Abstract
Aims: Patients with critical limb ischemia (CLI) have an increased risk of major amputation. The initial treatment approach for CLI may significantly impact the subsequent risk of major amputation or death. The objective of this study was to describe the initial treatment approaches of patients with CLI and the limb outcomes associated with each approach.
Methods: Data from MarketScan Commercial and Medicare Supplemental Databases from January 2006–December 2014 was utilized. Cohorts of CLI patients were defined as follows: (1) peripheral vascular intervention (PVI); (2) peripheral vascular surgery (PVS); (3) minor amputation without concomitant PVI or PVS (MinAMP); and (4) Patients without PVI, PVS, or MinAMP (conservative therapy). The odds of major amputation or inpatient death were estimated using the Cox proportional hazards model. For those patients requiring a major amputation, the incremental expenditures per member per month (PMPM) were estimated using a gamma log-link model.
Results: Conservative therapy was associated with significantly higher odds of major amputation or inpatient death compared to patients who underwent minor amputation (1.59-times), PVI (2.08-times), or PVS (2.12-times). Patients treated with an initial strategy of minor amputation also had higher odds of major amputation or inpatient death compared to PVS (1.31-times) or PVI (1.33-times). The estimated incremental expenditures PMPM for patients with a major amputation was $5,165.
Conclusions: Revascularization reduces the risk of a major amputation or inpatient death for patients with CLI when compared to conservative therapy. Major amputation is also associated with significantly higher healthcare expenditures.
Introduction
Critical limb ischemia (CLI) represents that most advanced form of peripheral artery disease (PAD), and is defined as chronic ischemic rest pain, ulcers, or gangrene attributable to arterial occlusive diseaseCitation1,Citation2. Estimates of the prevalence of PAD and CLI vary in the published literatureCitation3,Citation4. In a 2014 retrospective cohort study of insured adults who were at least 40 years of age, the mean annualized prevalence of PAD was 10.69%, and for CLI it was 1.33%Citation5. Patients with CLI are at high risk for either minor or major amputation: a recent population-based study in the UK found that major amputation-free survival rates at 5 years after a diagnosis of CLI were only 27%Citation6. Among people who undergo a major amputation, 5.7% undergo a major amputation in the contralateral limb within 1 year, with the percentage increasing to 11.5% at 5 yearsCitation7. Patients undergoing minor amputation for CLI also have a high risk for subsequent major amputation: at 5 years, 8.4% have had a major amputation of the contralateral limb and 14.2% have had a major amputation on the same limbCitation7. A prior single center study estimated the re-amputation rate at 1 year as high as 27%Citation8.
Amputation among CLI patients impairs quality-of-life and mobility, and is also associated with high subsequent healthcare costs. A study of the cost of amputations reported a median yearly cost of $32,129 (total charge in 2008 USD) with slightly less for a minor amputation ($27,377) and more for a major amputation ($39,512). According to an AHRQ analysis, an average diabetic patient with a lower limb amputation incurs an annual healthcare cost of $52,000 per year (2008 USD)Citation9. Prevention of major amputation, therefore, has the potential to be overall cost-saving to the healthcare system.
Current guidelines recommend revascularization for the majority of patients with CLI, while leaving the method of revascularization to the discretion of the treating physician and care teamCitation10–12. In this study, we describe the outcomes of patients with CLI based on an initial treatment strategy of endovascular intervention, surgical bypass, minor amputation, or conservative management. To address this question, we utilized a large payer database of individuals with commercial and Medicare supplemental insurance to estimate the risk of major amputation or inpatient death based on initial treatment strategy. For those patients undergoing major amputation, the incremental expenditure to the payer per member per month was also estimated.
Methods
Data source
Data for this study were derived from the Truven Health MarketScan Commercial Database (January 2006–December 2014) and the Medicare Supplemental and Coordination of Benefits Database (January 2006–December 2014)Citation13. These resources provide researchers access to fully integrated, de-identified, individual-level healthcare claims data, including complete payment records (insurance payments and patient payments) and specialty pharmacy and mail-order records for individuals covered by a variety of health plans located throughout the United States. Since the MarketScan databases contain inpatient, outpatient, and pharmacy claims, including any claims paid under a coordination-of-benefit arrangement, they provide a complete assessment of patients’ healthcare resource utilization and expenditures. A protocol describing the study was submitted to the New England Institutional Review Board (NEIRB) and deemed exempt from review (NEIRB#16-079).
Selection criteria and cohort definitions
To be eligible for inclusion in this study, patients had to have a minimum of two claims with an ICD-9 diagnosis of PAD. At least one of those claims had to have a diagnosis of atherosclerosis of either native vessels with rest pain, ulceration or gangrene, or bypass graft indicative of a clinical diagnosis of CLI (see Supplemental Table A for the list of ICD-9 diagnosis codes). In addition, patients had to have 6 months of continuous medical and pharmacy enrollment prior to diagnosis of CLI in order to ensure care within the healthcare system, and to improve the specificity of these inclusion criteria.
Patients meeting inclusion criteria were further categorized into the following cohorts based on the initial treatment pattern: (1) Peripheral vascular intervention (PVI); (2) Peripheral vascular surgery (PVS); (3) Minor Amputation without a record of PVI or PVS (MinAMP); and (4) Patients without a record of PVI, PVS, or MinAMP and CLI with gangrene (conservative therapy). PVI and PVS procedures were identified by the ICD-9 codes listed in Supplemental Table B; amputation was identified by the codes listed in Supplemental Table C. Since patients can undergo multiple procedures, rules were put in place for cohort assignment. First, any patient with a record of a peripheral vascular intervention was assigned to the PVI cohort. These patients could have a record of minor amputation and/or surgical revascularization. Second, patients with a record of peripheral vascular surgery without a record of PVI were assigned to the PVS cohort. Of note, the PVS cohort could also have a record of minor amputation, but never a record of PVI. Third, patients with a record of minor amputation (defined as an amputation below the ankle) without a record of either PVI or PVS were assigned to the MinAMP cohort. Finally, patients without a record of PVI, PVS, or MinAMP, and with diagnosis of atherosclerosis of native vessels with gangrene were assigned to the no PVI, PVS, or MinAMP cohort, referred to as conservative therapy, since no major revascularizations or minor amputations were performed on these patients. Patients without a record of PVI, PVS, MinAMP, and no record of having a diagnosis of atherosclerosis of native vessels with gangrene were excluded from this analysis, as they did not meet the criteria for a diagnosis of CLI, and may have had non-healing ulcerations due to other causes (e.g. primary infection).
Descriptive statistics
Outcome variables used in this analysis included a composite end-point for a patient having a major amputation or inpatient death, and total healthcare expenditures per patient per month. Inpatient death was defined as a record of death for any reason. Major amputation was defined as a record of amputation above the ankle (see Supplemental Table C for codes used to define major amputation). Healthcare expenditures included all inpatient and outpatient (emergency room, office visits, laboratory, radiology, and other procedures) services.
Every patient meeting inclusion and exclusion criteria within each cohort: (1) PVI, (2) PVS, (3) MinAMP, and (4) conservative therapy, were summarized prior to multivariable modeling by patient demographics, comorbid conditions, and risk factors for CLI. (see Supplemental Table D for ICD-9 Codes) The frequency of comorbidities and risk factors were determined based on ICD-9 codes. The Elixhauser Comorbidity Index (ECI) was used to calculate a composite comorbidity score for each cohortCitation14. The ECI score includes 31 categories of comorbidities that are associated with mortality (see Supplemental Table E for ICD-9 codes that comprise the Elixhauser categories). Continuous variables are presented as the mean and standard deviation; while categorical variables are summarized with the count and percentage in each category.
Multivariable models
The relative risk of major amputation or inpatient death for each treatment cohort was estimated using the proportional hazard Cox’s regression model. The Cox model was used because not all patients have the same follow-up for the outcome variable and, therefore, have different times at risk for the event. Furthermore, the Cox’s hazard regression model can be generalized to include time-dependent covariates, whose values for any given individual change over time. The multivariable Cox’s hazard model evaluated the association of PVI, PVS, and MinAMP treatments with the risk of our clinical composite (major amputation or death). Multivariable models were adjusted for patient demographics and comorbid conditions. Confidence intervals are reported graphically, where if the confidence interval includes 1, the hazard ratio is not statistically significant.
After the first diagnosis of CLI, average monthly healthcare expenditures (per patient per month) for patients with and without a major amputation were estimated with a generalized linear regression model with the gamma distribution log-link function. The log-gamma model was chosen because the outcome variable, healthcare expenditures, is often right-skewed. This model takes into account the distribution of the outcome variable without the need for complex retransformation procedures (e.g. natural logarithmic transformation). Since individual patients had different follow-up times upon which expenditures are calculated, enrollment months were included in the model as an offset. Two separate expenditure models were run with and without outpatient drug claims.
Results
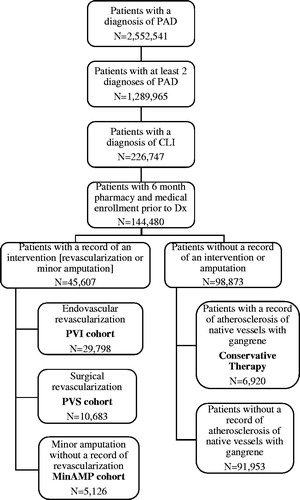
A total of 2,552,541 patients had at least one diagnosis of PAD. Of these PAD patients, ∼50% (1,289,965) met the more stringent criteria of having a minimum of two or more records with a diagnosis of PAD, and 226,747 (9%) had a record of CLI. Among those, 52,527 patients met the additional inclusion criteria of having either PVI, PVS, MinAMP, or no record of a PVI, PVS, or MinAMP with CLI. Final counts for each cohort were as follows: PVI patients = 29,798 (56.7%); PVS patients = 10,683 (20.3%), MinAMP patients = 5,126 (9.8%), conservative therapy = 6,920 (13.1%). The cohort description is shown in .
Figure 1. Attrition diagram. CLI, critical limb ischemia; MinAMP, Minor Amputation without a record of PVI or PVS; PAD, peripheral artery disease; PVI, Peripheral vascular interventions; PVS, Peripheral vascular surgery.
Patient demographics for the overall cohort and each sub-group are summarized in . The overall mean age was 68.6 years. Patients managed conservatively were older than the other sub-groups (average age of 70.9 years). The percentage of males ranges from 67.9% in the MinAMP group to 57.1% in the conservative therapy group. The majority of patients had Medicare insurance (59.7% overall), with the highest regional representation coming from the South, at 34.4% overall.
Table 1. Patient demographics.
The ECI, as well as the underlying comorbidity categories and risk factors for PAD, are summarized in . Patients managed conservatively had higher comorbidity scores than the PVI and PVS groups, but were similar to the MinAMP group, with an average score of 5.8 vs PVI 4.6; PVS 4.1; and MinAMP 5.4. Across all groups the risk factors for PAD, such as diabetes, chronic hypertension, chronic renal insufficiency, smoking, aortocoronary bypass status, and history of stroke were all highly prevalent. The highest prevalence of diabetes was in patients undergoing initial minor amputations ().
Table 2. Patient comorbidities.
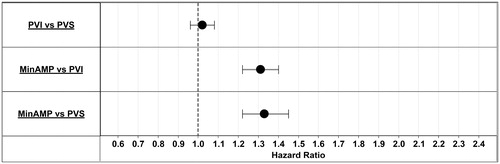
While adjusting for patient demographics and comorbid conditions, hazard ratios for the composite endpoint of inpatient death or major amputation were estimated for each intervention using the conservative therapy cohort as the reference. A patient was at higher risk of a major amputation or death when an intervention was not performed (). When patients without revascularization or minor amputation were compared to patients having minor amputations or revascularizations, the risk of a major amputation or inpatient death ranged from 1.59–2.12-times higher (conservative therapy vs MinAMP 1.59-times more at risk; conservative therapy vs PVI 2.08-times more at risk, and conservative therapy vs PVS 2.12-times more at risk). shows hazard ratios by intervention. Although there was no significant difference in risk between patients having a surgical (PVS) vs an endovascular revascularization (PVI); a primary strategy of minor amputation was associated with a higher risk of major amputation or death compared to endovascular or surgical revascularization (1.31- and 1.33-times more risk, respectively).
Figure 2. Hazard ratios by cohort. Hazard ratios for the composite endpoint of inpatient death or major amputation were estimated for each cohort using the conservative therapy cohort as the reference. MinAMP, Minor amputation without a record of PVI or PVS; PAD, peripheral artery disease; PVI, Peripheral vascular interventions; PVS, Peripheral vascular surgery.
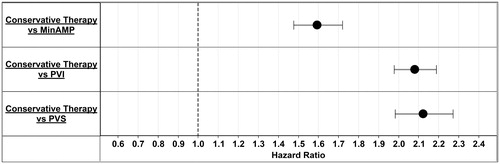
Figure 3. Hazard ratios by intervention. Hazard ratios for the composite end-point of inpatient death or major amputation were estimated for each intervention. MinAMP, Minor Amputation without a record of PVI or PVS; PAD, peripheral artery disease; PVI, Peripheral vascular interventions; PVS, Peripheral vascular surgery.
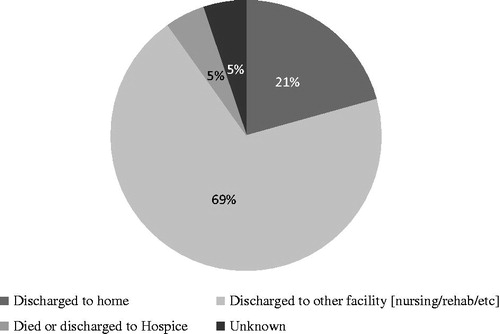
Most patients were not discharged to home after a major amputation. After a major amputation, 5% of patients died as an inpatient, 69% were transferred to another inpatient facility; and only 21% were discharged to home ().
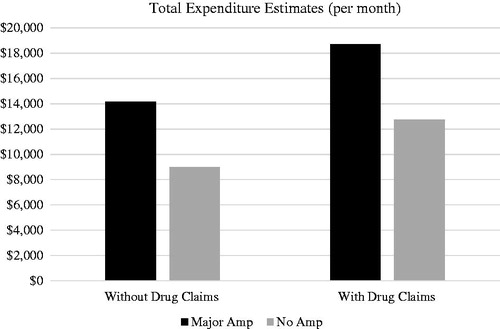
After all model adjustments, patients undergoing major amputations had significantly higher expenditures than those that did not require major amputation (p < .0001). Patients with a major amputation incurred an estimated additional ∼$5K per month in healthcare expenditures (). These expenditures included healthcare utilization from inpatient, outpatient, office visits, lab, and radiology visits. When pharmacy claims were included, the healthcare expenditure estimates increased to ∼$6K per month per patient with a major amputation.
Discussion
This study utilized a large payer database of individuals with commercial or Medicare supplemental insurance to estimate the relative risk of major amputation or inpatient death for CLI patients treated with endovascular intervention, lower extremity bypass surgery, or minor amputation vs patients with CLI who did not undergo any revascularization or minor amputation (conservative therapy). This study also estimated the incremental per patient per month healthcare burden after a CLI patient has a major amputation. Our study results indicated that a CLI patient is at higher risk of a major amputation or inpatient death when no intervention (surgery, endovascular intervention, or minor amputation) occurs. For those CLI patients undergoing an intervention, minor amputations without a revascularization procedure were associated with significantly more risk (∼1.3-times higher) for subsequent major amputation or death. When estimating the monthly per patient expenditures for CLI patients, our study found that patients undergoing major amputation have significantly higher expenditures than those that do not, with a difference of ∼$5K per month on average for healthcare utilization including inpatient, outpatient, office visits, lab, and radiology, and ∼$6K per month when outpatient pharmacy claims were included. Together, these findings emphasize the utility of intervention among patients presenting with CLI, both in preventing major amputation and minimizing the cost and subsequent morbidity associated with major amputation.
The majority of patients included in this cohort underwent some initial revascularization, with endovascular intervention (57%) more common than initial surgical bypass (20%), while the remaining 23% of patients were not offered revascularization but were instead managed with minor amputation or conservative therapy. These findings are consistent with recent studies demonstrating an increased utilization of endovascular intervention as a first-line approach for treatment of CLI, given the less invasive nature of such an approach and outcomes similar to bypass surgery in selected circumstancesCitation15. Interestingly, a recent study utilizing the nationwide inpatient sample demonstrated that there has been a significant increase in endovascular revascularization from 2003–2011, with an associated reduction in in-hospital mortality and major amputation over that time periodCitation16. A recent study from the Vascular Quality Initiative also found that endovascular revascularization was more frequently performed than surgical bypass, although there is significant variation in the percentage of cases performed at each siteCitation17,Citation18. Our findings confirm the increased trend toward utilization of endovascular intervention as a first-line therapy for the treatment of CLI, especially among patients with increased comorbidities.
Few studies have examined the initial treatment of patients with CLI among an overall representative cohort. In a recent German registry (CRITISCH) that included 27 vascular tertiary care centers, the initial treatment for CLI included endovascular intervention in 53%, bypass surgery in 24%, femoral endarterectomy in 10.5%, conservative treatment in 9.8%, and primary amputation in 2.5%Citation19. The overall revascularization rates in this cohort (77%) are lower than in the CRITISCH registry; these differences may be explained by a wider inclusion of all hospitals, as opposed to tertiary vascular centers. Medicare-based studies have also shown that a significant percentage of patients do not undergo lower extremity testing with ABIs or imaging prior to major amputation; it is possible that some patients in this cohort were not recognized clinically as having CLI, and were, therefore, not offered revascularizationCitation20. Importantly, studies of the regional intensity of revascularization rates have suggested that increased rates of revascularization are associated with low rates of major amputationCitation21,Citation22. Quality measures that ensure vascular consultation and/or lower extremity hemodynamic testing among patients with possible CLI, therefore, have the potential to identify patients who could benefit from revascularization, and thereby lower the overall rates of major amputation among patients with CLI.
Our study also found that, among patients with CLI, major amputation was associated with significantly increased healthcare costs, thus rendering it a less favorable 1st line strategy. Recent studies have suggested that revascularization is cost-effective compared to initial wound care alone among patients with CLI. Although the procedural costs of endovascular intervention are often higher than surgical bypass, the lower hospital costs and earlier patient discharge with endovascular intervention make the overall procedural costs comparableCitation23. A sub-study of the REACH registry also demonstrated that the annual costs of patients with prior major amputation are highCitation24. Given that the lifetime burden of a major amputation has been estimated to cost over $800,000, and that lower extremity amputations account for over 2.8 billion dollars of healthcare expenditures in the US per year, a cost-effective approach to revascularization, and especially recognition of the CLI earlier in the disease process, has the potential to decrease overall costs to the healthcare systemCitation25,Citation26.
Certain limitations exist to this study. First, claims-based data was used to identify patients with CLI. Administrative claims data are collected for the purpose of billing and reimbursement, not for coordinating medical care or conducting outcomes research. The data are subject to coding errors and under-reporting of clinical conditions that do not trigger a billable event. Laboratory results and physician notes are also absent, so specific medical details cannot be determined. Despite these shortcomings, administrative data have been widely used to evaluate the association between treatments and clinical outcomes, particularly when a portrayal of patient experience outside the controlled setting of the clinical trial is useful, such as in the case of PAD patients with CLI. Second, these data are limited to patients with healthcare insurance, and may not be generalizable to non-insured populations. Third, our data was not linked to long-term outcomes. It is well described that patients with CLI have high rates of re-admission as well as long-term mortality; the costs of these events could not be accurately estimated. However, these data provide a meaningful cross-sectional measure of CLI management and outcomes, based on initial treatment strategies.
Conclusion
This analysis of claims data included more than 2.5 million PAD patients, of which, 226,747 had a diagnosis of CLI. We found that patients with CLI have a significantly higher risk of a major amputation or inpatient death when no intervention occurs as part of the initial management. Furthermore, when minor amputations are performed without revascularization of the lower limb, CLI patients were at significantly higher risk (1.3-times higher) for subsequent major amputation or death. When estimating the monthly per patient healthcare burden to the payer for CLI patients having a major amputation, our study found that major amputations cost the payer ∼$5K to ∼$6K more per patient per month. In our study of a national healthcare database, we found that revascularization significantly reduces the risk of a major amputation or inpatient death for patients with CLI. More consistent guidelines are needed to enforce revascularization efforts for PAD patients suffering from critical limb ischemia.
Transparency
Declaration of funding
This study was sponsored by Cardiovascular Systems, Inc.
Declaration of financial/other interests
E.J. Armstrong is a consultant to Abbott Vascular, Boston Scientific, Cardiovascular Systems, Medtronic, and Spectranetics. B.J. Martinsen and H. Kotlarz are employees of and own stock in Cardiovascular Systems, Inc., the study sponsor. M.P. Ryan, E.R. Baker, and C. Gunnarsson are employees of CTI Clinical Trial and Consulting Services, which is a paid consultant to Cardiovascular Systems, Inc., the study sponsor.
SUPPLEMENTAL_MATERIAL.docx
Download MS Word (18.1 KB)Acknowledgments
No assistance in the preparation of this article is to be declared. The manuscript has not been submitted elsewhere, nor published elsewhere in whole or in part, except as an abstract.
References
- Foley TR, Armstrong EJ, Waldo SW. Contemporary evaluation and management of lower extremity peripheral artery disease. Heart 2016;102:1436-41
- Shishehbor MH, White CJ, Gray BH, et al. Critical limb ischemia: an expert statement. J Am Coll Cardiol 2016;68:2002-15
- Yost ML. Critical limb ischemia. United States epidemiology. Prevalence, market opportunities and analysis of the two most common comorbidities: diabetes and chronic kidney disease. Atlanta (GA): The Sage Group; 2010
- Yost ML. The diabetes method. A population-based method to estimate the prevalence of peripheral artery disease by age and glucose status. Atlanta (GA): The Sage Group; 2010
- Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg 2014;60:686-95, e682
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation 2015;132:1805-15
- Glaser JD, Bensley RP, Hurks R, et al. Fate of the contralateral limb after lower extremity amputation. J Vasc Surg 2013;58:1571-7, e1571
- Jindeel A, Narahara KA. Nontraumatic amputation: incidence and cost analysis. Int J Low Extrem Wounds 2012;11:177-9
- Margolis DJ, Malay DS, Hoffstad OJ, et al. Economic burden of diabetic foot ulcers and amputations. Diabetic Foot Ulcers. Data Points #3 (prepared by the University of Pennsylvania DEcIDE Center, under Contract No. HHSA290200500411). Rockville, MD: Agency for Healthcare Research and Quality; January 2011. AHRQ Publication No. 10(11)-EHC009-2-EF
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;69:e71-e126
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45(Suppl S):S5-S67
- Tendera M, Aboyans V, Bartelink ML, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851-906
- Hansen LG, Chang S. White Paper. Health research data for the real world: the MarketScan databases. Truven Health Analytics; 2012
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8-27
- Goodney PP, Tarulli M, Faerber AE, et al. Fifteen-year trends in lower limb amputation, revascularization, and preventive measures among medicare patients. JAMA Surg 2015;150:84-6
- Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003–2011. J Am Coll Cardiol 2016;67:1901-13
- Siracuse JJ, Menard MT, Eslami MH, et al. Comparison of open and endovascular treatment of patients with critical limb ischemia in the Vascular Quality Initiative. J Vasc Surg 2016;63:958-65, e951
- Soden PA, Zettervall SL, Curran T, et al. Regional variation in patient selection and treatment for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg 2017;65:108-18
- Bisdas T, Borowski M, Torsello G. Current practice of first-line treatment strategies in patients with critical limb ischemia. J Vasc Surg 2015;62:965-73, e963
- Vemulapalli S, Greiner MA, Jones WS, et al. Peripheral arterial testing before lower extremity amputation among Medicare beneficiaries, 2000 to 2010. Circ Cardiovasc Qual Outcomes 2014;7:142-50
- Goodney PP, Holman K, Henke PK, et al. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg 2013;57:1471-9, 1480, e1471–3; discussion 1479–80
- Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes 2012;5:94-102
- Barshes NR, Chambers JD, Cohen J, et al. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg 2012;56:1015-24, e1011
- Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes 2010;3:642-51
- MacKenzie EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am 2007;89:1685-92
- Elixhauser A, Andrews RM. Profile of inpatient operating room procedures in US hospitals in 2007. Arch Surg 2010;145:1201-8