Abstract
Aims: To evaluate the cost-effectiveness of real-time continuous glucose monitoring (CGM) compared to self-monitoring of blood glucose (SMBG) alone in people with type 1 diabetes (T1DM) using multiple daily injections (MDI) from the Canadian societal perspective.
Methods: The IMS CORE Diabetes Model (v.9.0) was used to assess the long-term (50 years) cost-effectiveness of real-time CGM (G5 Mobile CGM System; Dexcom, Inc., San Diego, CA) compared with SMBG alone for a cohort of adults with poorly-controlled T1DM. Treatment effects and baseline characteristics of patients were derived from the DIAMOND randomized controlled clinical trial; all other assumptions and costs were sourced from published research. The accuracy and clinical effectiveness of G5 Mobile CGM is the same as the G4 Platinum CGM used in the DIAMOND randomized clinical trial. Base case assumptions included (a) baseline HbA1c of 8.6%, (b) change in HbA1c of –1.0% for CGM users vs –0.4% for SMBG users, and (c) disutilities of –0.0142 for non-severe hypoglycemic events (NSHEs) and severe hypoglycemic events (SHEs) not requiring medical intervention, and –0.047 for SHEs requiring medical resources. Treatment costs and outcomes were discounted at 1.5% per year.
Results: The incremental cost-effectiveness ratio for the base case G5 Mobile CGM vs SMBG was $33,789 CAD/quality-adjusted life-year (QALY). Sensitivity analyses showed that base case results were most sensitive to changes in percentage reduction in hypoglycemic events and disutilities associated with hypoglycemic events. The base case results were minimally impacted by changes in baseline HbA1c level, incorporation of indirect costs, changes in the discount rate, and baseline utility of patients.
Conclusions: The results of this analysis demonstrate that G5 Mobile CGM is cost-effective within the population of adults with T1DM using MDI, assuming a Canadian willingness-to-pay threshold of $50,000 CAD per QALY.
Introduction
Diabetes is a complex, progressive, and costly disease. There are an estimated 3.4 million people living with diabetes in Canada, and diabetes prevalence is estimated to increase by 44% between 2015–2025Citation1. The economic burden of diabetes was estimated at ∼ CAD 12.2 billion in 2010, accounting for 3.5% of public healthcare spending in Canada, and is expected to increase in the coming years. Direct medical costs accounted for ∼17% of the total diabetes expenditure and costs associated with premature death due to diabetes accounted for about two thirds of the total costCitation2.
Diabetes is a chronic disease with significant long-term costs associated with disease-related complications. People with diabetes are more than 3-times as likely to be hospitalized with cardiovascular disease, 12-times more likely to be hospitalized with end-stage renal disease, and almost 20-times more likely to be hospitalized with non-traumatic lower limb amputations than the general populationCitation3. Diabetes is the leading cause of acquired blindness in Canadians under the age of 50, and diabetic retinopathy affects ∼500,000 CanadiansCitation4,Citation5. Given the chronic nature of diabetes and its high direct and indirect costs, long-term cost-effectiveness analyses are critically important to inform health technology assessment decision-makers regarding the reimbursement/funding for new therapeutic technologies intended to reduce the burden of disease.
Approximately 90% of all the diabetes cases in Canada are type 2 diabetes, and the remaining 10% are type 1 diabetesCitation2. People with type 1 diabetes (T1DM) require life-long treatment with insulin. Proper management of diabetes requires both achieving optimal glycemic control and avoiding hypoglycemia. Long-term follow-up data from studies such as the Diabetes Control and Complications Trial (DCCT) have demonstrated a beneficial effect of improved glycemic control on cardiovascular (CV) outcomes. After ∼11 years follow-up, compared with patients who received conventional diabetes management, patients who were intensively treated during the DCCT experienced a significant (42%) reduction in CV events, as well as a significant (57%) decrease in non-fatal myocardial infarctions, strokes, and CV deathsCitation6. Analyses performed 20 years after the DCCT showed that a mean of 6.5 years of intensive therapy aimed at achieving near-normal glucose levels reduced the risk of development and progression of retinopathy by as much as 76%, and was associated with a modestly lower all-cause mortality rate, compared with conventional therapyCitation7,Citation8.
The Canadian Diabetes Association 2013 Guidelines recommended that therapy for most people with type 1 or type 2 diabetes should be targeted to achieve HbA1c ≤7.0% to reduce the risk of microvascular (retinopathy, nephropathy, and neuropathy) and, if implemented early in the course of disease, macrovascular (angina, myocardial infarction, stroke, peripheral artery disease, and congestive heart failure) complications. To achieve this target, it is recommended that adults with T1DM receive insulin delivered as basal-bolus injections (or use of a pen) or via continuous subcutaneous insulin infusion (CSII) using an insulin pumpCitation9.
Despite advances in diabetes management and treatment, achieving optimal HbA1C levels still remains a challengeCitation10. Multi-center clinical trials, such as the Juvenile Diabetes Research Foundation (JDRF) CGM study and the SWITCH study, have demonstrated the effectiveness of real-time CGM over SMBG in improving glycemic controlCitation11,Citation12. Subsequent research has confirmed the efficacy of standalone real-time CGM when used in patients receiving multiple daily injections (MDI) of insulin to reduce HbA1c and glycemic variabilityCitation13,Citation14. In the recently conducted DIAMOND randomized controlled clinical trial in people with T1DM on multiple daily injections with a mean baseline HbA1c of 8.6%, there was a 1.0% reduction in HbA1c for the CGM group compared with 0.4% reduction in HbA1c for the SMBG group at 24 weeks from baseline (p < .001)Citation13. In the DIAMOND RCT, Dexcom G4 Platinum CGM system (with 505 software) was used which is equivalent to the Dexcom G5 Mobile CGM system in terms of accuracy and performanceCitation15–17.
The Dexcom G5 Mobile CGM System is unique by virtue of its indication for making treatment decisions without fingerstick blood glucose confirmation and integration with a smart phone device which obviates the need for a separate CGM receiverCitation16. These advantages result in less utilization of resources (fewer blood glucose testing strips) and improved patient usability and satisfaction, thus impacting the cost-effectiveness of CGM. Given the clinical benefits of standalone real-time CGM, a cost-effectiveness analysis of G5 Mobile CGM compared to self-monitoring blood glucose (SMBG) was performed from the Canadian societal perspective for adults with T1DM.
Methods
The QuintilesIMS CORE Diabetes Model (CDM; QuintilesIMS Health, Basel, Switzerland) version 9.0 was chosen to perform the cost-effectiveness analyses of Dexcom G5 Mobile compared to SMBG in T1DM patients on multiple daily injections in this assessment. The IMS CDM has been previously used by the National Institute of Health and Care Excellence (NICE)Citation18 and other health technology assessment bodies in their economic evaluations of new technologies for people with T1DM, and is a commonly used model in the literature that has been extensively validatedCitation19.
Model perspective, time-horizon, and discount rate
We used a cohort-based (bootstrap) model simulation over a 50-year time horizon as per convention with 1,000 simulation iterations containing 1,000 patients each; this approach was taken to create robust estimates and minimize errors.
Costs were estimated from the Canadian healthcare perspective. Consequences were expressed in quality-adjusted life years (QALYs) gained. As per Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines for cost-effectiveness analyses in Canada, clinical and cost outcomes were discounted at a rate of 1.5% ()Citation20.
Table 1. Key base case parameter values and sources for IMS CORE modelling.
Model description
The IMS CDM is an internet-based, interactive simulation model that predicts the long-term health outcomes and costs associated with the management of diabetes. The IMS CDM is widely validated, and the latest validation publication from 2014 is the basis for the technical model description provided in this reportCitation19. This description is consistent with the latest version of the model (version 9.0). Given the degree of validation of the model, it was deemed important not to use an alternative model or develop a de novo cost-effectiveness model for this evaluation.
The IMS CDM comprises 17 inter-dependent sub-models, which represent the most common diabetes-related complications: angina pectoris, myocardial infarction (MI), congestive heart failure (CHF), stroke, peripheral vascular disease (PVD), diabetic retinopathy, cataracts, hypoglycemia, diabetic ketoacidosis (DKA), nephropathy, neuropathy, foot ulcer/amputation, macular edema, lactic acidosis (T2DM only), peripheral edema (T2DM only), and depressionCitation19. A sub-model for non-specific mortality is also included. Each of these sub-models is a Markov model that includes different health states reflecting the severity/stage of the complication. Transition probabilities between the states of a complication sub-model can be dependent on time, demographics, health state, physiological factors, and diabetes typeCitation19. The analysis used QuintilesIMS CORE Diabetes model’s default ‘minimum approach’ for quality-adjusted life-year (QALY) estimation. In this approach, the quality-of-life for a patient with multiple complications is assumed to take the minimum of the utility values associated with these complicationsCitation32.
An important limitation of the model is that it is not suitable for modeling long-term outcomes for children or adolescent populations, because the background risk adjustment/risk factor progression equations are all based on adult populations. Hence, we had to limit all our analyses to the adult populationCitation19.
Model inputs and assumptions
Cohort patient characteristics
The DIAMOND clinical trial population had a mean baseline HbA1c of 8.6% (SD = 0.7%) for both the CGM and SMBG groups for people with Type 1 diabetes on multiple daily injections. The mean age of the patients in the clinical trial was 46 years, and the mean duration of diabetes for these patients was 19 yearsCitation13. The patient demographics and clinical characteristics in reflect those in the DIAMOND clinical trial.
Treatment effects
The treatment effects for this analysis were sourced from the DIAMOND RCT. Results from this trial demonstrated a 1.0% reduction in HbA1c for the CGM group compared to 0.4% reduction for the SMBG group at 24 weeks from baseline ()Citation13.
A post-hoc analysis was done of CGM data collected from patients in the DIAMOND RCT, where a 33% median reduction was seen in non-severe hypoglycemic events (NSHEs), which were defined as events with a glucose level <54 mg/dL with a duration of at least 20 minCitation33. By some standards, a hypoglycemic event with blood glucose <54 mg/dl lasting at least 20 min may be considered a severe hypoglycemic eventCitation34. However, for this cost-effectiveness analysis, we conservatively estimated that CGM would result in a 26% reduction in NSHEs, based on published data from earlier generation CGM devices.
The DIAMOND RCT was not designed or powered to detect severe hypoglycemic events (SHEs); therefore, in order to assess the effect of a reduction in severe hypoglycemic events, we conservatively assumed that CGM would result in a 50% reduction of severe hypoglycemia compared to SMBG alone. This is supported by data (83 individuals ≥25 years of age) demonstrating a 46% reduction in the rate of severe hypoglycemia in the “home use” continuation phase following the end-point of the Juvenile Diabetes Research Foundation (JDRF) randomized clinical trial. In this trial, the incidence rate of severe hypoglycemia was 21.8 per 100 person-years for the SMBG group and 7.1 events per 100 person-years for the CGM group in the first and last 6 months, respectively. As more recent CGM devices have demonstrated greater accuracy and are more “user friendly”, we assumed a 50% reduction in severe hypoglycemic events associated with CGM in the base-case analysisCitation24,Citation25. The IN CONTROL RCT, which evaluated the impact of the addition of CGM to MDI or pump in T1DM patients with hypoglycemia unawareness, found that there was a 59% reduction in severe hypoglycemic events for patients in the CGM group compared to the control group of SMBGCitation35.
Sensitivity analyses were performed around reduction in severe and non-severe hypoglycemic events to determine the robustness of the results.
Utilities and costs
The base-line utilities for the T1DM patient cohort and for acute events can be seen in . The utilities associated with each of the diabetes-related complications related health states were sourced from published literature and are available in Supplemental material 1.
According to a 2014 review of utility values in economic modeling for diabetesCitation28, the disutilities associated with acute hypoglycemic events vary widely. The disutilities associated with severe hypoglycemic event (SHE) and non-severe hypoglycemic event (NSHE) from the Marrett et al.Citation36 publication were –0.160 and –0.050, respectively, and the disutilities for SHE and NSHE from the Vexiau et al.Citation37 publication were –0.270 and –0.070, respectively. However, in this analysis (), we conservatively assumed the disutility associated with an SHE event to be –0.047 and with an NSHE to be –0.0142, based on Currie et al.Citation27,Citation28. Several studies have demonstrated that fear of hypoglycemia (FoH) is associated with decreases in health-related quality-of-life (HRQoL)Citation38–45. The Currie et al.Citation27 2006 study modeled the degree of FoH as well as changes in utility, with different levels of self-reported hypoglycemia by severity and frequency.
Only direct costs related to CGM, SMBG, and diabetes-related complications were included. All costs were adjusted to 2016 Canadian dollars (CAD). Costs related to insulin treatment were not included in the analyses, as those were assumed to be equivalent for both groups. The cost of CGM was based on the list price, and was sourced from the manufacturer (). The G5 Mobile CGM system is indicated as a replacement for fingerstick blood glucose testingCitation46. However, G5 Mobile CGM still requires two fingersticks per day for calibration. In this analysis, we conservatively considered 2.3 fingersticks/day for calibration with G5 Mobile CGM (). In the long-term DCCT trial, fingerstick testing was done at least 4-times per day to meet the target HbA1c level in the intensively treated group of diabetes patientsCitation47. In this analysis, we conservatively assumed that patients in the SMBG comparator group use four fingersticks per day for blood glucose testing (). All unit costs for diabetes-related complications were inflated to 2016 values, and were sourced from published literature and are available in Supplementary material 2.
Table 2. Price of Dexcom G5 Mobile CGM (list price).
Table 3. SMBG group list price.
Sensitivity analyses
One-way sensitivity analyses were conducted for key parameters, such as discount rate, baseline HbA1c level, hypoglycemia-related disutilities, HbA1c reduction conferred by CGM vs SMBG, percentage reduction in NSHEs and SHEs, starting utility of patients in the simulation cohort, and fingersticks per day, to determine the robustness of the results. Probabilistic sensitivity analysis was performed to derive the acceptability curve.
Results
The base-case results show that G5 Mobile CGM was associated with an improvement of 3.35 quality adjusted life-years (QALYs) compared to SMBG alone in T1DM adults receiving MDI. The total direct lifetime costs were $339,196 for the G5 Mobile CGM and $225,862 for SMBG alone. The incremental cost-effectiveness ratio (ICER) for G5 Mobile CGM compared to SMBG alone is $33,789/QALY in Canadian dollars (). The mean time to onset for each of the complications can be seen in Supplemental material 3.
Table 4. Base case cost-effectiveness results for G5 Mobile RTCGM vs SMBG alone for individuals with Type 1 diabetes in Canada (list price).
Extensive one-way sensitivity analysis was conducted on key input parameters (). Base-case results were not impacted by a change in the discount rate, baseline starting utility, or baseline starting %HbA1c level. However, in the sensitivity analysis, when the severe hypoglycemic event reduction rate on G5 Mobile CGM compared with SMBG alone is increased from 50% to 75%, the ICER becomes $29,140/QALY gained and,on the other hand, when this rate is decreased to 25%, the ICER becomes $39,662/QALY. Thus, the ICER was moderately impacted by the reduction in severe hypoglycemic events due to G5 Mobile CGM in this analysis. However, when the hypoglycemia-related disutilities were increased and decreased by 50%, it resulted in ICERs of $65,363/QALY gained and $22,783/QALY gained, respectively. Increasing the number of fingersticks used by patients per day in the SMBG group from 4 fingersticks to 6 and 8.2 fingersticks per day improved the cost-effectiveness for the G5 Mobile CGM compared to SMBG alone (). The cost-effectiveness acceptability curve for the G5 Mobile CGM can be seen in .
Figure 1. Probabilistic analysis cost-effectiveness analysis acceptability curve for G5 Mobile vs SMBG from the Canadian perspective.
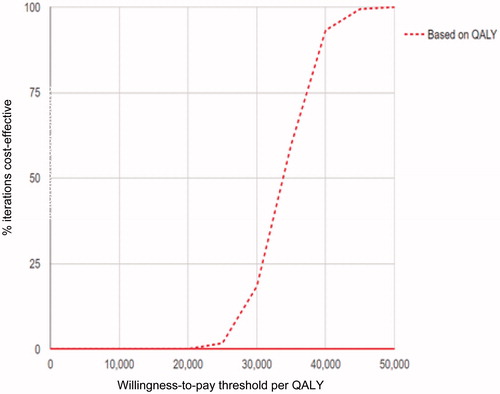
Table 5. Sensitivity analyses (base-case—G5 Mobile CGM vs SMBG alone).
Discussion
Cost-effectiveness analyses are important to consider for health technology assessment decision-making. Healthcare systems should consider the costs to society resulting from the failure to give patients access to CGM, including costs of managing severe and non-severe hypoglycemic episodes, costs of failing to achieve optimal glycemic control, and reductions in quality-of-life experienced by people suffering from diabetes. Costs are difficult to estimate because of the long-term nature of health outcomes in diabetes. CGM technology is improving (in accuracy and usability) at a rate which may make cost-effectiveness analyses obsolete by the time newer data are publishedCitation50. The accuracy of the CGM devices has improved significantly over time. For example, the G5 Mobile CGM system is approved for the replacement of confirmatory self-monitoring blood glucose measurements when making therapeutic decisions in Canada (CGM G5 still requires two fingersticks for calibration)Citation46. Evidence from REPLACE-BG, a multi-center, randomized, non-inferiority clinical trial, demonstrated that the use of the G5 Mobile CGM without confirmatory SMBG is as safe and effective as using CGM adjunctive to SMBG in adults with T1DM and an HbA1c close to targetCitation50. Also, a smart phone (or mobile device) can be used in lieu of the dedicated receiver. Both (1) the reduction in SMBG usage from 4–8-times a day to 2-times a day and (2) the ability to use a CGM with a mobile device instead of a receiver introduce cost savings for the healthcare system and impacts the cost-effectiveness of CGM. The ICER for G4 Platinum CGM that requires a receiver and SMBG confirmation is $40,120 CAD/QALY, compared with $33,789 CAD/QALY for G5 Mobile CGM.
An important aspect of cost-effectiveness analysis is determining the optimal “baseline” utility value and the appropriate set of dis-utilities, and this involves challenges comparable to those for other input parameters. For example, differences in reported utility values may be due to a variety of factors including cohort age, comorbiditiesCitation39, and use of different utility-assessment proceduresCitation40. Sensitivity analysis demonstrates that the results of this analysis were robust to changes in the baseline utility value of people with T1DM. In this analysis, the highest ICER was observed when the hypoglycemia-related disutilities were decreased by 50% (ICER = $65,363 CAD/QALY). Several studies have demonstrated that fear of hypoglycemia (FoH) can impact the health-related quality-of-life in diabetes patientsCitation38,Citation40,Citation42,Citation51. In Canada, 40% of the persons with diabetes are worried about hypoglycemia risks, and this negative impact of the worry about hypoglycemia was found to be independent of the type of diabetes and treatmentCitation52. Recent RCTs such as that in the Diamond clinical trial demonstrate that CGM significantly increases hypoglycemia related confidence compared with SMBG. The most striking group differences were seen in staying safe from serious hypoglycemic problems while sleeping and while drivingCitation53. This improvement in quality-of-life of patients with T1DM due to CGM use is reflected by the incremental quality adjusted life years (QALYS) seen for G5 Mobile compared with SMBG in this analysis. The Currie et al.Citation27 study was considered appropriate for deriving dis-utilities related to fear of hypoglycemia for this analysis, because the objective of the Currie et al.Citation27 study was to model the degree of fear of hypoglycemia experienced by individuals (n = 1305) with T1DM or T2DM (45% on insulin), as well as change in utility with different levels of self-reported hypoglycemia severity and frequency. Given these insights, the greatest value of CGM may be in the high-risk sub-group of patients with an increased risk and frequency of hypoglycemic events. These include sub-groups of people with T1DM, such as those with a history of hypoglycemic eventsCitation54; impaired awareness of hypoglycemia (risk of hypoglycemic events is 6-fold higher)Citation55; and those experiencing nocturnal hypoglycemia (since this is difficult to detect with SMBG)Citation56.
This review of published literature indicates that only two of all published studies assessed the cost-effectiveness of standalone CGMCitation57,Citation58, while the remainder assessed the cost-effectiveness of integrated insulin pump therapy and CGMCitation18,Citation59–62. The two published studies evaluating the cost-effectiveness of standalone CGM were from the US societal perspective, and were based on outcomes from the JDRF trial that included patients who used CGM integrated with an insulin pump. The ICER for the Huang et al.Citation57 study with lifetime time-horizon was $98,679 USD/QALY and for the McQueen et al.Citation58 study with a 33-year time-horizon was $45,033 USD/QALY. Other studies, such as Kamble et al.Citation59, assessed the cost-effectiveness of SAP (sensor augmented pump that includes the addition of two technologies: pump plus CGM) compared with MDI, while Ly et al.Citation60, Roze et al.Citation61 (France) and Roze et al.Citation62 (UK) assessed the cost-effectiveness of SAP (with low glucose suspend feature) compared with standard pump therapy, and the Riemsma et al.Citation18 study was an economic evaluation of integrated sensor augmented pumps.
To our knowledge, the current analysis is the first one to demonstrate the cost-effectiveness of standalone CGM compared with SMBG from the Canadian societal perspective. The base case ICER in the present study is more in line with the ICER reported in McQueen et al.Citation58, and the more favorable ICER seen in our analysis is more likely because of the improved CGM performance over time that results in greater patient trust in their device, promoting better adherence to CGM and sustained improvements in HbA1c.Citation63
Conclusions
The results of this cost-effectiveness analysis demonstrate that the base case incremental cost-effectiveness ratio (ICER) for G5 Mobile CGM compared to SMBG alone is $33,789 CAD/QALY (CI = $33,558–$34,079). The base case ICER is robust to changes in discount rate, baseline HbA1c level, and starting utility of the people with the T1DM cohort. The base case ICER is impacted by the increase in SMBG usage, increase or decrease in hypoglycemia-related disutilities, and increase or decrease in the reduction in the rate of severe hypoglycemic events associated with CGM. The results of this cost-effectiveness analysis conducted with IMS CORE Diabetes model for G5 Mobile CGM compared with SMBG alone in a cohort of people with T1DM over a 50-year time horizon demonstrate that G5 Mobile is a cost-effective intervention at a willingness-to-pay threshold of $50,000 CAD/QALYCitation64.
Transparency
Declaration of funding
This study was supported by funding from Dexcom, Inc.
Declaration of financial/other relationships
At the time of study completion and manuscript development, SC and CG were employees of Dexcom, Inc.
Supplemental material
Download Zip (42.8 KB)Acknowledgments
We would like to thank Dr Amy Bronstone for her editorial support.
References
- Diabetes Statistics in Canada. Diabetes Canada; 2015. http://www.diabetes.ca/how-you-can-help/advocate/why-federal-leadership-is-essential/diabetes-statistics-in-canada. [Last accessed 23 April 2017]
- Canadian Diabetes Association. An economic tsunami the cost of diabetes in Canada; 2009. https://www.diabetes.ca/CDA/media/documents/publications-and-newsletters/advocacy-reports/economic-tsunami-cost-of-diabetes-in-canada-english.pdf. [Last accessed 23 April 2017]
- Public Health Agency of Canada. Diabetes in Canada: Facts and figures from a public health perspective; 2011. http://www.phac-aspc.gc.ca/cd-mc/publications/diabetes-diabete/facts-figures-faits-chiffres-2011/highlights-saillants-eng.php#chp1. [Last accessed 13 January 2017]
- CNIB. About diabetic retinopathy; 2017. http://www.cnib.ca/en/your-eyes/eye-conditions/eye-connect/DR/About/Pages/default.aspx. [Last accessed 13 January 2017]
- CNIB. Eye connect: diabetic retinopathy, 2017. http://www.cnib.ca/en/your-eyes/eye-conditions/eye-connect/DR/Pages/default.aspx. [Last accessed 13 January 2017]
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53
- Lachin JM, White NH, Hainsworth DP, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631-42
- Writing Group for the DERG, Orchard TJ, Nathan DM, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45-53
- Canadian Diabetes Association Clinical Practice Guidelines Expert C, Booth G, Cheng AY. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2013;37(Suppl1):S1-S212
- Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971-8
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464-76
- Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia 2012;55:3155-62
- Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371-8
- Marcus Lind WP, Hirsch IB, Heise T, et al. Continuous glucose monitoring vs. conventional therapy for glycemic control in adults with Type 1 diabetes treated with multiple daily injections The GOLD Randomized Clinical Trial. JAMA 2017;317:1-10
- Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol 2015;9:209-14
- Dexcom G5 Mobile Continuous Glucose Monitoring System User Guide. San Diego, CA: Dexcom, Inc.; 2016. https://dexcompdf.s3.amazonaws.com/G5-Mobile-UG-OUS-ENCA-mmol.pdf. [Last accessed 16 July 2017]
- Dexcom G4 PLATINUM Continuous Glucose Monitoring Systems User Guide. San Diego, CA: Dexcom, Inc.; 2016. https://s3-us-west-2.amazonaws.com/dexcompdf/LBL-012080+G4+PLATINUM+with+Spritz.pdf. [Last accessed 16 July 2017]
- Riemsma R, Corro Ramos I, Birnie R, et al. Integrated sensor-augmented pump therapy systems [the MiniMed(R) Paradigm Veo system and the Vibe and G4(R) PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess 2016;20:v–xxxi,1–251
- McEwan P, Foos V, Palmer JL, et al. Validation of the IMS CORE Diabetes Model. Val Health 2014;17:714-24
- Guidelines for the Economic Evaluation of Health Technologies. Canada; 4th Edition. 2017. https://cadth.ca/guidelines-economic-evaluation-health-technologies-canada-4th-edition. [Last accessed 17 July 2017]
- UK Hypoglycemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140-7
- Foos V, Varol N, Curtis BH, et al. Economic impact of severe and non-severe hypoglycemia in patients with type 1 and type 2 diabetes in the United States. J Med Economics 2015;18:420-32
- Battelino T, Liabat S, Veeze HJ, et al. Routine use of continuous glucose monitoring in 10,501 people with diabetes mellitus. Diabet Med 2015;32:1568-74
- JDRF Continuous Glucose Monitoring Study Group, Bode B, Beck RW, et al. Sustained benefit of continuous glucose monitoring on A1C, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care 2009;32:2047-9
- JDRF Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17-22
- Solli O, Stavem K, Kristiansen IS. Health-related quality of life in diabetes: the associations of complications with EQ-5D scores. Health Qual Life Outcomes 2010;8:18
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523-34
- Beaudet A, Clegg J, Thuressom P-O, et al. Review of utility values for economic modeling in type 2 diabetes. Val Health 2014;17:462-70
- Harris SB, Yale J-F, Chiasson J-L, et al. Out-of-pocket costs of managing hyperglycemia and hypoglycemia in patients with type 1 diabetes and insulin-treated type 2 diabetes. Can J Diabetes 2007;31:25-33
- O’Brien JA, Patrick AR, Caro JJ. Cost of managing complications resulting from type 2 diabetes mellitus in Canada. BMC Health Serv Res 2003;3:7
- CADTH. CADTH Optimal Use report; United Kingdom, 2013
- IMS Health. Cost-Effectiveness analysis of dulaglutide 1.5mg vs. exenatide once weekly using the IMS CORE Diabetes Model in the Slovak setting; 2016. https://kategorizacia.mzsr.sk/Lieky/Download/ObjectionRequestAttachment/1176. [Last accessed 14 July 2017]
- Riddlesworth T, Price D, Cohen N, et al. Hypoglycemic event frequency and the effect of continuous glucose monitoring in adults with type 1 diabetes using multiple daily insulin injections. Diabetes Ther 2017;8:947-951
- International Hypoglycaemia Study G. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40:155-7
- van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol 2016;4:893-902
- Marrett E, Stargardt T, Mavros P, et al. Patient-reported outcomes in a survey of patients treated with oral antihyperglycaemic medications: associations with hypoglycaemia and weight gain. Diabetes Obes Metab 2009;11:1138-44
- Vexiau P, Mavros P, Krishnarajah G, et al. Hypoglycemia in patients with type 2 diabetes treated with a combination of metformin and sulfonylurea therapy in France. Diabetes Obes Metab 2008;10:16-24
- Stargardt T, Gonder-Frederick L, Krobot KJ, et al. Fear of hypoglycaemia: defining a minimum clinically important difference in patients with type 2 diabetes. Health and Quality of Life Outcomes 2009;7:91
- Shingler S, Fordham B, Evans M, et al. Utilities for treatment-related adverse events in type 2 diabetes. J Med Econ 2015;18:45-55
- Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251-65
- Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ 2011;14:646-55
- Gilet H, Gruenberger J-B, Bader G, et al. Demonstrating the burden of hypoglycemia on patients’ quality of life in diabetes clinical trials: measurement considerations for hypoglycemia. Val Health 2012;15:1036-41
- Wild D, von Maltzahn R, Brohan E, et al. A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 2007;68:10-15
- Edelman SV, Blose JS. The impact of nocturnal hypoglycemia on clinical and cost-related issues in patients with type 1 and type 2 diabetes. Diabetes Educ 2014;40:269-79
- Shi L, Shao H, Zhao Y, et al. Is hypoglycemia fear independently associated with health-related quality of life. Health Qual Life Outcomes 2014;12:167
- FDA Press release. FDA expands indication for continuous glucose monitoring system, first to replace fingerstick testing for diabetes treatment decision; 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm534056.htm. [Last accessed 14 July 2017]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977-86
- Ontario Drug Benefit Formulary/Comparative Drug Index; 2014. Edition 42. Ministry of Health and Long-Term Care, Ontario, Canada
- Ontario Public Drug Programs - Reimbursement levels for Blood Glucose Test Strips. Ontario Ministry of Health and Long-term Care 2013. http://www.health.gov.on.ca/en/pro/programs/drugs/teststrips/bg_teststrips.aspx. [Last accessed 23 April 2017]
- Aleppo G, Ruedy KJ, Riddlesworth TD, et al. REPLACE-BG: A randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in well-controlled adults with type 1 diabetes. Diabetes Care 2017;40:538-45
- McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract 2013;19:792-9
- Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med 2013;30:767-77
- Polonsky WH, Hessler D, Ruedy KJ, et al. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017;40:736-41
- Cariou B, Fontaine P, Eschwege E, et al. Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: results from the DIALOG study. Diabetes Metab 2015;41:116-25
- Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697-703
- Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocrine Pract 2010;16:244-8
- Huang ES, O’Grady M, Basu A, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care 2010;33:1269-74
- McQueen RB, Ellis SL, Campbell JD, et al. Cost-effectiveness of continuous glucose monitoring and intensive insulin therapy for type 1 diabetes. Cost Effectiveness and Resource Allocation 2011;9:13
- Kamble S, Schulman KA, Reed SD. Cost-effectiveness of sensor-augmented pump therapy in adults with type 1 diabetes in the United States. Value Health 2012;15:632-8
- Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health 2014;17:561-9
- Roze S, Smith-Palmer J, Valentine W, et al. Cost-effectiveness of sensor-augmented pump therapy with low glucose suspend versus standard insulin pump therapy in two different patient populations with type 1 diabetes in France. Diabetes Technol Ther 2016;18:75-84
- Roze S, Smith-Palmer J, Valentine WJ, et al. Long-term health economic benefits of sensor-augmented pump therapy vs continuous subcutaneous insulin infusion alone in type 1 diabetes: a U.K. perspective. J Med Econ 2016;19:236-42
- Giani E, Snelgrove R, Volkening LK, et al. Continuous glucose monitoring (CGM) adherence in youth with type 1 diabetes: associations with biomedical and psychosocial variables. J Diabetes Sci Technol 2016;11:476-483
- Jaswal A. Canada 2020 analytical commentary No.3: valuing health in Canada. Who, how, and how much? 2013. http://canada2020.ca/wp-content/uploads/2013/06/Canada-2020-Analytical-Commentary-No-3-Valuing-Health-in-Canada-FINAL.pdf. [Last accessed 12 July 2017]
