Abstract
Objective: This study describes the symptom and economic burden associated with brain metastases (BM) in patients with non-small cell lung cancer (NSCLC) receiving epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (EGFR-TKIs).
Methods: This retrospective study included adults with ≥2 medical claims, within 90 days, for lung cancer and ≥1 administration of EGFR-TKIs. Based on ICD-9 codes, patients were stratified into cohorts by type of metastases (BM, other metastases [OM], or no metastases [NM]), and by when the metastasis diagnosis occurred (synchronous or asynchronous).
Results: The population (synchronous BM [SBM] = 24, synchronous OM [SOM] = 23, asynchronous BM [ASBM] = 15, asynchronous OM [ASOM] = 49, NM = 85) was mostly female (57%), average age 69 years (SD = 11). SBM patients experienced more fatigue and nausea/vomiting compared with SOM and NM patients and more headaches and loss of appetite than NM patients. ASBM was associated with more fatigue, nausea/vomiting, headaches, pain/numbness, altered mental status, and seizures than NM, and more headaches and pain/numbness than ASOM. SBM patients experienced a greater increase in per-member-per-month all-cause total healthcare costs after diagnosis ($20,301) vs SOM ($9,131, p = .001) and NM ($2,493, p = .001). ASBM’s cost increase between baseline and follow-up ($7,867) did not differ from ASOM’s ($4,947, p = .195); both were larger than NM ($2,493, p = .001 and p = .009, respectively).
Limitations: EGFR mutation status was inferred based on EGFR-TKI treatment, not by molecular testing. Patients were from US commercial insurance plans; results may not be generalizable to other populations.
Conclusions: Among patients with EGFR-TKI-treated NSCLC, patients with BM experienced more symptoms and, when diagnosed synchronously, had significant increases in total medical costs vs patients with OM and NM. Therapeutic options with central nervous system activity may offer advantages in symptomatology and costs in EGFR-mutated patients with BM.
Introduction
Brain metastases (BM) are found in about a quarter of patients at the time of non-small cell lung cancer (NSCLC) diagnosis, and as many as half of the patients will eventually develop BM during the course of their diseaseCitation1–6. Metastatic NSCLC is difficult to treat in general, and median overall survival for patients with NSCLC who have BM ranges from 3–15 months, depending on the specific diagnosis and treatmentCitation7,Citation8. Furthermore, results suggest that patients who are diagnosed with a brain metastasis at the time of diagnosis of the primary lesion have worse survival than those with a brain metastasis that develops after the lung primary tumorCitation9,Citation10.
The sequelae associated with BM are dependent on the location of the lesion(s) in the brain and the pressure they place on surrounding tissue, and include neurologic symptoms such as fatigue, headaches, pain or numbness, seizures, nausea/vomiting, limb weakness, or appetite disturbances. Studies have reported that BM severely impact a patient’s quality-of-life (physical, cognitive, and functional impairments), as well as result in high healthcare resource utilization and substantial clinical, economic, and caregiver burdenCitation4,Citation11–16. A meta-analysis showed that NSCLC patients with even a single brain metastasis experience the greatest decline in quality-of-life compared with other sites of metastasis, such as those located in the adrenals, liver, or boneCitation17,Citation18.
Patients with epidermal growth factor receptor (EGFR) mutation-positive NSCLC comprise a distinct sub-group of the disease, with clinically relevant mutations found in 7–23% of Western populations and 30–50% of Asian populationsCitation19–24. The literature regarding the association of EGFR-positive status with incidence of BM has been mixed. Studies have shown that patients with EGFR-positive tumors demonstrate a higher incidence of BM at initial diagnosis compared with patients with wild-type EGFRCitation6,Citation25. However, Stanic et al.Citation26 found that, except for a non-significant increase in frequency of BM at diagnosis in patients positive for EGFR mutations, EGFR status had no influence upon the cumulative incidence of BMCitation26. Their study also showed that patients with EGFR-positive tumors had a longer time to central nervous system (CNS) progression, and those with BM at NSCLC diagnosis had longer overall survival, but EGFR status had no influence on time to death in patients who developed BM after NSCLC diagnosisCitation26. Another study found that EGFR mutations in metastatic NSCLC patients were associated with a high likelihood of developing BM, but a higher median survival after BM diagnosis, especially in patients with BM at the time of NSCLC diagnosisCitation27.
EGFR tyrosine kinase inhibitor (EGFR-TKI) therapy is the recommended first-line option in patients with EGFR-sensitizing mutation (exon 19 deletion and exon 21 [L858R] substitution)-positive metastatic NSCLC, providing improved overall response rates and progression-free survival (PFS) compared with chemotherapyCitation28–31. However, ∼ 30% of patients with EGFR-positive NSCLC develop CNS metastases after initial response to EGFR-TKIsCitation32–34. Nevertheless, a study in patients treated with gefitinib or erlotinib first-line showed that the rate of CNS progression was less for the EGFR-TKIs than for upfront chemotherapyCitation32.
Few studies have estimated the burden of BM in NSCLC, and no studies have investigated this in patients treated with EGFR-TKIsCitation35–38. This information could help better manage patient symptoms and assist in developing therapies to address BM in patients with EGFR mutations.
The current study was designed to quantify the symptom and economic burden of BM among patients with NSCLC treated with EGFR-TKI therapies. This study also took into consideration burden based on when the metastasis was diagnosed relative to the initial NSCLC diagnosis (i.e. at the same time as NSCLC diagnosis [synchronous] or if the NSCLC diagnosis preceded the metastasis diagnosis [asynchronous]), as these different groups could have different symptom burden and associated costs.
Method
Data sources
Data from the HealthCore Integrated Research Environment (HIRE) were used to conduct the study. The HIRE is a single commercial payer insurance database that contains claims integrated across data sources and types (i.e. professional claims, facility claims, outpatient pharmacy claims, outpatient laboratory results, and enrollment information). Data are geographically dispersed across the US. HealthCore accesses the data in a manner that complies with federal and state laws and regulations, including those related to privacy and security of individually identifiable health information.
Study design
This retrospective study included patients aged ≥18 years with at least two International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) diagnosis codes for lung cancer (198.3x) within 90 days of each other, and at least one administration of an EGFR-TKI between May 1, 2012 and March 31, 2015. EGFR-TKI therapy could have occurred at any time following the cancer diagnosis, and patients may have received standard chemotherapy. Patients with NSCLC were identified using a validated treatment-based algorithm that excluded those with small cell lung cancerCitation39.
Patients were classified with BM based on the presence of two or more codes for secondary malignant neoplasm of brain and spinal cord (ICD-9 CM 198.3x). The other metastases cohort included patients diagnosed with secondary malignant neoplasm of lymph nodes, respiratory and digestive systems, kidney, urinary organs, skin, nervous system, bone and bone marrow, ovaries, adrenal gland, breast, genital organs, and other unspecified sites (ICD-9 CM 196.0x–196.9x, 197.1x–197.8x, 198.0x–198.7x, 198.81, 198.82, 198.89, 199.xx, 200.xx–208.9x). The no-metastases cohort assignment was defined by the absence of codes for any metastatic condition. In order to include all patients with metastases, the time of metastasis diagnosis was taken into account, and cohorts were further classified as either synchronous or asynchronousCitation5. Synchronous status was based on cancer diagnosis and metastasis diagnosis occurring within ±15 days of each other. Asynchronous status was based on cancer diagnosis occurring at least 30 days before metastasis diagnosis. Thus, the study design contained five cohorts: synchronous BM (SBM), synchronous other metastases (SOM), asynchronous BM (ASBM), asynchronous other metastases (ASOM), and no metastases (NM). provides an overview of the study index dates (NSCLC and metastasis diagnosis dates) and measurement phases for the synchronous metastatic and the non-metastatic control cohorts () and asynchronous metastatic cohorts ().
Figure 1. Study design. (A) No metastases and synchronous (NSCLC and metastasis diagnosis ± 15 days) cohorts. (B) Asynchronous (NSCLC diagnosis before metastasis diagnosis).
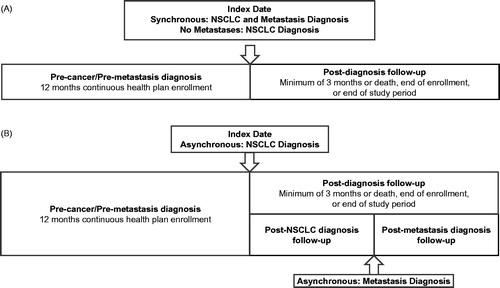
The diagnosis date was defined as the first observed ICD-9 code for diagnosis of lung cancer. Patients were required to have at least 12 months of continuous health plan enrollment prior to and at least 3 months post-diagnosis follow-up after the diagnosis date. For all five cohorts, the post-diagnosis follow-up period continued until the end of the study period, the end of health plan enrollment, or death. In addition, to allow for supplemental analyses, the post-diagnosis follow-up period for the ASBM and ASOM cohorts was divided into two segments: (1) symptoms and costs during the post-NSCLC diagnosis, but before metastasis diagnosis phase, and (2) the post-metastasis phase, as is shown in .
EGFR-TKI status was based on an observation of at least one pharmacy claim for either afatinib (General Product Identifier [GPI] 21534006x) or erlotinib (GPI 21534025x). Gefitinib was not included because, at the time of the study, the US Food and Drug Administration had not yet approved it as a first-line treatment for metastatic EGFR-positive NSCLC. All patients were required to have received EGFR-TKI therapy. For patients with synchronous disease, EGFR-TKI therapy could have occurred at any time during the post-diagnosis follow-up after the conjoint lung cancer and BM diagnosis. For asynchronous disease patients, whose metastasis was diagnosed after their cancer diagnosis, EGFR-TKI therapy was required to occur before the diagnosis of metastasis.
Study measures
Patient demographics and clinical characteristics were collected on the diagnosis date. Comorbidity burden was evaluated using the Quan-Charlson Comorbidity Index (QCI) during the 12-month pre-diagnosis period.
Select symptoms commonly associated with metastasesCitation37 were evaluated during the pre-diagnosis and post-diagnosis follow-up periods (). The number of patients with each symptom was reported for each cohort. Because the length of follow-up varied for each patient, symptoms could not be reported as percentages, but were reported as rates per 1,000 patient-years for the pre-diagnosis and post-diagnosis follow-up periods. For the asynchronous cohorts, the post-diagnosis follow-up period was broken into two time periods (post-NSCLC diagnosis and post-metastasis diagnosis; ), and symptom rates were calculated separately and reported in the supplemental analysis section.
Table 1. ICD-9 (CM) codes for symptoms assessed.
Healthcare costs for total all-cause healthcare (medical plus pharmacy) were assessed during the pre-diagnosis and post-diagnosis follow-up periods. In addition, for the asynchronous cohorts, costs were assessed post-NSCLC diagnosis and post-metastasis diagnosis to permit the supplemental analyses during these separate periods. Due to varying follow-up times, healthcare costs for the pre-diagnosis and post-diagnosis periods were reported in a per-member-per-month (PMPM) metric. All costs were adjusted to 2016 dollars, based on consumer price index information provided by the Bureau of Labor Statistics for Medical Care Services (MCS)Citation40.
Analyses
Statistical significance was set at p < .05. All analyses, except for costs, were conducted using paired comparisons. Continuously scaled demographic variables were assessed using t-tests, while categorical variables were assessed for significance using either the Chi-square test or Fischer’s exact test. A Generalized Linear Model (GLM) approach with a logit distribution and log-link was used to analyze the differences in symptom rates between cohorts at each measurement phase. For symptoms, paired comparisons were conducted among the synchronous cohorts (SBM, SOM, and NM), and a similar approach was used for the asynchronous (ASBM, ASOM, and NM) cohorts. Repeated-measures analyses for costs were conducted using the Generalized Estimating Equation (GEE) approach using a Gamma distribution with log-link. For cost variables, the SBM, SOM, and NM cohorts were compared as a single factor, and ASBM, ASOM, and NM were treated similarly. All costs are reported as least squares values.
The GEE repeated-measures analysis allowed for three main independent comparisons. First, an omnibus test for overall slope (change over time) for all cohorts averaged together was conducted. This provides information about whether costs went up, stayed the same, or went down for all three cohorts between pre-diagnosis and post-diagnosis. The second statistical test assessed the overall cohort × time interaction that determined if the cohorts had different slopes (i.e. different changes in cost trajectories) between pre-diagnosis and post-diagnosis. When significant cohort × time interactions effects were obtained, post-hoc, paired comparisons were conducted to determine which specific cohorts differed from each other in the magnitude of the cost increase. The test for a main effect among cohorts was divided into an overall comparison among the three cohorts at pre-diagnosis and an overall comparison among the cohorts for differences at post-diagnosis. Significant findings for these two sets of comparisons were followed up with pairwise comparisons between each. The statistical tests for the supplemental analyses of ASBM and ASOM were the same as those used for symptom rates and costs in the primary analyses.
Results
Patient characteristics
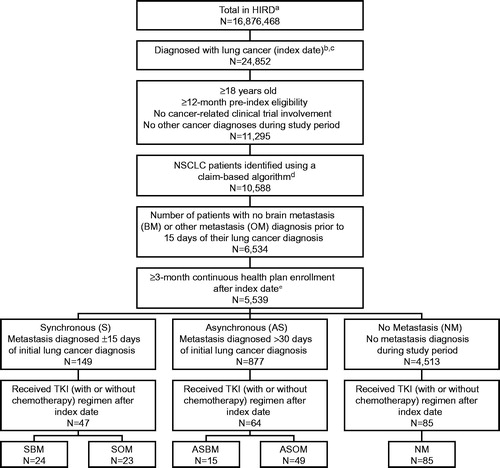
Of 24,852 patients diagnosed with lung cancer during the study period, 5,539 patients met the study inclusion criteria (). Of these, 149 patients had a synchronous diagnosis of NSCLC, with a metastasis diagnosed within ±15 days; 877 patients were classified as asynchronous; and 4,513 patients were diagnosed with NSCLC and did not have a metastasis diagnosed. From these, patients who were treated with a TKI after cancer diagnosis were selected for the study cohorts. There were 47 synchronously diagnosed patients (SBM, n = 24; SOM, n = 23), 64 asynchronously diagnosed patients (ASBM, n = 15; ASOM, n = 49), and 85 patients with no metastases diagnosis during the study observation period.
Figure 2. Study attrition. (a) HIRD, HealthCore Integrated Research Database. (b) ≥ 2 medical claims, within 90 days of each other, with an ICD-9 diagnosis (162.2x–162.5x, 162.8x, 162.9x, 231.2x) at any diagnostic position for lung cancer. (c) The date of the first lung cancer diagnosis was recorded as the patients’ index date. (d) Excluded small-cell lung cancer patients. (e) Patients who died within 3-month post-diagnosis follow-up period were included.
Synchronous cohorts
For the synchronous cohort comparisons, the mean ages of the SBM (60.7 years) and SOM (64.4 years) cohorts were significantly younger (p < .001) than the NM cohort (72.3 years), with no significant differences in gender among cohorts (). However, comorbidity burden as measured by the mean QCI score was significantly higher (p = .003) for the NM cohort (3.5) than for the SOM cohort (1.9), which was significantly higher (p < .001) than the BM cohort (1.0). With regard to health plan type, the SBM cohort had a higher percentage of patients enrolled in consumer-driven health plans (12.5%) than NM (2.4%). There were no significant differences among the cohorts in US region of residence, but NM did have a significantly longer amount of follow-up months (14.6) than did either SBM (11.1) or SOM (10.1).
Table 2. Demographic characteristics.
Asynchronous cohorts
Patients in the ASBM and ASOM cohorts were similar in terms of age (). However, statistically significant differences were observed regarding type of health plan insurance coverage. A higher proportion of the ASBM cohort had consumer-driven health plans (26.7%) compared with the ASOM (4.1%) or with the NM (2.4%) cohorts. In contrast, both the ASOM (73.5%) and NM (74.1%) cohorts had a higher proportion with preferred provider organization coverage than did the ASBM cohort (46.7%; p = .03 and p < .01, respectively). There were no statistically significant differences observed for region of residence or months of follow-up. Finally, there were no significant differences among the asynchronous cohorts for mean scores on the QCI.
Symptoms
Symptoms are reported in rate per 1,000 patient-years. The rates for the synchronous cohorts are reported in and the rates for the asynchronous cohorts are shown in . Of the symptoms assessed (), fatigue, shortness of breath, nausea or vomiting, headaches, pain or numbness, altered mental status, stroke or transient ischemic attack (TIA), anxiety, seizures, and loss of appetite occurred in sufficient frequency to analyze and report.
Figure 3. Symptom rate per 1,000 patient-years for synchronous patients. All significance is at the p ≤ .05 level: (a) NM vs SBM, (b) SBM vs SOM & NM, (c) SBM vs NM.
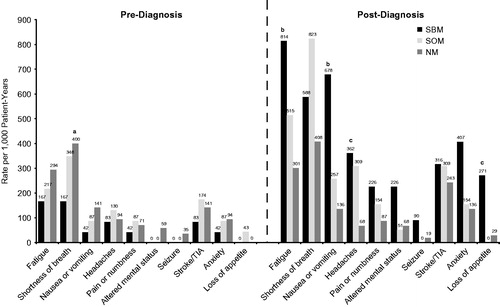
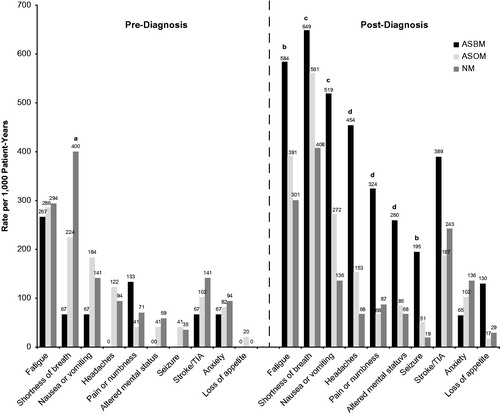
Figure 4. Symptom rate per 1,000 patient-years for asynchronous patients. All significance is at the p ≤ .05 level: (a) NM vs ASBM & ASOM, (b) ASBM vs NM, (c) ASBM & ASOM vs NM, (d) ASBM vs ASOM & NM.
Synchronous cohorts
The rates (per 1,000 patient-years) of symptoms during the pre-diagnosis period were largely similar across the synchronous cohorts and the NM control cohort. Only shortness of breath was significantly higher in the NM cohort (400) compared with the SBM cohort (167; p = .04), as shown in . At follow-up, significant effects were found for fatigue, nausea or vomiting, headaches, and loss of appetite. SBM had a higher rate of fatigue (814) than SOM (515; p = .03) and NM (301; p = .01), and SBM suffered a higher rate per 1,000 patient-years of nausea or vomiting during the follow-up period (678) than did SOM (257; p = .01) and NM (136; p < .001). In addition, SBM experienced a higher rate of headaches (362) than NM (68) (p = .01), but neither SBM nor NM were significantly different on headache rate from SOM (257) (p = .65 and p = .08, respectively). Finally, SBM’s rate of loss of appetite was significantly higher (271) than that for NM (29; p = .01).
Asynchronous cohort
The symptom findings for the asynchronous cohorts are shown in . At baseline, NM had a significantly higher rate of shortness of breath (400) than did either the ASBM (67; p = .01) or the ASOM cohort (224; p = .04). There were no other significant findings during the pre-diagnosis period. During the post-diagnosis follow-up period, patients in the ASBM cohort experienced higher rates of fatigue (584) than did NM (301; p = .05), as well as seizures (195 vs 19; p = .05). ASBM (649) and ASOM (561) experienced a significantly higher rate of shortness of breath than did NM (301; p = .02 and p = .03, respectively), but the ASBM and ASOM cohorts were not significantly different (p = .87). The comparisons for nausea and vomiting indicated that both ASBM (519) and ASOM (272) had significantly higher post-diagnosis rates than NM (136) did (p = .02 and .03, respectively), but that ASBM and ASOM did not differ (p = .09). ASBM had a higher rate of headaches (454) than either ASOM (153; p = .02) or NM (68; p = .0001); ASOM’s rate did not differ from NM (p = .08). ASBM (324) experienced significantly more pain or numbness than ASOM (68; p = .01) and NM (87; p = .01). Finally, ASBM had a significantly higher rate of altered mental status (260) than did ASOM (85; p = .049) and NM (68; p = .03). ASOM and NM rates of altered mental status were not significantly different from each other (p = .69). No statistically significant differences were detected among cohorts for stroke or TIA, anxiety, or loss of appetite.
Healthcare costs
Synchronous cohorts
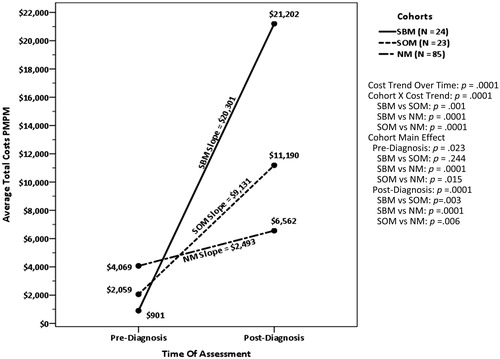
The total healthcare costs for the pre-diagnosis baseline and post-diagnosis follow-up periods for the synchronous cohorts and the NM control are presented in . All values are given in average PMPM 2016 US dollars. During the 12-month pre-diagnosis baseline period, total healthcare costs (medical + prescription costs) were significantly different between SBM ($901) vs NM ($4,069; p = .0001) and SOM ($2,059) vs NM (p = .015). The Time main effect, which reflects the change in costs from pre- to post-diagnosis for the average of all three cohorts, was significant (p = .0001), indicating total PMPM healthcare costs increased following patients’ cancer diagnosis, regardless of metastatic status. The average increase in cost was $10,642. The Time × Cohort effect was also significant, indicating the cohorts differed in the amount of cost increases they incurred over time. Post-hoc tests showed the cost increase for SBM ($20,301) was significantly larger than for SOM ($9,131; p = .001) and NM ($2,493; p = .001). The difference between SOM and NM was also significant (p = .001). As shows, during the post-diagnosis period, SBM ($21,202) had higher final PMPM total healthcare costs than SOM ($11,190; p = .003) and NM ($6,562; p = .001). The post-diagnosis total cost difference between SOM and NM was also significant (p = .006). Post-diagnosis of NSCLC and metastasis, medical costs were the primary drivers of total costs for both SBM (79.5% of total costs) and SOM (65.3% of total costs), but not for NM (33.4% of total costs). The difference between SBM and SOM was not significant (p = .308), but the percentage of total costs attributable to medical care was significantly greater for both BM and OM than for NM (p < .001 for both).
Asynchronous cohorts
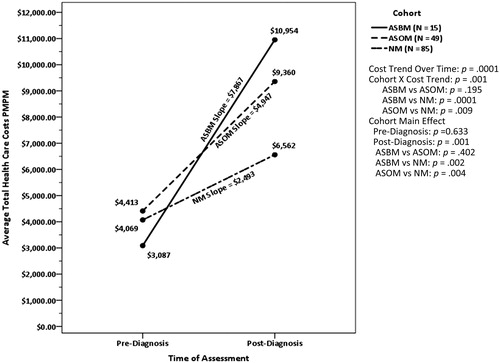
The total healthcare cost outcomes for the asynchronous cohorts and the NM control for the pre-diagnosis baseline and post-diagnosis follow-up periods are presented in . Unlike the synchronous cohorts, no significant differences were detected among the ASBM ($3,087), ASOM ($4,413), and NM ($4,069) cohorts during the 12-month pre-diagnosis period. The Time main effect for the average increase in total healthcare costs across AS cohorts was $5,102 (p = .001), indicating total PMPM healthcare costs increased following patients’ cancer diagnosis, regardless of metastatic status. The Time × Cohort interaction was also significant, as shown in . The slopes for ASBM ($7,867) and ASOM ($4,947) were significantly steeper than for NM ($2,493) (p = .001 and p = .009, respectively). However, the difference in the cost increases for ASBM and ASOM was not significant (p = .195). At follow-up, subsequent to the diagnosis of NSCLC and later metastasis, ASBM ($10,954) and ASOM ($9,360) experienced higher costs of care than NM ($6,562) (p = .002 and p = .004, respectively). However, the contrast between ASBM and ASOM at post-diagnosis (p = .402) did not reach statistical significance. Post-diagnosis of NSCLC and metastasis, medical costs were the primary drivers of total costs for both ASBM (77.5% of total costs) and ASOM (65.7% of total costs), but not for NM (33.4% of total costs). The difference between ASBM and ASOM was not significant (p = .401), but the percentage of total costs attributable to medical care was significantly greater for both BM and OM than for NM (p < .001 for both).
Supplemental analyses of ASBM and ASOM for pre-diagnosis, post-cancer diagnosis, and post-metastasis diagnosis
As described in the Methods section, a supplemental analysis of the follow-up period for the ASBM and ASOM cohorts was conducted. Follow-up was divided into two segments: (1) following the NSCLC diagnosis, but before the metastasis diagnosis, and (2) following the metastasis diagnosis ().
Symptoms
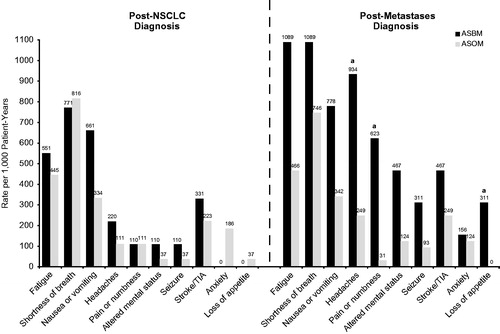
The symptom rates per 1,000 patient-years for the follow-up period divided into the post-cancer diagnosis and post-metastasis diagnosis phases for the AS cohorts are shown in . There were no significant differences between ASBM and ASOM during the post-cancer and pre-metastasis diagnosis phase. However, following the metastasis diagnosis, ASBM patients experienced a significantly higher rate of headaches (934 vs 249 per 1,000 patient-years), pain or numbness (623 vs 31), and loss of appetite (311 vs 3), than did ASOM (p = .01, p < .001, and p = .04, respectively).
Costs
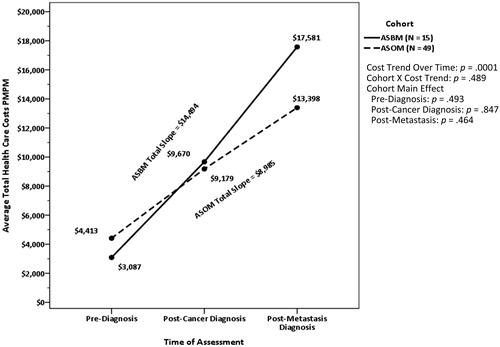
There was a significant Cost-Trend-Over-Time effect for all-cause total healthcare costs over the three assessment periods (p < .001), as displayed in . The total increase in the average PMPM healthcare cost for both cohorts taken together between pre-diagnosis and post-metastasis diagnosis was $11,739. Following the cancer diagnosis, the costs for both cohorts significantly increased over baseline by $5,674 (p < .001). After the metastasis diagnosis, the costs for both cohorts together increased by $6,065, which was also significant (p = .03; the overall values are not shown in the figure). shows the total slope values for each cohort, and even though ASBM’s increase in cost was $5,508 more than ASOM, the difference was not statistically significant (p = .489). There were no other statistically significant findings for healthcare costs within the supplemental analyses. Following the cancer diagnosis, but prior to the diagnosis of metastasis, the medical costs for ASBM (79.5%) and ASOM (72.3%) were again the primary drivers of total costs, and the cohorts were not significantly different from each other (p = .571). Similarly, following the metastasis diagnosis, medical cost contributed the larger percentage to total costs for ASBM (85.0%) and ASOM (75.0%) (p = .418).
Discussion
The results of this real-world observational study demonstrated that, among patients with NSCLC treated with EGFR-TKIs, patients with BM experienced more symptoms and, for synchronous metastases, there was an increase in economic burden compared with those with no and/or other metastases. These results confirm an earlier study of data from an insurance claims database and cancer registry out of central Serbia that found that, at diagnosis, CNS cancers (mainly brain tumors) were the most expensive-to-treat malignancy, although the authors did not differentiate whether these were metastases or primary lesionsCitation41. Even for asynchronous metastases, the cost was significantly higher for ASBM over NM, and was numerically higher over ASOM. The patient population classification is similar to other NSCLC studies of BM that have taken into account the time of metastasis diagnoses relative to initial NSCLC diagnosisCitation5,Citation25,Citation42. In addition, this study ensured that all patients with metastases were captured in the analysis by including both patients with synchronous and asynchronous metastases. This analysis was not designed to compare the differences between the synchronous and asynchronous cohorts, but to explore whether these groups demonstrated any differences in symptom burden and related costs, based on timing of metastasis in relation to lung cancer.
Among patients with synchronous disease, those in the SBM cohort experienced higher rates of fatigue and nausea or vomiting compared with both SOM and NM cohorts during the post-diagnosis follow-up period, and a higher incidence of headaches and loss of appetite than NM. This pattern was similar for the asynchronous patients during the post-diagnosis follow-up period. ASBM suffered significantly higher rates of fatigue, shortness of breath, nausea/vomiting, headaches, pain/numbness, altered mental status, shortness of breath, and seizures than those in the NM group, and more headaches, pain/numbness, and altered mental status than those in the ASOM cohort. The asynchronous follow-up period was divided into a post-NSCLC diagnosis period and a post-metastasis diagnosis period to assess the impact of the development of metastases on symptoms over and above the cancer diagnosis alone. There were no statistically significant differences in symptoms between ASBM and ASOM during the post-cancer diagnosis phase, but ASBM experienced significantly more headaches and pain or numbness than the ASOM cohort after the metastasis diagnosis occurred. These findings are consistent with research by Guerin et al.Citation37 on anaplastic lymphoma kinase (ALK)-positive NSCLC, in which they found that patients experienced a substantially higher number of symptoms after BM diagnosis. In this study, the most frequent symptoms after diagnosis were fatigue (39.0%), shortness of breath (38.0%), nausea or vomiting (33.3%), and headaches (23.9%), which was similar to our findingsCitation37.
Synchronous NSCLC patients with BM incur a significantly higher increase in their total PMPM cost of care compared with the time before cancer and metastasis diagnosis when contrasted with those who developed other metastatic conditions or who did not develop metastases. Specifically, the PMPM all-cause total healthcare cost increase for SBM was $20,301 compared with $9,131 for SOM and $2,493 for NM patients. The cost increase for ASBM was $7,867 over the pre-cancer diagnosis period, which was significantly greater than for NM ($2,493) and $2,920 more than the ASOM increase ($4,947), although not statistically significant. Stronger results for the synchronous cohorts lend support for a greater cost burden for BM patients. During the post-diagnosis follow-up period, for SBM the $21,202 PMPM costs were greater than for SOM ($11,190) and NM ($6,562). The lack of consistent results among the asynchronous cohorts limits the overall conclusion about relative cost burden being higher for BM. During the full post-diagnosis follow-up period, the contrast between ASBM and ASOM did not achieve statistical significance ($10,954–ASBM vs $9,360–ASOM). In addition, when we examined the post-cancer diagnosis and post-metastasis diagnosis periods separately for the asynchronous patients, the increase in the average total cost for both cohorts between pre-diagnosis and post-cancer diagnosis was $5,674, and the increase between post-cancer diagnosis and post-metastasis diagnosis was $6,065. The post-cancer diagnosis costs for ASBM were $9,670 vs $9,179 for ASOM, whereas the post-metastasis diagnosis costs were $17,581 for ASBM and $13,398 for ASOM. Despite the $4,183 PMPM difference in post-metastasis costs between ASBM and ASOM, this did not achieve statistical significance. The failure to achieve statistical significance for the ASBM vs ASOM comparison could be due to the small sample size of the ASBM cohort (n = 15). Although not a focus of the study, it is noted that the SOM and ASOM cohorts also showed an increase in cost burden as compared with NM. This adds a validity to the general conclusions.
One thing to note regarding the cost data is that, for BM and OM (synchronous and asynchronous), medical costs made up two-thirds or more of the total cost spend, but, for those with NM, medical costs made up only one-third of the total cost spend. This might suggest that patients with advanced-stage NSCLC and metastases are not treated as aggressively with chemotherapy as non-metastatic patients. However, while the claims data used in this study fully captured outpatient pharmacy cost, they did not capture inpatient pharmacy utilization and costs. Thus, no firm conclusions can be drawn.
The finding that NSCLC patients on EGFR-TKI with BM incur steep increases in post-metastasis healthcare costs is congruent with other studies. For instance, Ray et al.Citation12 reported that healthcare utilization and PMPM costs after BM diagnosis among lung cancer patients were substantial and in the range of $17,000. In the cohort study conducted by Guérin et al.Citation37, the economic burden of BM among ALK-positive NSCLC, treated with crizotinib, was $5,983 prior to diagnosis of BM, but increased to $22,645 following diagnosis of BM. Our findings are consistent with the previous reports and extend the results to patients receiving EGFR-TKI treatment with SBM. We believe our findings aid in building a consensus that BM lead to increased symptom burden and healthcare costs, irrespective of the molecular sub-types, in lung cancer.
While encouraging, the results from our study are subject to limitations. The data were obtained from administrative claims, which are associated with methodological challenges that may have contributed to undetected errors in codingCitation43. With respect to symptoms, those that were not perceived to fall into a particular ICD-9 code may have been misrepresented or not captured at all on the insurance billing claim form. In addition, symptom-related information on onset or duration that may be clinically relevant was not obtained. Because the guidelines at the time this study was conducted recommended EGFR mutation testing prior to initiating treatment with an EGFR-TKI, the positive EGFR status of the patients in this study was inferred based on their treatment with an EGFR-TKI. However, mutational status for each patient was not available in this database, so it is possible that not all patients were EGFR mutation-positive. Lastly, all patients included in the study were members of large commercial health insurance plans, so these results may not be generalizable to patients with other types of health insurance or to those living outside the US.
Conclusions
The current study demonstrated that both synchronously and asynchronously diagnosed BM, in patients with NSCLC treated with the EGFR-TKIs available at the time of this study, were associated with more symptom burden compared to patients with OM and to those with NM. In addition, patients with BM experienced steeper healthcare costs than those with OM and NM, although statistical significance was only reached in the synchronous cohort. These findings underscore the need for novel approaches to target NSCLC-related BM beyond the EGFR-TKI therapy options available during the study period. For instance, therapies with activity in the CNS may offer advantages for reduced symptoms and cost burden.
While previous studies primarily focused on patients with ALK-positive NSCLC or a non-differentiated patient population, the current findings extend the research to NSCLC patients treated with an EGFR-TKI, which is the recommended first-line therapy for patients with EGFR mutations. Future research comparing clinical and economic disease burden in patients with BM vs those without BM should take into account the distinct molecular sub-types of NSCLC (such as EGFR-positive patients).
Transparency
Declaration of funding
Funding for this study was provided by AstraZeneca.
Declaration of financial/other interests
RMT reports funding from AstraZeneca, during the conduct of the study; and employment from Healthcore, Inc. BW reports funding from AstraZeneca and employment from Healthcore, Inc. during the conduct of the study; BW is a current employee of Janssen Pharmaceuticals, Inc. AWF reports employment from AstraZeneca. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentation
Poster presented at the Academy of Managed Care Pharmacy (AMCP) Nexus, October 3–6, 2016, National Harbor, MD.
Acknowledgments
The authors thank Meredith Rogers, MS, of The Lockwood Group (Stamford, CT) for providing medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE).
References
- Edelman MJ, Belani CP, Socinski MA, et al. Outcomes associated with brain metastases in a three-arm phase III trial of gemcitabine-containing regimens versus paclitaxel plus carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 2010;5:110-16
- Shi AA, Digumarthy SR, Temel JS, et al. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? J Thorac Oncol 2006;1:205-10
- Zietemann V, Duell T. Every-day clinical practice in patients with advanced non-small-cell lung cancer. Lung Cancer 2010;68:273-7
- Sorensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung, risk groups, and prognosis. J Clin Oncol 1988;6:1474-80
- Duell T, Kappler S, Knoferi B, et al. Prevalence and risk factors of brain metastases in patients with newly diagnosed advanced non-small cell lung cancer. Cancer Treat Commun 2015;4:106-12
- Han G, Bi J, Tan W, et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget 2016;7:56998-7010
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25
- Ahluwalia MS, Winkler F. Targeted and immunotherapeutic approaches in brain metastases. ASCO, Chicago, IL: Educational Book, 2015. p 67-74
- Andrews RJ, Gluck DS, Konchingeri RH. Surgical resection of brain metastases from lung cancer. Acta Neurochir (Wien) 1996;138:382-9
- Thomas AJ, Rock JP, Johnson CC, et al. Survival of patients with synchronous brain metastases: an epidemiological study in southeastern Michigan. J Neurosurg 2000;93:927-31
- Nieder C, Norum J, Stemland JG, et al. Resource utilization in patients with brain metastases managed with best supportive care, radiotherapy and/or surgical resection: a Markov analysis. Oncology 2010;78:348-55
- Ray S, Dacosta-Byfield S, Ganguli A, et al. Comparative analysis of survival, treatment, cost and resource use among patients newly diagnosed with brain metastasis by initial primary cancer. J Neurooncol 2013;114:117-25
- Schubart JR, Kinzie MB, Farace E. Caring for the brain tumor patient: family caregiver burden and unmet needs. Neuro Oncol 2008;10:61-72
- Tang V, Rathbone M, Park Dorsay J, et al. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol 2008;255:820-7
- Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys 2008;72:1311-18
- Jakovljevic M, Zugic A, Rankovic A, et al. Radiation therapy remains the key cost driver of oncology inpatient treatment. J Med Econ 2015;18:29-36
- Peters S, Bexelius C, Munk V, et al. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev 2016;45:139-62
- Roughley A, Damonte E, Taylor-Stokes G, et al. Impact of brain metastases on quality of life and estimated life expectancy in patients with advanced non-small cell lung cancer. Value Health 2014;17:A650
- Sekine I, Yamamoto N, Nishio K, et al. Emerging ethnic differences in lung cancer therapy. Br J Cancer 2008;99:1757-62
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46
- D’Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 2011;29:2066-70
- Tomasini P, Serdjebi C, Khobta N, et al. EGFR and KRAS mutations predict the incidence and outcome of brain metastases in non-small cell lung cancer. Int J Mol Sci 2016;17:pii: E2132. doi: 10.3390/ijms17122132
- Stanic K, Zwitter M, Turnsek N, et al. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol 2014;48:173-83
- Baek MY, Ahn HK, Park KR, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med 2016 doi: 10.3904/kjim.2015.158. [Epub ahead of print]
- NCCN. National Comprehensive Cancer Network non-small cell lung cancer version 8.2017. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Fort Washington, PA: NCCN; 2017
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8
- Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42
- Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 2012;18:4406-14
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010;16:5873-82
- Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 2005;103:2344-8
- Lange A, Prenzler A, Frank M, et al. A systematic review of the cost-effectiveness of targeted therapies for metastatic non-small cell lung cancer (NSCLC). BMC Pulm Med 2014;14:192
- Fox KM, Brooks JM, Kim J. Metastatic non-small cell lung cancer: costs associated with disease progression. Am J Manag Care 2008;14:565-71
- Guerin A, Sasane M, Zhang J, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ 2015;18:312-22
- Vera-Llonch M, Weycker D, Glass A, et al. Healthcare costs in patients with metastatic lung cancer receiving chemotherapy. BMC Health Serv Res 2011;11:305
- Duh MS, Reynolds Weiner J, Lefebvre P, et al. Costs associated with intravenous chemotherapy administration in patients with small cell lung cancer: a retrospective claims database analysis. Curr Med Res Opin 2008;24:967-74
- Bureau of Labor Statistics. Consumer price index. Washington, DC: United States Department of Labor; 2016
- Dagovic A, Matter Walstra K, Gutzwiller FS, et al. Resource use and costs of newly diagnosed cancer initial medical care. Eur J Oncol 2014;19:166-84
- Ali A, Goffin JR, Arnold A, et al. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol 2013;20:e300-6
- Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies—report of the ISPOR Task Force on Retrospective Databases. Value Health 2003;6:90-7