its complications also impose dramatic impacts on patients and their families. Effective interventions or initiatives are, therefore, urgently needed to reduce the incidence and financial impact of diabetes complications in the Thai population. These interventions would also help improve life expectancy and quality-of-life of patients with diabetes.
According to the Thai Clinical Practice Guideline for DiabetesCitation9, insulin is recommended to be an add-on therapy when patients with T2DM receiving the first-line oral anti-diabetic drug, i.e. metformin, are unable to achieve glycemic control. In addition to insulin, other add-on therapies include thiazolidinedione, dipeptidyl peptidase-4 inhibitors, alpha-glucosidase inhibitors, and repaglinide. The target population in the study, therefore, included patients with T2DM who had uncontrolled blood glucose with oral anti-diabetic drugs and received a combination therapy.
Long-acting human insulin analogs, such as insulin glargine (IGlar), have shown potential benefits in patients in lowering severe nocturnal hypoglycemiaCitation10. Included in the National List of Essential Medicine (NLEM), insulin glargine (IGlar) is indicated for patients with type 1 diabetes who have frequent severe hypoglycemia or nocturnal hypoglycemia from Neutral Protamine Hagedorn (NPH) insulin. Recently, insulin detemir (IDet) has become available in Thailand; however, the cost of this medication is high. The decision to use and pay for this medication would depend on whether potential incremental benefits gained from this product can outweigh its costs. Previous studies have reported the value for money of IDet compared to IGlar; however, their findings were inconsistent. Guillermin et al.Citation11 suggested that treating T2DM patients with IDet was associated with comparable HbA1c change, but higher costs compared with IGlar in Canada. On the other hand, changing from IGlar to IDet was found to be a cost-saving option in the GermanCitation12 and ChineseCitation13 settings. Given this inconsistent evidence, there is a need to estimate the long-term cost-effectiveness of IDet compared with IGlar for the treatment of patients with T2DM to guide the IDet reimbursement decision-making process from a payer’s perspective. This study was, therefore, conducted to evaluate the long-term cost-effectiveness of IDet compared to IGlar from the perspective of the payer in Thailand. The selection of the comparator (IGlar) for this study was made based on the recommendation by Thai stakeholders, including endocrinologists, policy-makers, and patients who were interested in identifying a new and cost-effective insulin analog that can be used as an alternative to NPH insulin.
Methods
IMS CORE diabetes model
We used a validated computer simulation model of diabetes, namely The IMS Center for Outcomes Research (IMS CORE) model version 8.5, to estimate the long-term costs and clinical outcomes. Details of this model are described elsewhereCitation14,Citation15. The IMS CORE model composed of 15 sub-models which simulate the diabetic complications of angina, myocardial infarction, congestive heart failure, stroke, peripheral vascular disease, neuropathy, foot care, retinopathy, macular edema, cataract, nephropathy, hypoglycemia, ketoacidosis, lactic acidosis, and non-specific mortality. Each sub-model encompasses a Markov model using Monte Carlo simulation which incorporates a treatment sequence. All-cause mortality rate used in the model was adjusted with the age-specific mortality rate of Thai peopleCitation16.
A series of inter-connected time-dependent models represent patient characteristics, clinical parameters, risk factors, treatment effects, complications, and economic parameters over time. This model has been validated and tested in various settings to ensure the reliability of simulated outcomesCitation15,Citation17.
Simulation cohorts
A hypothetical simulation cohort was patients with T2DM. Baseline characteristics and clinical data were derived from Thai Diabetes Registry (TDR) Reports in 2003Citation18 and 2006Citation19, the National Health Security Office (NHSO)Citation20, the National Statistical OfficeCitation21, the Center for Alcohol StudiesCitation22, and other published studiesCitation23–27 ().
Table 1. Baseline demographics and complications of patients in a simulated cohort.
Treatment effects
We obtained the treatment effect of IGlar from The Thai Insulin Therapy Assessment (TITAN) ProgramCitation28, which evaluated efficacy and adverse events of insulin use in 41 hospitals in Thailand. We performed a systemic search using MEDLINE, EMBASE, and Clinicaltrial.gov from their inception to August 2014. However, we did not identify any randomized controlled trials (RCTs) comparing IDet and IGlar in Thailand, but found a meta-analysis reporting weighted mean difference (WMD) of IDet conducted by Swinnen et al.Citation29. The systematic review included four RCTs comparing insulin detemir with insulin glargine, with a duration of 12 weeks or longerCitation30–33. We obtained the baseline rate of hypoglycemia of IGlar from the TITAN ProgramCitation28 and calculated the IDet hypoglycemia rate based on a meta-analysis by Swinnen et al.Citation29. Because none of the patients receiving IGlar in the TITAN Program experienced severe hypoglycemiaCitation28, we derived this data from the report by the Canadian Agency for Drugs and Technologies in Health (CADTH)Citation34. The same approach was used to derive the rate of severe hypoglycemia in our previous studyCitation35. Severe hypoglycemia of IDet was also calculated from the study conducted by Swinnen et al.Citation29. We pooled the change in body mass index (BMI) from baseline from four RCT studiesCitation30–33, and found that patients receiving IDet had significantly less BMI change than those receiving IGlar (mean difference = −0.35) (). This is attributable to significantly less weight gain found in patients treated with IDet compared to patients treated with IGlar (mean difference = −0.91, 95% CI = −1.21 to −0.61)Citation29.
Table 2. Treatment efficacy and adverse events.
Costs and perspective
Considering the costs involved in the treatment of diabetes, from the perspective of the payer, only direct medical costs, namely the cost of insulin and costs associated with diabetes-related complications and management, were included. Insulin treatment costs were estimated from the daily dose and unit costs. Unit costs of IDet and IGlar were obtained from the Drug and Medical Supply Information Center (DMSIC)Citation36, Ministry of Public Health, which provides qualified and unbiased information about medicines and medical supplies to the Thai population. According to the Thai Health Technology Assessment GuidelinesCitation37, the use of median drug price is recommended for a study on cost-effectiveness. If each drug has more than one brand name, the median drug price from all the listed original drugs should be used. Both IDet and IGlar had only one brand, with the median price of 2,801.26 THB (77.64 USD) and 2,792.70 THB (77.40 USD), respectively. The drug daily dose was pooled from four RCT studiesCitation30–33. IGlar was used once daily, with 0.57 (95% CI =0.46–0.69) units per kilogram. IDet, on the other hand, was classified into mixed dose, once, and twice daily doses. The mixed dose of 0.83 (95% CI =0.77–0.89) units per kilogram was used for the base-case scenario. The once-daily dose was 0.50 (95% CI =0.11–0.89) units per kilogram, and the twice-daily dose was 0.93 (95% CI =0.88–0.98) units per kilogram. The IDet doses were varied in the sensitivity analysis as well. The average weight of 69.67 kilograms was calculated from 25.7 kilograms per square meter BMI of Thai diabetesCitation19 and the average height of 1.65 metersCitation38. The daily doses of IDet and IGlar were 57.83 units per day and 39.71 units per day, respectively. Based on the daily dose and the unit price listed above, the acquisition cost was 539.97 THB (14.97 USD) per day, or 197,087.79 THB (5,462.52 USD) per year, for IDet, and 369.69 THB (10.25 USD) per day, or 134,935.85 THB (3,739.91 USD) per year, for IGlar. Costs associated with diabetes-related complications, management, and insulin adverse events were primarily derived from hospital databases and Thai published literatureCitation39–44 (). Data regarding other oral anti-diabetic drugs were not taken into consideration, because we assumed that their costs were equal for patients receiving both insulin alternatives and, therefore, cancelled out from the analysis. Hospital databases contain four sub-databases, which are inpatient, outpatient, pharmacy, and financial databases. Information regarding age, gender, health insurance coverage, date of birth, and diagnosis codes (International Statistical Classification of Diseases 10th Revision; ICD-10) are found in the inpatient and outpatient databases. The pharmacy database includes the medication name, regimen for each prescription, and amount of medication prescribed per prescription. Lastly, the financial database contains charges for medication, laboratory, and medical services. All the databases are linked using a unique encrypted identification code for each patient. All the costs were taken for calculation after being adjusted for inflation with the consumer price indexCitation45, and presented in the year 2015. The costs were converted at the rate of 36.08 THB per USD as of December 30, 2015Citation46.
Table 3. Costs of diabetes complications and management.
Discounting and time horizon
All the costs and outcomes were discounted at the rate of 3% per annum in accordance with the Thai Health Technology Assessment GuidelineCitation47. To capture the long-term costs and outcomes, the time horizon was expanded to 50 years, as the starting age of our study cohort was 61 years.
Sensitivity analyses
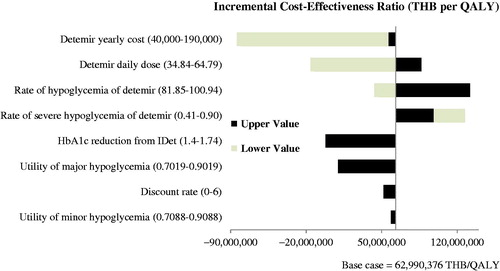
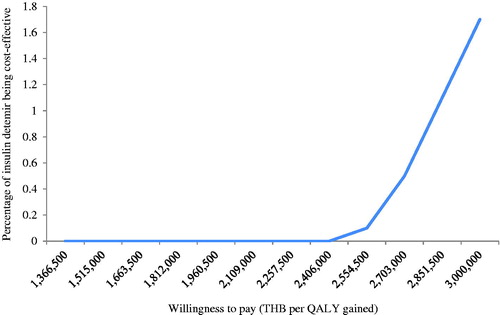
A series of one-way sensitivity analyses were performed to examine the influence of key input parameters on the outcomes projected by the model, including change in HbA1c, rate of hypoglycemia, discount rate, daily dose of IDet, costs, and utilities. The results are displayed as a tornado diagram. Furthermore, a probabilistic sensitivity analysis was performed using a non-parametric bootstrapping approach. It is a re-sampling procedure that estimates an empirical sampling distribution for costs and effects. Bootstrap samples of the same size as the original data are drawn with replacement from the original sample. Repeating the process many times generates bootstrap replicates of costs and effects, which can be used to calculate the incremental cost-effectiveness ratio (ICER)Citation48. In this model, a cohort of 1,000 non-identical patients was set. Each patient with different baseline characteristics was simulated 1,000 times. The mean and the standard deviation of costs, life expectancy, and quality-adjusted life expectancy were calculated. The result was presented as a cost-effectiveness acceptability curve (CEAC). As recommended by the Health Economic Working Group (HEWG) under the Sub-committee for Development of the NLEMCitation49, a ceiling threshold of 160,000 THB per QALY (4,434.59 USD per QALY) gained was used to justify the cost-effectiveness of new intervention in Thailand in this study.
Results
Base-case analysis
Our study suggests that IDet incurred much higher total costs (3,262,268 THB vs 2,405,599 THB, or 90,417.63 USD vs 66,674.03 USD), but led to minimal improvement in life years (LYs) and QALYs compared with IGlar (13.119 LYs vs 13.116 LYs; 8.921 QALYs vs 8.908 QALYs), leading to an extremely high ICER of ∼1.7 million USD per QALY. However, if IDet was used as a single dose, the result became cost-saving, indicating the lower cost, but higher effectiveness of IDet compared with IGlar. On the contrary, the ICER was increasing when a double dose of IDet was used, as shown in . The large cost component was primarily driven by the medication cost. The acquisition cost of IDet was 2,701,682 THB (74,880.32 USD), which was greater than that of IGlar (1,849,320 THB or 51,256.10 USD). The costs of treatment complications were virtually similar between IDet and IGlar ().
Table 4. Summary of base-case analysis.
Table 5. Cost component associated with insulin detemir and insulin glargine.
Sensitivity analyses
The cost of IDet showed the greatest impact on the ICER estimates (). When the cost of IDet declined from the base case by 34% (from 197,087.79 THB (5,462.52 USD) per year to 130,000 THB (3,603.10 USD) per year), IDet became a cost-effective option compared to the ceiling threshold of 160,000 THB per QALY (). When IDet was used as a once-daily dose (118,727.58 THB (3,290.68 USD) per year), it became a cost-saving strategy. illustrates that IDet is unlikely to be a cost-effective strategy, even if the ceiling ratio was set at 3 million THB.
Table 6. Threshold analysis varying cost of insulin detemir.
Discussion
Our study reported the long-term effectiveness and cost-effectiveness of IDet compared with IGlar from the Thai payer’s perspective. We found that IDet was associated with higher costs and better QALYs than IGlar; however, its benefit, measured as QALY gained, did not offset its high acquisition costs. This conclusion is due mainly to the extremely high total cost of IDet compared with IGlar (3,262,268 THB vs 2,405,599 THB or 90,417.63 USD vs 66,674.03 USD) and a negligible gain in QALYs (8.921 vs 8.908 for IDet and IGlar, respectively). The difference in total costs was mainly from the medication. Our findings indicated that IDet has become a cost-saving alternative compared with IGlar when it is used as a once-daily treatment. Treatment effects, such as HbA1c reduction and the incidence of severe hypoglycemia, were not significantly different between IDet and IGlar.
Despite different healthcare system, our findings were in line with a previous study based on the CORE Diabetes Model, suggesting that treatment of -T2DM patients with IDet led to higher lifetime costs and insignificant change in HbA1c compared with IGlar, regardless of IDet regimensCitation11. Consistent findings may in part be due to that of Guillermin et al.Citation11, who pooled the relative efficacy and safety of IDet and IGlar from the same head-to-head RCTsCitation30–33 as our study.
Few studies reported that IDet was economically attractive compared to IGlar. IDet was cost-effectiveness from the perspective of AmericanCitation50 and German payersCitation12. Similarly, Yang et al.Citation13 suggested that switching from IGlar to IDet was a cost-saving option for Chinese T2DM patients. It should be noted that the studies reporting a favorable cost-effectiveness profile obtained the efficacy of IDet from a single RCT or observational study that showed the larger reduction in HbA1c, the lower increase in BMI, and the fewer rates of major hypoglycemic events in IDet than IGlar. The American study derived the efficacy of IDet from a head-to-head RCT of IDet + Insulin Aspart (IAsp) and IGlar + IAsp in type 1 diabetes patients, while the GermanCitation12 and ChineseCitation13 studies obtained the treatment effect of IDet from the Predictable Results and Experience in Diabetes through Intensification and Control to Target: An International Variability Evaluation (PREDICTIVE) study, which was an international, multi-center, open-label observational study involving more than 35,000 patients with T1DM or T2DM transferred to an IDet regimen.
Our study was conducted to evaluate the cost-effectiveness between IGlar and IDet within the Thai context. The baseline demographics, treatment effects, and complications were mainly derived from the TDR database, which enrolled 9,419 diabetes patients from different parts of Thailand. Besides drug acquisition costs and diabetes complications, treatment costs were estimated as closely to costs incurred by the Thai healthcare payer as possible. Those costs were mostly derived from hospital databases, which reflect the actual practice of diabetes treatment in Thailand. Even though the treatment effects of insulin analogs were derived from a meta-analysis of RCTs conducted in other countriesCitation29, the efficacy and adverse events of IGlar were derived from a large Thai cohort studyCitation28.
Our study adds the following contributions to the existing literature. First, as a previous study demonstrated, IDet leads to lower weight gain than IGlarCitation29: we incorporated the change in the BMI from baseline into the CORE diabetes model. Second, several scenarios of data analysis were performed based on the different daily injection doses of IDet. The findings indicated that ICER was sensitive to the daily dose of IDet. Finally, the total lifetime direct costs were estimated for each insulin treatment, which reflected the insulin costs incurred by the payer. The key strength of our study was that the findings from this study were considered as part of the economic evidence that can be used to support the selection of long-acting insulin analogs in Thailand. In addition, findings from our study can be used to inform the decision-making process in other countries, with similar socioeconomics levels and healthcare systems to Thailand, as they might have comparable costs of treatments, baseline demographic characteristics, and willingness to pay values to our study.
In Thailand, the medicines that are high cost but are important for particular groups of patients, economic evaluation, and budget impact analysis information is required for the sub-committee for the development of the NLEM to consider in decision-making. The health technology assessment (HTA) process for pharmaceutical reimbursement involves prioritizing the list of HTA topics according to the burden of disease and the degree of severity by the sub-committee for the development of the NLEM, performing a health economic evaluation by a Health Economic Working Group commissioned by the sub-committee, appraising the methodological quality of a health economic evaluation report by experts in the field, and considering the health economic evaluation evidence during the reimbursement decision by the sub-committeeCitation51.
This study encompasses a certain number of limitations. First, given the lack of local efficacy data, we assumed that the treatment effects of IDet observed in the published RCTs were generalizable to the Thai T2DM patients. We acknowledged that the daily dose of both types of insulin in other countries might be slightly higher than that being used in general practice in Thailand. Second, the utility values were mostly derived from the data from other countries; however, the result of sensitivity analysis suggested that the impact of utility on cost-effective findings was negligible. Third, we assumed that T2DM patients would use the same insulin for lifetime; however, it is possible that patients may switch to another insulin for a variety of reasons in real practice setting. Finally, our study did not take into account the more recent meta-analysis by Freemantle et al.Citation52, showing that IGlar 300 units/mL (Gla-300) was associated with a reduction in HbA1c, but an increase in body weight over IDet; however, none of these changes were statistically significant. Similarly, this meta-analysis also shows that IGlar-300 was associated with a numerically lower rate of documented symptomatic and nocturnal hypoglycemic events. This recent meta-analysis was omitted from our study because it was not available at the time of our systematic search. If the findings of Freemantle et al.Citation52 had been used in this study, IGlar might have shown a cost-saving option compared to IDet, due to the lower rate of hypoglycemia.
Based on the findings of the study, IDet is not economically attractive compared to IGlar from the Thai payer’s perspective. However, the decision to make an appropriate choice needed to consider other factors such as drug allergy, adherence, frequency of administration, ease of use, and availability and accessibility of the insulin among Thai T2DM patients who are usually advanced in age.
Conclusions
From the Thai payer’s perspective, treatment with IDet in patients with T2DM who had uncontrolled blood glucose with oral anti-diabetic drugs was not a cost-effective insulin analog compared with IGlar treatment. Cost of IDet was the most influential factor that played a major role in the study findings. Our findings could be generalized to other countries, with similar socioeconomics levels and healthcare systems.
Transparency
Declaration of funding
This study was supported by the CMU Mid-Career Research Fellow Program, Chiang Mai University, Thailand.
Declaration of financial and other relationships
The authors report no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the IMS Health team for support and training in the use of the IMS CORE Diabetes Model.
References
- Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34:1249-57
- Temelkova-Kurktschiev T, Stefanov T. Lifestyle and genetics in obesity and type 2 diabetes. Exp Clin Endocrinol Diabetes 2012;120:1-6
- Papier K, Jordan S, D'Este C, et al. Incidence and risk factors for type 2 diabetes mellitus in transitional Thailand: results from the Thai cohort study. BMJ Open. 2016;6:e014102
- Aekplakorn W. Prevalence and management of diabetes and associated risk factors by regions of Thailand, Third National Health Examination Survey 2004. Diabetes Care 2007;30:2007-12
- Aekplakorn W, Stolk RP, Neal B, et al. The prevalence and management of diabetes in Thai adults: the international collaborative study of cardiovascular disease in Asia. Diabetes Care 2003;26:2758-63
- Aekplakorn W, Chariyalertsak S, Kessomboon P, et al. Prevalence and management of diabetes and metabolic risk factors in Thai adults: the Thai National Health Examination Survey IV, 2009. Diabetes Care 2011;34:1980-5
- Rawdaree P, Ngarmukos C, Deerochanawong C, et al. Thailand diabetes registry (TDR) project: clinical status and long term vascular complications in diabetic patients. J Med Assoc Thai 2006;89(Suppl1):S1-S9
- Bureau of Policy and Strategy, Ministry of Public Health, Thailand. Health Profile Report 2007–2013; August 25, 2015. http://bps2.moph.go.th/ 2015 Aug 25
- Diabetes Association of Thailand, The Endocrine Society of Thailand, Department of Medical Services Ministry of Public Health, National Health Security Office. Clinical Practice Guideline for Diabetes 2014. Bangkok Aroonkarnpim; 2014
- Home P, Fritsche A, Schinzel S, et al. Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with type 2 diabetes using NPH insulin or insulin glargine. Diabetes Obes Metab 2010;12:772-9
- Guillermin A, Samyshkin Y, Wright D, et al. Modeling the lifetime costs of insulin glargine and insulin detemir in type 1 and type 2 diabetes patients in Canada: a meta-analysis and a cost-minimization analysis. J Med Econ 2011;14:207-16
- Valentine W, Goodall G, Aagren M, et al. Evaluating the cost-effectiveness of therapy conversion to insulin detemir in patients with type 2 diabetes in Germany: a modeling study of long-term clinical and cost outcomes. Adv Ther 2008;25:567-84
- Yang L, Christensen T, Sun F, et al. Cost-effectiveness of switching patients with type 2 diabetes from insulin glargine to insulin detemir in chinese setting: a health economic model based on the PREDICTIVE study. Value Health 2012;15(1Suppl):S56-S9
- Palmer A, Roze S, Valentine W, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (type 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(Suppl1):S5-S26
- Palmer A, Roze S, Valentine W, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin 2004;20(Suppl1):S27-S40
- World Health Organization. Life tables by country: Thailand 2014. October 15, 2014. http://apps.who.int/gho/data/?theme=main&vid=61640 2014 Oct 15
- McEwan P, Foos V, Palmer JL, et al. Validation of the IMS CORE Diabetes Model. Value Health 2014;17:714-24
- The Endocrine Society of Thailand. Diabetes Registry Project 2003. Health Systems Research Institute; 2004 Bangkok
- Kosachunhanun N, Benjasuratwong Y, Mongkolsomlit S, et al Thailand diabetes registry project: glycemic control in Thai type 2 diabetes and its relation to hypoglycemic agent usage. J Med Assoc Thai 2006;89(Supp1):S66-S71
- Rangsin R, Tatsanavivat P, MedResNet. An assessment on quality of care among patients diagnosed with type 2 diabetes and hypertension visiting hospitals of Ministry of Public Health and Bangkok Metropolitan Administration in Thailand, 2012. Thailand: National Health Security Office (NHSO); 2012 Bangkok
- National Statistical Office. Health 2007; October 15, 2014. http://web.nso.go.th/en/survey/bts/datafiles/560619_09_Health.pdf 2014 Oct 15
- Thamarangsi T. The situation of alcohol beaverage consumption and impact in Thailand 2013. Center for Alcohol Studies, International Health Policy Program, Ministry of Public Health; 2013 Bangkok
- Krairittichai U, Potisat S, Jongsareejit A, et al. Prevalence and risk factors of diabetic nephropathy among Thai patients with type2 diabetes mellitus. J Med Assoc Thai 2011;94(Suppl2):S1-S5
- Nitiyanant W, Chetthakul T, Sang-A-Kad P, et al. A survey study on diabetes management and complication status in primary care setting in Thailand. J Med Assoc Thai 2007;90:65-71
- Supapluksakul S, Ruamviboonsuk P, Chaowakul W. The prevalence of diabetic retinopathy in Trang province determined by retinal photography and comprehensive eye examination. J Med Assoc Thai 2008;91:716-22
- Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in Thai patients with type 2 diabetes. J Diabetes Complications 2014;28:124-9
- Thaneerat T, Tangwongchai S. Prevalence of depression, hemoglobin A1c level, and associated factors in outpatients with type 2 diabetes. Asian Biomed (Res Rev News) 2009;3:383-90
- Rawdaree P, Sarinnapakorn V, Pattanaungkul S, et al. A prospective, longitudinal, multicenter, observational study to assess insulin treatment patterns in diabetic patients in Thailand: Results from the TITAN study. J Med Assoc Thai 2014;97:1140-50
- Swinnen S, Simon A, Holleman F, et al. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011:CD006383
- Swinnen S, Dain M, Aronson R, et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care 2010;33:1176-8
- Rosenstock J, Davies M, Home P, et al. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408-16
- Raskin P, Gylvin T, Weng W, et al. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev 2009;25:542-8
- Hollander P, Cooper J, Bregnhoi J, et al. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial cmparing insulin detemir with insulin glargine in a basal-bolous regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther 2008;30:1976-87
- Canadian Agency for Drugs and Technologies in Health. Long-acting insulin analogues for the treatment of diabetes mellitus: meta-analyses of clinical outcomes. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health; 2008
- Permsuwan U, Chaiyakunapruk N, Dilokthornsakul P, et al. Long-term cost-effectiveness of insulin glargine versus neutral protamine Hagedorn insulin for type 2 diabetes in Thailand. Appl Health Econ Health Policy 2016;14:281-92
- Drug and Medical Supply Information Center. 2015; April 15, 2016. http://dmsic.moph.go.th 2016 Apr 15
- Riewpaiboon A. Measurement of costs for health economic evaluation. J Med Assoc Thai 2014;97(Suppl5):S17-S26
- Jordan S, Lim L, Seubsman S, et al. Secular changes and predictors of adult height for 86105 male and female members of the Thai Cohort Study born between 1940 and 1990. J Epidemiol Commun Health 2012;66:75-80
- Maharaj Nakorn Chiang Mai Hospital. Pharmacy and Healthcare Service Fees. Chiang Mai; 2014
- Pornpinatepong S. Cost-effectiveness analysis of diabetic retinopathy screening in type2 diabetes mellitus. Master’s Thesis. Bangkok: Mahidol University; 2005
- Riewpaiboon A. Standard cost lists for health technology assessment. Health Intervention and Technology Assessment Program (HITAP); 2011 Nonthaburi, Thailand
- Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health 2007;10:61-72
- King Chulalongkorn Memorial Hospital. Pharmacy and Healthcare Service Fees. Bangkok: King Chulalongkorn Memorial Hospital; 2014
- National Health Security Office. Schedule of health benefits and fees. Bangkok: National Health Security Office; 2014.
- Bureau of Trade and Economics Indices, Ministry of Commerce. CPI 2016; July 8, 2016. www.price.moc.go.th/price/cpi/index_new_e.asp 2016 Jul 8
- Bank of Thailand. Rates of Exchange of Commercial Banks in Bangkon Metropolis (2002–present); January 7, 2016. http://www2.bot.or.th/statistics/ReportPage.aspx?reportID =123&language=eng 2016 Jan 7
- Permsuwan U, Guntawongwan K, Buddhawongsa P. Handling time in economic evaluation studies. J Med Assoc Thai 2014;97(Suppl5):S50-S8
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68
- Health Economic Working Group. The second meeting report of Health Economic Working Group in 2011. Bangkok: Food and Drug Administration, Ministry of Public Health; 2011
- Valentine W, Palmer A, Erny-Albrecht K, et al. Cost-effectiveness of basal insulin from a US Health System perspective: comparative analyses of detemir, glargine, and NPH. Adv Ther 2006;23:191-207
- Teerawattananon Y, Tritasavit N, Suchonwanich N, et al. The use of economic evaluation for guiding the pharmaceutical reimbursement list in Thailand. Z Evid Fortbild Qual Gesundhwesen 2014;108:397-404
- Freemantle N, Chou E, Frois C, et al. Safety and efficacy of insulin glargine 300 u/mL compared with other basal insulin therapies in patients with type 2 diabetes mellitus: a network meta-analysis. BMJ Open 2016;6:e009421