Abstract
Aims: This study explored the use of a value-based pricing approach for the new calcimimetic etelcalcetide indicated for the treatment of secondary hyperparathyroidism (SHPT) in patients receiving hemodialysis. It used the US payer perspective and applied the cost-effectiveness framework. Because etelcalcetide is an intravenous therapy that can be titrated for individual patients, and because its utilization is yet to be assessed in real world settings, a range of plausible doses were estimated for etelcalcetide to define a range of prices. These were either in relation to the existing oral calcimimetic cinacalcet or compared to no calcimimetic treatment.
Materials and methods: The value-based price of etelcalcetide was determined via a Markov model. This model combined data from the etelcalcetide trials and previously published cost-effectiveness models in SHPT, and allowed extrapolation of treatment effects on mortality, cardiovascular events, fracture, and parathyroidectomy. Several dosing scenarios were explored covering the dose ranges of 30.0–64.18 mg per day for cinacalcet and 1.07–3.11 mg per day for etelcalcetide. These included the mean dose from the etelcalcetide trials, the preliminary defined daily dose, and the expected most common dose in real world. An acceptable price range for etelcalcetide was assessed by comparing the incremental cost-effectiveness ratios obtained with the willingness-to-pay threshold range of $100,000–$300,000/quality-adjusted life-years.
Results: Cost-effectiveness analysis supported value-based prices for etelcalcetide ranging from $21.15–$49.97/mg vs cinacalcet, and $13.79–$119.45/mg vs no calcimimetics.
Limitations: There is uncertainty around what the real-world dosing will be for etelcalcetide. Another important nuance is that no studies have examined etelcalcetide effects on hard outcomes and, therefore, this modeling exercise relied on an extrapolation approach.
Conclusions: This cost-effectiveness analysis, including scenarios to address uncertainties, allowed estimation of a value-based price range to aid reimbursement decisions in the US.
Introduction
Healthcare providers in the US are increasingly concerned with the rising costs of pharmaceuticals. In a context where prices for brand-name drugs are typically higher in the US than other developed countriesCitation1, US payers increasingly focus on the economic value of treatments in addition to their clinical value. On a practical level, this implies explaining the price based on the value, especially for innovative products, which is similar to the existing practice in the healthcare system of several European countries for some years. An example of this trend is provided by the increasingly influential role played by the Institute for Clinical and Economic Review (ICER), an independent non-profit organization that seeks to improve healthcare value by providing comprehensive clinical and cost-effectiveness analyses of treatments, tests, and procedures. Given this trend, it is important that the price for novel treatments in the US be determined on the basis of their clinical and economic value.
In this study, we aimed to explore the use of a value-based pricing approach for a new calcimimetic etelcalcetide using the framework of the cost-effectiveness analysis. We aimed to compare against (a) the existing oral calcimimetic, cinacalcet, as well as against (b) no calcimimetic treatment, whereas for all three treatment options we assumed that they were in addition to phosphate binders (PB) and vitamin D (VD). These therapies are indicated for the treatment of secondary hyperparathyroidism (SHPT), a disease characterized by the excessive secretion of parathyroid hormone (PTH) and associated with hyperplasia of the parathyroid glands. This disorder is a frequent complication for patients with end stage renal disease (ESRD) receiving hemodialysis therapy. SHPT is linked to the risk of extra skeletal calcification, reduced bone density and strengthCitation2, bone fracturesCitation3, morbidity, and mortalityCitation4–6. SHPT is highly prevalent among the ∼2 million people throughout the world who are receiving dialysis, including 450,000 people in the US. Treatment of SHPT is traditionally accomplished through treatment with an active form of vitamin D and/or phosphate binders. Parathyroidectomy, either with or without auto implantation, is also an optionCitation7. Over the last decade, cinacalcet, a calcimimetic agent, has been added to conventional therapies for this indication. Calcimimetics have been shown to control the key parameters of SHPT (PTH, calcium, and phosphate). Evidence suggests that calcimimetics may have a beneficial impact on long-term outcomes, although it has not been proven definitivelyCitation8,Citation9. Importantly, non-compliance is a major problem with oral calcimimetic in the real world settingsCitation10. Etelcalcetide is a novel peptide calcimimetic for the treatment of SHPT which is administered intravenously three times per week at dialysis sessions. The phase 3 trials for etelcalcetide demonstrated superior control of PTH vs placebo or cinacalcet when added to standard of careCitation11,Citation12. Additionally, treatment with etelcalcetide also reduced levels of calcium, phosphate, and fibroblast growth factor 23 to a greater extent than cinacalcet. Elevated levels of each of these biomarkers has been associated with increased risk of adverse outcomes in patients on dialysisCitation4,Citation13.
The objective of our study was to assess a value-based price range for etelcalcetide by performing a cost-effectiveness analysis on various drug utilization assumptions, and comparing the outcome with commonly applied willingness-to-pay (WTP) thresholds.
Methods
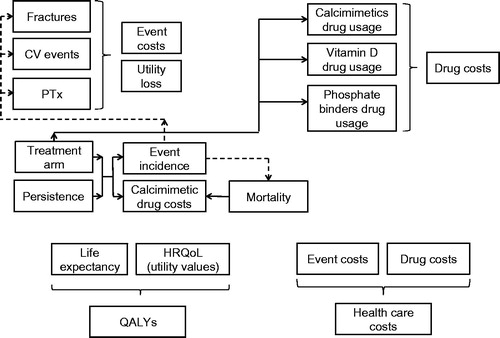
We applied a cost-effectiveness model described elsewhereCitation14 in order to assess various utilization and outcomes scenarios. Briefly, this was a Markov cohort state transition model with 3% discount rates on costs and outcomes. The model allowed the simulation of death, occurrence of cardiovascular events and fractures, and parathyroidectomy over a life-time horizon (). Patients could either be persistent with calcimimetic treatment, or discontinue it. Etelcalcetide, in addition to PB/VD, was compared to cinacalcet (also in addition to PB/VD) and to no calcimimetic treatment, i.e. PB/VD alone. The analysis covered the long-term outcomes produced, i.e. survival and quality-adjusted life-years (QALYs), as well as overall costs (). The rates of clinical events for cinacalcet were derived from the “EValuation Of Cinacalcet Hydrochloride (HCl) Therapy to Lower CardioVascular Events” (EVOLVE) trial using pre-specified analyses adjusted for baseline covariates and drug discontinuationCitation8,Citation15. The lag-censored analyses of hazard ratios (i.e. the follow-up time was censored at 6 months after patients stopped using a study drug) from the EVOLVE study were preferred because they were believed to be more accurate, in view of the fact that the course of the EVOLVE study was characterized by frequent study drug discontinuation and crossing-over between the study’s arms. Because no studies have tested the direct effects of etelcalcetide on clinical outcomes, the rates of clinical events for etelcalcetide were indirectly derived by extrapolating the effects of etelcalcetide on surrogate end-points (i.e. laboratory measures—30% reduction in PTH) from the phase 3 program onto the EVOLVE hazard ratios for cardiovascular events, fractures, parathyroidectomy, and death ()Citation16. The interruption of cinacalcet treatment and switch to the vitamin D and phosphate binders alone was modeled based on parametric extrapolation of real-world persistence dataCitation10. No real-world data is available for etelcalcetide, and no significant persistence difference could be observed within the etelcalcetide head-to-head trial vs cinacalcet. For this reason, we assumed that, in the base case, etelcalcetide and cinacalcet have the same discontinuation profile. The utility values that characterize the health states in the model were derived from a published analysis, where the EQ-5D dimensions were converted into a single utility index using the Dolan algorithmCitation17,Citation18.
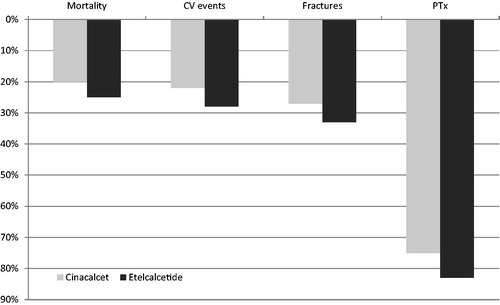
Figure 2. The ability of calcimimetics in persistent subjects to reduce event incidence rates (percent reduction). Based on Belozeroff et al.Citation13 and on Stollenwerk et al.Citation14. CV, cardiovascular; PTx, parathyroidectomy.
The total cost was estimated as the sum of drug consumption (calcimimetics, vitamin D, and phosphate binders), management of clinical events (cardiovascular events, fractures, and parathyroidectomy), and routine monitoring. Drug prices of vitamin D and phosphate binders were based on Red BookCitation13,Citation19. A cinacalcet wholesale acquisition cost (WAC) price of $0.90 per mg was applied. The costs of cardiovascular events, fractures, and parathyroidectomy were derived from a previous health economic analysisCitation13. We combined real-world and trial-based data to inform doses for our cost-effectiveness analysesCitation20, Several studies have demonstrated that the real-world utilization of cinacalcet is substantially different from the doses set in experimental settingsCitation21–23. A similar trend is consequently expected for etelcalcetide, as demonstrated in the open label extension study of the etelcalcetide trials ()Citation24. However, robust real-world data are still unavailable. Therefore, we treated the calcimimetic doses as variations in scenarios for our cost utility analysis. The following dosing scenarios were explored ():
The calcimimetic trial dose. This dose is based on the efficacy assessment phase (EAP) of the etelcalcetide trials, i.e. the period post-calcimimetic titration (Weeks 20–27) of the etelcalcetide trialsCitation11,Citation12;
The defined daily dose (DDD), as published by the World Health Organization (WHO)Citation25. Whereas the cinacalcet DDD is released, the status of the etelcalcetide DDD is temporary, and expected to be implemented in 2018.
The dose which is expected to be most commonly used in real world. This dose could be observed for cinacalcet, and was estimated for etelcalcetide based on the most frequently used dose in the open-label extension study. This coincided with the lowest available dose for each product per their respective labels. Consequently, this scenario illustrated the case where the calcimimetic doses in the real-world practice are used most sparingly. This is a possibility under emerging payment conditions where strong incentives against over-utilization are introduced as part of a payment policy, e.g. US bundling processCitation26.
The value-based price range was determined as the etelcalcetide unit prices that yielded ICERs coinciding with the lower and upper thresholds of a $100,000–$300,000/QALY WTP range. Furthermore, in-between a threshold of $150,000/QALY has been assessed. In the US, there is no commonly agreed WTP, however thresholds in the area ranging from $100,000–$300,000 have been appliedCitation13,Citation27–31.
Table 1. Calcimimetic doses in the scenarios explored.
Results
The value-based pricing approach based on the cost-effectiveness method and a $100,000–$300,000/QALY WTP range supported a unit price for etelcalcetide ranging from $21.15–$49.97/mg vs cinacalcet and $13.79–$119.45/mg vs no calcimimetics, i.e. PB/VD alone (). In the scenario with the highest etelcalcetide utilization, depending on WTP, using trial doses the maximum value based unit price for etelcalcetide ranged from $21.15–$26.97/mg vs cinacalcet and from $13.79–$41.15/mg vs no calcimimetics. When the lowest (most common) doses were used, the value-based etelcalcetide unit price, depending on WTP, ranged from $33.06–$49.97/mg vs cinacalcet, and from $40.04–$119.45 vs no calcimimetics. The health outcome by treatment arm and a cost breakdown is provided in .
Table 2. Value-based price ranges for etelcalcetide, calculated in the dosing scenarios.
Table 3. Health outcomes and cost breakdown by treatment arm.
Discussion
In this study, we used an existing cost-effectiveness model for etelcalcetide, and applied it to the US context in order to estimate a range of value-based prices for etelcalcetide over several dosing scenarios that may be expected in the real world practice in the absence of the real-world utilization data. This value-based pricing approach, based on the cost-effectiveness method, represents a novel framework for exploring the potential value of a new medicine, given the best data available. In doing this, we tested a series of assumptions around dose relationship to describe a range of acceptable, or value-based, prices. This approach is distinct from the perspective normally adopted in a cost-effectiveness analysis. Conventional economic evaluations start from the price of the investigational product and the costs of healthcare resources to assess the cost-effectiveness profile to make a yes/no decision on whether a therapy may be considered cost-effective. In this study, we explored the range of potential dose relationships between the novel therapy and a comparator to estimate the drug prices, leading to the commonly accepted WTP thresholds in a cost-effectiveness model. We believe that our analysis will help inform coverage decisions for the US payers, given the uncertainty around the expected utilization, and, consequently, costs of a new therapy. Lastly, our paper illustrates the tension between clinical trials (internal validity) and real-world trials (external validity), with specific focus on a key element of the economic evaluation such as the drug utilization, and offers a potential analytical solution to bridge this gap.
The design of the economic model we used for this study is in-line with previous calcimimetics cost-effectiveness analyses, as all previous models covered similar types of events, i.e. mortality, cardiovascular events, fractures, and parathyroidectomyCitation4,Citation13,Citation32,Citation33. Furthermore, our model is aligned with the inputs that have been used in previous US-based economic analysesCitation13.
The results of our study suggest that a unit price for etelcalcetide ranging from $21.15–$49.97/mg (vs cinacalcet) or from $13.79–$119.45/mg (vs no calcimimetics) should be an acceptable result for healthcare decision-makers and payers in the US, if compared with a $100,000–$300,000/QALY WTP range. The upper end of the price range is linked to the estimated real-world dosing, around which uncertainty remains. This uncertainty will be reduced by future studies of the real-world utilization and effectiveness.
These value-based price estimates were based on incremental QALY gains of 0.039 vs cinacalcet and of 0.184 vs no calcimimetics. The magnitude of this incremental health gain may appear small, but it reflects (a) the short life expectancy of SHPT patients, which is ∼7 years, and (b) that this (average) health gain is not distributed evenly, but concentrated on patients where one or more events could be avoided. For these patients, the changes can be very meaningful.
While our analysis implied dose equivalence from various sources for the value-based pricing on a population level, these are not to be taken as dose conversion ratios for individual patients, as this would require additional clinical studies. Of note, the actual cost of etelcalcetide will also depend on the reimbursement philosophy applied. In this study, we assumed the same schema for both calcimimetics, with cinacalcet and etelcalcetide being prescribed to the patient and paid for through a combination of patient funds and insurance. The application of a different philosophy may clearly alter the results of the study and influence the conclusions.
Other limitations of this study are linked to the treatment effect size and the economic model used. One important aspect is that the etelcalcetide investigational program did not include testing its effects on clinical outcomes. In the absence of a direct measure of the effectiveness of etelcalcetide on clinical outcomes, the model made use of pre-specified analyses from the prior outcomes study to extrapolate etelcalcetide effects on outcomes, which have been subject to controversyCitation14. However, economic evaluations of cinacalcet have been done using similar indirect approaches, and, thus, we believe this is reasonableCitation27,Citation30,Citation31.
The current analysis depends on a comparison of the efficacy of etelcalcetide compared to other therapies with regards to lowering PTH levels in patients with SHPT on dialysis. Despite the fact that calcimimetics have been found to be very effective in treating SHPT, the mechanisms leading to the efficacy are currently only partially understoodCitation34. Furthermore, the potential benefit of etelcalcetide could be independent of PTH reduction related to concomitant changes in other markers of CKD-MBD. In this regard, reductions in FGF-23 during calcimimetic treatment have been associated with a lower rate of mortality and non-fatal cardiovascular events in the EVOLVE trialCitation35.
A further limitation in our study is that we only varied the calcimimetic drug usage, but not the efficacy observed in the trial. On the one hand, one may argue that a lower dose should yield a lower efficacy. On the other hand, we know that the impact of dosing on efficacy is limited: the ability to lower PTH or to reduce clinical events cannot infinitely be increased by increasing the calcimimetic dose. Clinical trials may have the tendency to over use the investigational product to prove efficacy: First, PTH levels may be decreased beyond the threshold needed to reduce clinical events. For example, in clinical trials a PTH level of 300 pg/mL might be targeted, whereas in clinical practice a threshold of 600 pg/mL may be judged as clinically efficient. Second, calcimimetic drugs in trials may, furthermore, be used beyond their efficacy to lower PTH. Currently there is no empirical evidence that would demonstrate a calcimimetics dose-dependency. The usage of trial drug, however, is expected to over-estimate calcimimetic costs, and, therefore, under-estimate cost-effectiveness. Therefore, it might be valuable to consider alternative dosing scenarios.
A further consideration is related to the assumption of the same persistence and compliance between cinacalcet and etelcalcetide. Due to the IV route of administration it is likely that the real-world persistence and compliance of patients treated with etelcalcetide will be higher. In particular, the IV route of administration may lead to a better control by the general practitioner. Furthermore, it was expected that, compared to cinacalcet, the adverse effects on the gastrointestinal tract could be reduced. However, in the head-to-head trial, neither persistence differences, nor a reduction of gastrointestinal adverse events could be observedCitation12. There is no robust data available to quantify the benefits due to potential persistence and compliance gains. Therefore, for this analysis, potential improvements were not modeled.
Finally, it may be regarded as a limitation that the analysis, despite including background medication costs such as phosphate binders and vitamin D, does not include dialysis costs. This approach is consistent with previous economic evaluationsCitation30, and is based on the fact that the high dialyses costs have been revealed as acceptable from a societal perspective. The inclusion of dialysis costs in cost-effectiveness analysis would panelize any intervention in dialysis patients which increase life-timeCitation36. The approach undertaken here is in line with the UK NICE guidelinesCitation33,Citation37.
The current analyses are based on the cost-effectiveness approach. However, we would like to acknowledge that cost-effectiveness is just one of several methods that might be used to achieve a value-based price range. For example, alternatively the net-health-benefit approach, social value analyses, or other approaches could be used.
We also want to acknowledge that analyses such as the one presented cannot be the only criterion for setting a price, but such analyses are meant to inform the price that may be considered efficient by society at large. Models are never suitable to capture all aspects that should be considered for decision-making.
Conclusions
The value-based pricing approach using the cost-effectiveness method represents a novel framework to provide healthcare decision-makers and payers evidence of the potential value of a new medicine. The application of this method to the case of etelcalcetide, a novel calcimimetic for the treatment of SHPT, yielded the definition of an acceptable price range of $21.15–$49.97/mg (vs cinacalcet) or $13.79–$119.45/mg (vs no calcimimetics), which accounted for the uncertainty around real-world efficacy, utilization, willingness-to-pay, and model assumptions. Further research would be helpful to assess the drug real-world utilization and effectiveness.
Transparency
Declaration of funding
Financial support for this study was provided by Amgen. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. SI received consulting fees for support to the development of the study and for the writing of the manuscript.
Declaration of financial/other interests
BS, KC, and VB are employees and shareholders of Amgen. SI received consulting fees from Amgen, Incyte, Roche, and Boehringer Ingelheim. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
A preliminary version of this study has been presented at the European ISPOR Congress, which took place in Vienna, Austria, in November 2016.
Acknowledgments
We would like to thank Kirsten Westerhout for the helpful discussions around the development of this article.
References
- Bloomberg News. The U.S. pays a lot more for top drugs than other countries; 2015. http://www.bloomberg.com/graphics/2015-drug-prices/ [Last accessed July 2016]
- Goodman WG. The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin Dial 2004;17:209-16
- Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int 2006;70:1358-66
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208-18
- Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 2011;26:1948-55
- Natoli JL, Boer R, Nathanson BH, et al. Is there an association between elevated or low serum levels of phosphorus, parathyroid hormone, and calcium and mortality in patients with end stage renal disease? A meta-analysis. BMC Nephrol 2013;14:88
- Conzo G, Della Pietra C, Tartaglia E, et al. Long-term function of parathyroid subcutaneous autoimplantation after presumed total parathyroidectomy in the treatment of secondary hyperparathyroidism. A clinical retrospective study. Int J Surg 2014;12(Suppl1):S165-S9
- Evolve Trial Investigators, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012;367:2482-94
- Bensink ME, Lozano-Ortega G, Block GA, et al. A Bayesian meta-analysis of clinical and observational studies of cinacalcet and clinical outcomes in secondary hyperparathyroidism. American Society of Nephrology Kidney Week, San Diego (CA); November 3–8, 2015
- Reams BD, et al. Dynamics of cinacalcet use and biochemical control in hemodialysis patients: a retrospective new-user cohort design. BMC Nephrol 2015;16:175
- Block GA, Bushinsky DA, Cunningham J, et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017;317:146-55
- Block GA, Bushinsky DA, Cheng S, et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017;317:156-64
- Gutiérrez OM1, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008;359:584-92
- Stollenwerk B, Iannazzo S, Akehurst R, et al. Assessing the cost-utility of etelcalcetide: a Markov model. ISPOR 19th Annual European Congress; 2016
- Belozeroff V, Chertow GM, Graham CN, et al. Economic evaluation of cinacalcet in the United States: the EVOLVE trial. Value Health 2015;18:1079-87
- Stollenwerk B, Dehmel B, Akehurst R, et al. Modelling etelcalcetide effectiveness on health outcomes: relating biochemical outcomes to mortality, cardiovascular events, fractures and parathyroidectomy. Submitted to: Society for Medical Decision Making (SMDM), 16th Biennial European Conference. London, 14 June 2016
- Briggs AH, Parfrey PS, Khan N, et al. Analyzing health-related quality of life in the EVOLVE trial: the joint impact of treatment and clinical events. Med Decis Making 2016;36:965-72
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108
- Red Book; 2013. www.micromedexsolutions.com. [Last accessed July 2013]
- Garrison Jr LP, Neumann PJ, Erickson P, et al. Using real-world data for coverage and payment decisions: The ISPOR real-world data task force report. Value Health 2007;10:326-35
- Amgen Inc. Data on File, IMS Health; 2015. US data
- ARO – “Analyzing Data, Recognizing Excellence and Optimizing Outcome” database, data on file
- St Peter WL, Li Q, Liu J, et al. Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 2009;4:354-60
- Martin KJ, Block G, Cheng S, et al. Interim analysis of a multicenter single-arm extension study to describe the long-term safety of etelcalcetide (AMG 416) in the treatment of secondary hyperparathyroidism in subjects with chronic kidney disease on hemodialysis. Chicago (IL): American Society of Nephrology (ASN); 2016
- New DDDs. World Health Organization. https://www.whocc.no/atc/lists_of_new_atc_ddds_and_altera/new_ddds/. [Last accessed June 1, 2017]
- Federal Register/Vol. 75, No. 155/Thursday, August 12, 2010/Rules and Regulations. Department of Health and Human Services. Centers for Medicare & Medicaid Services. 42 CFR Parts 410, 413 and 414 [CMS–1418–F] RIN 0938–AP57. Medicare Program; End-Stage Renal Disease Prospective Payment System
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796-7
- Institute for Clinical and Economic Review. PCSK9 inhibitors for treatment of high cholesterol: effectiveness, value, and value-based price benchmarks. Draft report. September 8, 2015. [Last accessed November 2015]
- Boer R, Lalla AM, Belozeroff V. Cost-effectiveness of cinacalcet in secondary hyperparathyroidism in the United States. J Med Econ 2012;15:509-20
- Ubel PA, Berry SR, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood) 2012;31:709-17
- Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist 2006;11:90-5
- Garside R, Pitt M, Anderson R, et al. The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation. Health Technol Assess 2007;11:iii, xi-xiii, 1-167
- Eandi M, Pradelli L, Iannazzo S, et al. Economic evaluation of cinacalcet in the treatment of secondary hyperparathyroidism in Italy. Pharmacoeconomics 2010;28:1041-54
- Conzo G, Perna AF, Napolitano S, et al. Partial response to cinacalcet treatment in a patient with secondary hyperparathyroidism undergoing hemodialysis: a case report. J Med Case Rep 2012;6:417
- Moe SM, Chertow GM, Parfrey PS, et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 2015;132:27-39
- Grima DT, Bernard LM, Dunn ES, et al. Cost-effectiveness analysis of therapies for chronic kidney disease patients on dialysis: a case for excluding dialysis costs. Pharmacoeconomics 2012;30:981-9
- NICE. Guide to the methods of technology appraisal: National Institute for Health and Care Excellence (NICE); 2013