Abstract
Aims: Bringing patients with type 2 diabetes to recommended glycated hemoglobin (HbA1c) treatment targets can reduce the risk of developing diabetes-related complications. The aim of the present analysis was to evaluate the short-term cost-effectiveness of once-daily liraglutide 1.8 mg vs once-daily lixisenatide 20 μg as an add-on to metformin for treatment of type 2 diabetes in the US by assessing the cost per patient achieving HbA1c-focused and composite treatment targets.
Materials and methods: Percentages of patients achieving recommended targets were obtained from the LIRA-LIXI trial, which compared the efficacy and safety of once-daily liraglutide 1.8 mg and once-daily lixisenatide 20 μg as an add-on to metformin in patients with type 2 diabetes failing to achieve glycemic control with metformin. Annual costs were estimated from a healthcare payer perspective. An economic model was developed to evaluate the annual cost per patient achieving target (cost of control) with liraglutide 1.8 mg vs lixisenatide 20 μg for five end-points.
Results: Annual treatment costs were higher with liraglutide 1.8 mg than lixisenatide 20 μg, but this was offset by greater clinical efficacy, and the cost of control was lower with liraglutide 1.8 mg than lixisenatide 20 μg for all five end-points. The annual cost of control was USD 3,850, USD 11,404, USD 3,807, USD 4,299, and USD 6,901 lower for liraglutide 1.8 mg than lixisenatide 20 μg for targets of HbA1c < 7.0%, HbA1c ≤ 6.5%, HbA1c < 7.0% and no weight gain, HbA1c < 7.0% with no weight gain and no confirmed hypoglycemia, and HbA1c < 7.0% with no weight gain and systolic blood pressure <140 mmHg, respectively.
Conclusions: Once-daily liraglutide 1.8 mg was associated with greater clinical efficacy than once-daily lixisenatide 20 μg, which resulted in a lower annual cost of control for HbA1c-focused and composite treatment targets.
Introduction
In 2015, 29.3 million people in the US were estimated to have diabetes, and this is predicted to increase to 35.1 million by 2040Citation1. The direct costs of diabetes are substantial, at USD 320–561 billion in 2015, and are projected to increase to USD 349–621 billion in 2040Citation1. The majority of the total cost is due to diabetes-related complications, and this can be reduced by improving treatment for patients with diabetesCitation2.
The importance of achieving and maintaining glycemic control in patients with type 2 diabetes has been well documented and, therefore, forms the primary goal of treatmentCitation3–5. However, data has also shown that patients benefit from a multi-factorial treatment approach, where treatment also aims to minimize the risk of hypoglycemia, control blood pressure, and reduce or control body weightCitation6–10. Based on this evidence, the American Diabetes Association (ADA) has released a number of treatment guidelines. A glycated hemoglobin (HbA1c) target of <7% is recommended for the majority of patients, with a more stringent target of HbA1c ≤6.5% if this can be achieved without significant adverse effects of treatmentCitation3. A systolic blood pressure target of <140 mmHg is recommended, and it is also stated that the effect of medications on body weight and hypoglycemia risk should be considered when making treatment decisionsCitation11–13.
Glucagon-like peptide-1 (GLP-1) receptor agonists represent an attractive treatment option for patients with type 2 diabetes, as they are associated with reductions in HbA1c, weight loss, and low risk of hypoglycemiaCitation14,Citation15. Liraglutide and lixisenatide are two once-daily GLP-1 receptor agonists currently approved for use in the US.
The aim of the present analysis was to evaluate the short-term cost-effectiveness of liraglutide 1.8 mg vs lixisenatide 20 μg in the US setting for treatment of patients with type 2 diabetes failing to achieve HbA1c targets using metformin monotherapy. The analysis assessed the annual cost per patient achieving HbA1c-focused and composite (capturing hypoglycemia, blood pressure, and body weight) treatment targets.
Methods
Clinical data
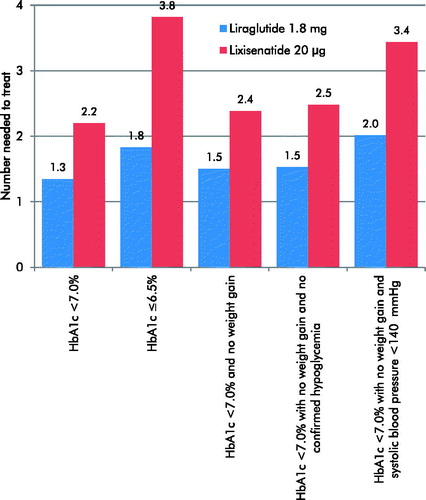
Clinical data used in the analysis were obtained from the LIRA-LIXI trial, which compared the efficacy and safety of once-daily liraglutide 1.8 mg and once-daily lixisenatide 20 μg in patients with type 2 diabetes failing to achieve HbA1c targets using metformin monotherapyCitation16. Pre-specified end-points of the study included the percentage of patients achieving treatment targets of HbA1c < 7.0%, HbA1c ≤6.5%, HbA1c < 7.0% and no weight gain, HbA1c < 7.0% with no weight gain and no confirmed hypoglycemia, and HbA1c < 7.0% with no weight gain and systolic blood pressure <140 mmHg (). The number needed to treat was calculated in terms of the number of patients requiring treatment, with each GLP-1 receptor agonist to bring one patient to each treatment target.
Table 1. Percentage of patients achieving treatment targets.
Cost data
Annual costs were estimated from a healthcare payer perspective in 2017 US dollars (USD). Costs captured in the analysis included the study drug (liraglutide 1.8 mg or lixisenatide 20 μg) and needles for injection (equal in both arms). No other costs, such as the cost of diabetes-associated complications, were included in the analysis. Annual costs were calculated by multiplying the daily cost by 365.25.
Evaluation of cost-effectiveness
The annual cost per patient achieving target (cost of control) was evaluated for the five end-points assessed in the LIRA-LIXI trial. The annual cost of control was calculated using an economic model developed in Microsoft Excel. An example calculation for the cost per patient achieving a target of HbA1c < 7.0% with no weight gain and no confirmed hypoglycemia is shown in . The analysis was performed over a 1-year time horizon and, therefore, no discounting was applied.
Table 2. Example cost of control calculation for HbA1c < 7.0% with no weight gain and no confirmed hypoglycaemia.
Results
Annual cost outcomes
Annual treatment costs per patient were higher with liraglutide 1.8 mg than lixisenatide 20 μg (USD 9,270 vs USD 7,436, respectively).
Number needed to treat
Liraglutide 1.8 mg was associated with a lower number needed to treat to bring one patient to target for all five end-points included in the analysis (). Differences were greatest for the strict glycemic control target of HbA1c ≤ 6.5%.
Cost of control
The annual cost per patient achieving each of the five targets was lower with liraglutide 1.8 mg than lixisenatide 20 μg (). The annual cost per patient achieving the recommended target of HbA1c < 7.0% was USD 12,493 with liraglutide 1.8 mg vs USD 16,343 with lixisenatide 20 μg. Relative to spending USD 1 on liraglutide 1.8 mg, spending of USD 1.31 was required with lixisenatide 20 μg to achieve an equivalent outcome (reflecting a 31% increase in spending to achieve comparable care). When the stricter target of HbA1c ≤6.5% was considered, the difference between the two treatment arms was greater, with liraglutide 1.8 mg associated with an annual cost of control of USD 16,978 compared with USD 28,381 with lixisenatide.
Figure 2. Annual cost of control for liraglutide 1.8 mg vs lixisenatide 20 μg.
HbA1c, glycated hemoglobin; USD, 2017 US dollars.
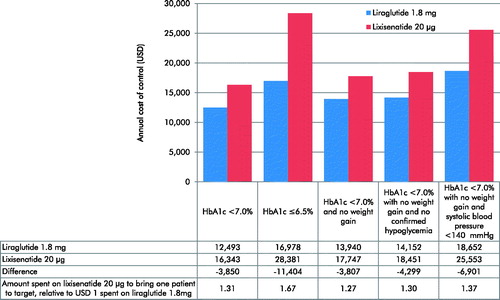
When a treatment target of HbA1c < 7.0% and no weight gain was assessed, the annual cost of control was USD 3,807 lower with liraglutide 1.8 mg than with lixisenatide 20 μg. Inclusion of hypoglycemia in the end-point resulted in a larger benefit, with the annual cost of control USD 4,299 lower with liraglutide 1.8 mg than with lixisenatide 20 μg when a target of HbA1c < 7.0% with no weight gain and no confirmed hypoglycemia was considered. Bringing one patient to a target of HbA1c < 7.0% with no weight gain and no confirmed hypoglycemia required spending of USD 1.30 on lixisenatide for every USD 1 spent on liraglutide 1.8 mg (reflecting a 30% increase in spending to achieve comparable care).
The annual cost per patient reaching the target of HbA1c < 7.0% with no weight gain and systolic blood pressure <140 mmHg was USD 18,652 for patients treated with liraglutide 1.8 mg vs USD 25,553 for patients treated with lixisenatide 20 μg. USD 1.37 would be required to be spent on lixisenatide 20 μg to bring one patient to this composite target, relative to USD 1 spent on liraglutide 1.8 mg.
Discussion
Based on data from the LIRA-LIXI trial, once-daily liraglutide 1.8 mg was more efficacious than once-daily lixisenatide 20 μg (both as an add-on to metformin) in terms of bringing patients with type 2 diabetes not achieving glycemic control targets on metformin to recommended treatment targets. While liraglutide 1.8 mg was associated with increased annual per patient treatment costs, the greater clinical efficacy resulted in a lower cost of control than lixisenatide 20 μg for all five end-points included in the analysis. The greatest differences in cost of control were for composite end-points and the strict glycemic control target of HbA1c ≤6.5%, reflecting the range of benefits that can be derived from treatment with liraglutide 1.8 mg.
An advantage of the present analysis is the simple and transparent approach. Therefore, the analysis can be easily replicated and updated when acquisition costs of interventions change or when new clinical data become available. Analyses conducted using a similar approach to the present analysis have been published previouslyCitation17,Citation18. A further advantage of the present study was the end-points assessed, as all treatment targets were based on guidelines released by the ADACitation11–13. The use of composite end-points capturing HbA1c, hypoglycemia, blood pressure, and body weight allows the multi-factorial benefits of treatment with GLP-1 receptor agonists to be assessed. A multi-factorial treatment approach has been shown to improve outcomes for patients with type 2 diabetesCitation7–9,Citation11.
However, the approach taken is not without limitations. The analysis does not offer a willingness-to-pay context, as the questions of how much a healthcare payer is willing to pay per patient achieving control is open. Therefore, it is not possible to generalize across analyses or therapeutic areas, which is a key advantage of the more conventional approach to cost-effectiveness analysis in which the cost per quality-adjusted life year (QALY) gained is calculated. As such, the approach described is not intended to replace conventional modeling, but to provide complementary information to assist decision-makers over a short-term time horizon. Additionally, the LIRA-LIXI trial was conducted in nine European countries rather than in the US and, therefore, the transferability of the data may represent a potential limitation.
Conclusions
The present analysis assessed the short-term cost-effectiveness of liraglutide 1.8 mg vs lixisenatide 20 μg in patients failing to achieve HbA1c targets using metformin monotherapy in the US setting. The greater clinical efficacy associated with liraglutide 1.8 mg resulted in a lower cost of control compared with lixisenatide 20 μg for all end-points, both HbA1c-focused and composite targets.
Transparency
Declaration of funding
The present cost-effectiveness analysis was supported by funding from Novo Nordisk Inc.
Declaration of financial/other relationships
BH and CM are employees of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk Inc. to support preparation of the analysis. CG and TDT are employees of Novo Nordisk Inc.
Acknowledgments
No assistance in the preparation of this article is to be declared.
References
- International Diabetes Federation. IDF Diabetes Atlas, 7th edition. http://www.diabetesatlas.org/across-the-globe.html. [Last accessed 16 February 2017].
- Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med 2013;45:253-61
- American Diabetes Association. Glycemic targets. Diabetes Care 2017;40(Supp1):S48-S56
- Benhalima K, Standl E, Mathieu C. The importance of glycemic control: how low should we go with HbA1c? Start early, go safe, go low. J Diabetes Complications 2011;25:202-7
- Eldor R, Raz I. The individualized target HbA1c: a new method for improving macrovascular risk and glycemia without hypoglycemia and weight gain. Rev Diabet Stud 2009;6:6-12
- Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383-93
- Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580-91
- Gæde P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016;59:2298-307
- Webb DR, Khunti K, Gray LJ, et al. Intensive multifactorial intervention improves modelled coronary heart disease risk in screen-detected Type 2 diabetes mellitus: a cluster randomized controlled trial. Diabet Med 2012;29:531-40
- Sandbæk A, Griffin SJ, Sharp SJ, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: the ADDITION-Europe Study. Diabetes Care 2014;37:2015-23
- American Diabetes Association. Obesity management for the treatment of type 2 diabetes. Diabetes Care 2017;40(Supp1):S57-S63
- American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care 2017;40(Supp1):S64-S74
- American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care 2017;40(Supp1):S75-S87
- Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 2011;34(Suppl2):S279-S84
- Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 2015;4:212283
- Nauk M, Rizzo M, Johson A, et al. Once-daily liraglutide versus lixisenatide as add-on to metformin in type 2 diabetes: a 26-week randomized controlled clinical trial. Diabetes Care 2016;39:1501-9
- Langer J, Hunt B, Valentine WJ. Evaluating the short-term cost-effectiveness of liraglutide versus sitagliptin in patients with type 2 diabetes failing metformin monotherapy in the United States. J Manag Care Pharm 2013;19:237-46
- Skovgaard R, Jon Ploug U, Hunt B, et al. Evaluating the cost of bringing people with type 2 diabetes mellitus to multiple targets of treatment in Canada. Clin Ther 2015;37:1677-88