Abstract
Aims: This study aimed to evaluate the economic value for leuprorelin acetate 6-month depot compared with leuprorelin acetate 3-month depot from a societal perspective in Japanese prostate cancer patients.
Methods: The cost analysis estimated the reduction in direct and indirect costs as well as intangible costs saved by having one less injection. Claims data were used for the analyses of direct and indirect costs reduction. A discrete choice experiment based on a web-based survey estimated the monetary value of the intangible costs for one injection. Another web-based survey of prostate cancer patients, who had received treatment with leuprorelin acetate injections, was carried out to calibrate the results of the discrete choice experiment.
Results: Reductions in medical costs and loss of productivity for having one less injection in prostate cancer patients receiving leuprorelin acetate were JPY 5,670 and JPY 1,723, respectively. Intangible costs saved by using a 6-month depot formulation instead of a 3-month depot formulation for the injection of leuprorelin acetate were estimated to be JPY 19,872, including the values for a reduction in pain (JPY 3,131), injection site reactions (JPY 11,545), waiting time (JPY 9,479), and subtracting the value of medical consultation (JPY 4,283). The total cost reduction for having one less injection was JPY 27,265.
Limitations: The respondents from the internet panel provided by a survey company are not necessarily a representative population of Japanese society.
Conclusions: Leuprorelin acetate 6-month depot has an advantage in monetary value in the reduction in medical costs, loss of productivity, and intangible costs for having one less injection in prostate cancer patients compared with leuprorelin acetate 3-month depot. In the costs for treating with leuprorelin acetate, the percentage of intangible costs might not be negligible. The intangible costs will probably be actively evaluated to proceed to patient-centered healthcare in society.
Introduction
Prostate cancer is the most commonly diagnosed cancer in males in 87 countries according to GLOBOCAN 2012Citation1,Citation2. Approximately 1.1 million males worldwide were diagnosed with prostate cancer in 2012Citation2, and 12.9% of males are expected to be diagnosed with prostate cancer during their lifetimeCitation3. The risk of developing prostate cancer increases with age, and the majority (60%) of newly-diagnosed patients are older than 65 yearsCitation3. The average age of prostate cancer patients in Japan is 72.5 yearsCitation4. As prostate cancer is relatively slow-growing, it needs long-term continuing care, leading to high medical costs. As the population ages, the number of patients with prostate cancer has been steadily increasing, and the associated costs have also been increasingCitation5. In the US, the total medical costs of prostate cancer were estimated to be $12 billion in 2010, which is the fifth highest in national expenditures for cancer careCitation5. Therefore, prostate cancer is an important public health issue from the viewpoint of both personal and economic burdensCitation6. Hormone therapy, which is also called androgen deprivation therapy, is used for patients with metastatic or recurrent prostate cancer, and sometimes it is combined with radiotherapy. In Japan, luteinizing hormone-releasing hormone (LH-RH) agonists either as monotherapy or in combination regimens with anti-androgen drugs are most commonly used for hormone therapyCitation7,Citation8. One of the currently available LH–RH agonists, leuprorelin acetate, is prescribed as a long-acting depot formulation with different durations of action. Currently, 1-, 3-, and 6-month depot formulations are available for the treatment of prostate cancerCitation9. The 6-month depot formulation has recently been launched in Japan, and is expected to decrease the burden on patients by reducing the frequency of injections compared with those with shorter acting durationsCitation9.
It has been reported that the 3-month and 6-month depot formulations are both equally effective in suppressing serum testosterone levels in patients with prostate cancerCitation10. Regarding safety, frequencies of adverse events and tolerability are not significantly different between the two treatment optionsCitation10. Therefore, the 6-month depot formulation is considered to be comparable with the 3-month depot formulation in both efficacy and safety. As patients can skip one injection by using a 6-month depot formulation instead of a 3-month depot formulation, the 6-month depot formulation has many potential advantages for the patients. Patients using the 6-month depot formulation would visit hospitals less frequently, which would save time, as well as out-of-pocket costs associated with medical examinations. In addition, patients would be free from pain and injection site reactions which often accompany the use of injections. However, patients view a visit to a physician as having valueCitation11. In this case, having one less injection could be perceived as less beneficial for the patient.
Recently, economic evaluation has been widely adopted for healthcare assessment, and has become increasingly important for both the payers and society in the evaluation of healthcare outcomesCitation12–14. In cost analysis, not only direct costs, but also indirect and intangible costs, such as loss of productivity and the emotional costs of the patients, are consideredCitation15. In the case of leuprorelin acetate, direct costs include fees for medical examinations, indirect costs include loss of productivity of patients and attendants, and intangible costs include pain, injection site reactions, and mental burden of patients, such as waiting for a doctor’s consultation. These intangible factors are important in order to achieve “patient-centered” medical care. However, such analyses have not been conducted in the evaluation of prostate cancer treatments.
The aim of this study was to evaluate the costs saved by having one less injection of leuprorelin acetate in prostate cancer patients. In this study, we conducted a discrete choice experiment to assess intangible costs to patients, such as pain, injection site reactions, and waiting time. We calculated direct medical costs as well as indirect medical costs caused by loss of productivity of patients by using real world settings. By combining the results obtained, we evaluated the total cost saved by having one less injection of leuprorelin acetate in Japanese prostate cancer patients.
Methods
Study design
We conducted a cost analysis for having one less injection of leuprorelin acetate in Japanese prostate cancer patients to evaluate the costs of leuprorelin acetate 6-month depot formulation compared with the 3-month depot formulation. The cost analysis consisted of three elements: direct costs reduction, indirect costs reduction, and intangible costs saving. Direct costs were defined as direct medical costs, and did not include long-term care costs. Indirect costs included the loss of productivity, and did not include transportation fee or the loss of productivity for a carer. Loss of productivity for a carer was not included, because there were only a few respondents who required a carer. Transportation fee was not included because of the variation in the distance from patients’ homes to the treating hospital. Intangible costs included the monetary value for patients’ emotional value, such as pain and injection site reactions. Claims data and a Medical Fee Schedule Table were used for the analyses of reduction in direct costs. Claims data and the Japanese Basic Survey on Wage Structure in 2016Citation16 were used for the analyses of indirect costs. The Medical Fee Schedule Table is standardized under the social health-insurance system that covers all Japanese citizens, and we used this table. Outpatient care in our study was applied to this payment system. The fee schedule was nationally standardized, with negotiations between providers and payers, and the fee will be revised by considering actual costs. Japanese guidelines of economic evaluations of healthcare recommend using the Medical Fee Schedule Table in payment for medical services as guidance for medical costsCitation17. A discrete choice experiment was conducted to estimate monetary value in the intangible costs for a general population. Another survey on prostate cancer patients who had experienced injections of leuprorelin acetate was carried out to calibrate the results of the discrete choice experiment. We estimated the intangible costs for one injection of leuprorelin acetate by fitting the results of emotional value for patients with injections of leuprorelin acetate in a calibration survey to the results of the monetary value of the emotional value due to injection in the discrete choice experiment.
The study was approved by the Ethics Committee, Research Institute of Healthcare Data Science (RI2016005), and registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN000024759).
Data source and participants
For the discrete choice experiment and its calibration, the respondents were included in an online survey panel managed by ANTERIO Inc. (Tokyo, Japan). The respondents for the discrete choice experiment in a general population were Japanese men aged ≥50 years who had received more than one injection in a hospital in the past year. Injections included vaccination, medication, anesthesia, blood donation or other reasons taking part in a hospital in the past year. In the discrete choice experiments’ survey, we sampled 313 men of the general population (respondents without prostate cancer) randomly selected from an internet panel which included 7.5 million people. We also sampled 108 prostate cancer patients, preferentially including those with hormone therapy, from an internet panel. The response rate was 72.3% in the discrete choice experiment. In the calibration survey, we sampled prostate cancer patients with injections of leuprorelin acetate from an internet panel. The web-based surveys were conducted from November 2016 to January 2017.
A claims database from April 2008 to November 2016 provided by Medical Data Vision (Tokyo, Japan) was used to analyze the direct and indirect cost reductions. The prostate cancer patients were identified and selected according to the diagnosis under the International Classification of Diseases 10th revision (ICD-10) code C61Citation18.
Survey for a discrete choice experiment
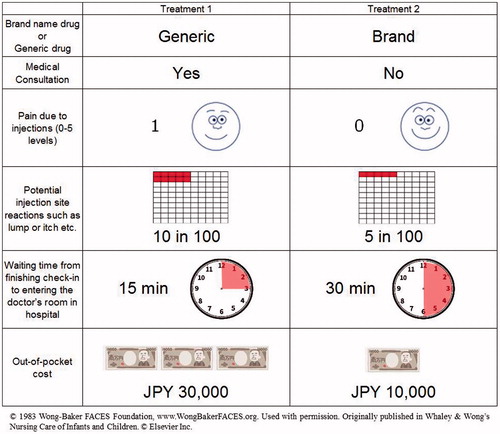
A discrete choice experiment was conducted to estimate the intangible costs saved for one injection. In the discrete choice experiment, six attributes and their levels were selected after a discussion with physicians. shows the attributes: drugs (brand-name or generic), medical consultation by doctors, pain due to injection, frequency of injection site reactions, and waiting time from finishing check-in to entering the doctor’s room in a hospital, and the level for each attribute. The Wong-Baker FACES Pain Rating Scale was used to evaluate the pain due to injection. In addition, the out-of-pocket payment was included as an attribute to calculate the monetary values of intangible costs for one injection from the answer for the attributes. The orthogonal design resulted in the creation of 27 choice tasks from a total of 648 possible combinations. In choice task questions, respondents were asked to choose which treatment would be preferred between two options. A representative example of choice task questions is shown in . Each respondent completed 20 choice tasks. The survey for choice task questions was performed via the internet.
Table 1. Attributes and attribute levels in discrete choice experiment.
Survey for calibration
A survey for prostate cancer patients who have received injections of leuprorelin acetate was conducted to estimate the intangible costs of having one less injection from the results of the discrete choice experiment. The respondents for the calibration survey were asked the following four questions. They had to answer by selecting one option from a list for questions 1 and 2, and particular numbers for questions 3 and 4. Questions and possible answers included: Question 1: Have you consulted a doctor? (possible answers: every time, almost every time (∼ 80%), one in two, few (∼ 20%), never); Question 2: How did you feel your pain due to injection? (0, 1, 2, 3, 4, 5 level of Wong-Baker FACES Pain Rating Scale); Question 3: How many times did you have injection site reactions in 100 injections?; Question 4: How long did you wait from finishing check-in to entering the doctor’s room in the hospital?
Estimation of direct and indirect costs
To analyze the direct costs for having one less injection of leuprorelin acetate, we identified two profiles of prostate cancer patients who had been treated with injection of leuprorelin acetate 6-month depot. One patient had filed no claim at 3 months after injection of leuprorelin acetate 6-month depot. The other patient filed some claims at 3 months after injection of leuprorelin acetate 6-month depot. The patients who were prescribed leuprorelin acetate 6-month depot formulation and 3-month depot formulation were identified by having the claim receipt named LEUPLIN PRO for injection kit 22.5 mg and LEUPLIN SR for injection kit 11.25 mg, respectively. The medical costs included a follow-up consultation fee, medical costs related to diagnosis, and medical costs related to injection (these are described in ). For prostate cancer patients receiving the leuprorelin acetate 6-month depot formulation, all medical costs would be reduced if they had not had a hospital visit and, if a visit were required, then only the medical costs related to injection would be reduced. These medical costs were estimated as follows: (1) identified medical activities found in the guidelines on prostate cancerCitation8 for those patients who receive leuprorelin acetate 6-month depot formulation and had no hospital visits at 3 months after the injection, (2) assumed a claim was filed corresponding to the medical activities in step 1, (3) calculated the medical cost corresponding to the claim from the Medical Fee Schedule Table, and (4) evaluated the medical cost reductions by using the weighted average value in both patients.
Table 2. Medical cost reduction for prostate cancer patients with injections of leuprorelin acetate.
For the indirect costs, we analyzed productivity loss in patients. According to the Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical CouncilCitation17, we assumed that the monthly average wage for prostate cancer patients prescribed leuprorelin acetate 3-month depot formulation was JPY 304,000. This value is the average wage across all industries, all ages, and for both genders obtained from the Japanese Basic Survey on Wage Structure in 2016Citation19. Estimated time for productivity loss was assumed to be one half day within a 30 day period. The indirect cost reductions were evaluated by using the weighted average value from the monetary values in patients with or without receiving medical service at 3 months after the leuprorelin acetate injection.
Statistical analysis
We applied a conditional logit model to analyze the data of choice task questions in the discrete choice experiment. The model reveals the hidden utility behind each level of each attribute by the inverse estimation, based on each choice of the two choice task cards. In the conditional logit model, the utility function is assumed to be the same among people. The ratio of the coefficient of each attribute to the coefficient of out-of-pocket attribute is the monetary value of each level of each attribute. This ratio is marginal rate of substation (MRS) in economics and reflects willingness-to-pay (WTP) for the change of an attributeCitation20. From the results of the survey for calibration, we calculated the average value of each attribute in one injection of leuprorelin acetate and, then, we estimated the intangible costs for having one less injection of leuprorelin acetate.
We conducted sensitivity analyses on the intangible costs to evaluate the difference among patient sub-groups which were the respondents with or without prostate cancer, those with prostate cancer treated with hormone therapy, and those aged 50–59, 60–69, and ≥70 years. We conducted sensitivity analyses to estimate both the total cost saved for having one less injection of leuprorelin acetate by changing the percentage of prostate cancer patients with medical service 3 months after the leuprorelin acetate injection and for having one less injection of leuprorelin acetate.
Cross-sectional analysis was carried out to analyze the direct and indirect costs reduction.
Results
Patient identification
The respondents for the discrete choice experiment were 421 men, who consisted of 313 men without prostate cancer and 108 men with prostate cancer. Among the prostate cancer patients, 87 individuals had received injection for hormone therapy. The numbers of all respondents, including prostate cancer patients, in each age group of 50–59, 60–69, and ≥70 years, were 194, 146, and 81, respectively. The respondents for the calibrations were 42 prostate cancer patients, who had received injections of leuprorelin acetate. The mean (standard deviation, SD) age of the respondents without prostate cancer in the discrete choice experiments was 58.9 years (7.1). The mean age of the respondents in the calibration survey was 69.0 years (7.3).
We identified 37,058 and 2,857 prostate cancer patients with prescribed leuprorelin acetate 3-month depot and 6-month depot, respectively, in the Medical Data Vision database. The mean (SD) age of the patients with leuprorelin acetate 3-month depot and 6-month depot was 77.1 years (7.5) and 79.2 years (7.4), respectively.
Intangible costs for one injection by discrete choice experiment
Intangible costs for having one injection in each attribute of the discrete choice experiment were estimated from Logit model’s coefficients (Supplementary Table 1). The amount of intangible costs for reducing the frequency of injection site reactions from 10 to one in 100 injections was the highest among all the attributes, which means that respondents were willing to pay the most out-of-pocket costs for reducing the frequency of injection site reactions from 10 to one in 100 injections ().
Table 3. Intangible costs for one injection by discrete choice experiment.
indicates the average value of each attribute for one injection of leuprorelin acetate among the patients who had experienced the therapy with leuprorelin acetate. Based on these results, intangible costs saved for having one less injection of leuprorelin acetate was estimated to be JPY 19,872 () including the value in reducing pain (JPY 3,131), injection site reactions (JPY 11,545), waiting time (JPY 9,479), and subtracting the value of medical consultation (JPY 4,283).
Table 4. Intangible costs saved for having one less injection in prostate cancer patients with injections of leuprorelin acetate. Note that intangible costs saved for having one less injection were calculated by the results of discrete choice experiment on respondents without prostate cancer.
To estimate the costs from the society perspective in primary results, we selected the respondents without prostate cancer as a general population. We next performed sensitivity analysis to evaluate the difference in intangible costs between patients’ and societal perspective. Sensitivity analyses indicated that the amount of intangible costs for one injection in respondents with and without prostate cancer was JPY 24,934 and JPY 19,872, respectively ().
Table 5. Sensitivity analysis of intangible costs saved for having one less injection in prostate cancer patients with injections of leuprorelin acetate.
Total costs for having one less injection of leuprorelin acetate
We analyzed the reduction in direct medical costs by having one less injection in prostate cancer patients receiving treatment with leuprorelin acetate injections 6-month depot. For 34% of patients, who did not receive medical service at 3 months after injections, the medical cost reduction was JPY 10,620 (). The cost for 66% of patients, who received medical service at 3 months, was JPY 3,120. Thus, reduction in direct costs for having one less injection in prostate cancer patients receiving treatment with leuprorelin acetate injections was JPY 5,670.
We also analyzed the reduction in indirect costs by having one less injection in prostate cancer patients receiving treatment with leuprorelin acetate 6-month depot injections. The reduction of productivity loss for prostate cancer patients with and without medical service at 3 months after injections was JPY 0 (66% of patients) and JPY 5,067 (34% of patients), respectively. Thus, the indirect cost reduction for having one less injection in prostate cancer patients receiving treatment with leuprorelin acetate injections was JPY 1,723.
The reduction in total costs for prostate cancer patients with and without medical service at 3 months after injections was JPY 22,992 and JPY 35,559, respectively. Therefore, the reduction in total costs was JPY 27,265, which was calculated by the sum of the reduction in medical costs (JPY 5,670), productivity loss (JPY 1,723), and intangible costs (JPY 19,872) ().
Table 6. Total costs reduction for having one less injection in prostate cancer patients with injections of leuprorelin acetate.
Discussion
Recently the American Society of Clinical Oncology (ACSO) provided a value framework that would enable a physician and patient to evaluate the value of a specific cancer treatmentCitation21. The framework includes values of efficacy, safety, and medical costs, and also adds the new value of the treatment-free interval. In this study, we analyzed medical costs, productivity loss, and intangible costs saved by having one less injection, in order to evaluate economic values in leuprorelin acetate 6-month depot as compared to leuprorelin acetate 3-month depot. The intangible cost included various values such as pain, injection site reactions, and waiting for the doctor’s consultations for an injection. Thus, our results may provide useful information for the value framework for Japanese prostate cancer patients.
In this study, we conducted cost analyses to evaluate economic value in leuprorelin acetate 6-month depot by comparison with leuprorelin acetate 3-month depot. If prostate cancer patients are treated with either leuprorelin acetate 3-month depot or 6-month depot over 1 year, they receive four or two injections, respectively. Since the cost of leuprorelin acetate 3-month depot and 6-month depot is JPY 66,891 and JPY 102,414, respectivelyCitation22, treatment of patients with leuprorelin acetate 6-month depot can save drug costs (JPY 62,736) in 1 year as compared to treatment with leuprorelin 3-month depot. As a result, the total cost reduction for having one less injection of leuprorelin acetate in Japanese prostate cancer patients is JPY 27,265. In 1 year, the saved costs for patients treated with injection of leuprorelin acetate 6-month depot is JPY 54,530. Thus, treatment with leuprorelin acetate 6-month depot in Japanese prostate cancer patients has an advantage monetary value in lower drug prices and also the cost reduction for having one less injection compared with treatment with leuprorelin acetate 3-month depot. The ratio of reduction in medical costs, reduction in productivity loss, and savings for intangible costs in total cost reduction was 21%, 6%, and 73%, respectively. To obtain patients-centered care, the intangible costs saved is not negligible for society. Although a reduction in medical costs is important for payers, intangible costs saved would be useful for payers because society has been increasingly focused on patient-centered care.
We applied a discrete choice experiment on respondents without prostate cancer for evaluation of intangible costs for having one less injection of leuprorelin acetate as a primary analysis. Subject groups for the discrete choice experiment were overall respondents, respondents without prostate cancer, prostate cancer patients, and prostate cancer patients with hormone therapy. To evaluate intangible costs from the perspective of society, the discrete choice experiment needed to perform an analysis on a group that was a close representative of the general population. Since overall respondents included 12.9% of prostate cancer patients, overall respondents in this study are not suitable as representative of the general population. Therefore, we selected respondents without prostate cancer patients for the evaluation of intangible costs calculated from the discrete choice experiment. In fact, sensitivity analysis showed that the lowest intangible costs were in the group of respondents without prostate cancer patients (JPY 19,872), followed by the group of overall respondents (JPY 21,035), the group of prostate cancer patients (JPY 24,943), and the group of prostate cancer patients with hormone therapy (JPY 26,044). These results suggested that intangible costs from the discrete choice experiment in specific prostate cancer patients were estimated higher than the general population.
In this study, we conducted a discrete choice experiment to determine intangible costs for having one less injection. Discrete choice experiments have been widely used for evaluation of patients’ preference or willingness to pay in health economics, outcomes research, and health services researchCitation23. There is no published literature which has focused on intangible costs for having one less injection by using a discrete choice experiment. On the other hand, there are several reports focusing on patients’ preference for injection device attributes by using a discrete choice experiment approachCitation24,Citation25. Brett Hauber et al.Citation24 noted that respondents preferred to decrease injection frequency from daily to weekly, switch from a longer and thicker needle to a shorter and thinner needle, and eliminate injection-site reactions. In the present study, we showed that intangible costs for reducing the frequency of injection site reactions from 10 to one in 100 injections was the highest value. Thus, our results suggested that reduction of injection-site reactions was an important value for prostate patients who required injections constantly.
The savings in medical costs and productivity loss for having one less injection of leuprorelin acetate were estimated from the percentage of prostate cancer patients with medical service 3 months after the injection. Our results demonstrated that 34% of prostate cancer patients did not receive medical service at 3 months after injections, and the total cost saved was JPY 27,265. If 10 and 50% of prostate cancer patients did not receive medical service at 3 months after injections, the total cost saved was JPY 24,249 and JPY 29,276, respectively. These results indicated that there was no big difference in the total cost saved for having one less injection of leuprorelin acetate to change the percentage of patients with medical service at 3 months after the injection.
Productivity loss for having one less injection of leuprorelin acetate was estimated according to the Japanese Guideline for Cost-Effectiveness EvaluationCitation13. To estimate productivity loss, the average monthly wage for prostate cancer patients prescribed leuprorelin acetate was assumed to be JPY 304,000. This is the average wage across all industries, for all ages, and for both genders, and it was obtained from the Japanese Basic Survey on Wage Structure in 2016Citation10. The cost reduction in productivity loss is JPY 1,723, and the total cost saved was JPY 27,265. The average age of prostate cancer patients with leuprorelin acetate is ∼70 years. If we assume the average wage for prostate cancer patients as the average wage in men aged 65–69 years (JPY 264,600), the cost saving in productivity loss and the total cost saved will be JPY 1,499 and 27,041. These results suggested that there is little difference in the total cost saved for having one less injection of leuprorelin acetate between the estimation from the average wage across all industries, for all ages, and for both genders and the average wage in men aged 65–69 years.
For the discrete choice experiment and the calibrations, there is potential bias in respondents with a self-reported physician’s diagnosis of prostate cancer, because they were recruited from an internet panel survey company. Suzuki et al.Citation10 reported that the incidence of adverse events of injection site induration in prostate cancer patients with leuprorelin acetate 3-month depot was 13.9%. In the present study, the frequency of injection site reactions in respondents with prostate cancer was 13%, which is in agreement with previous reports.
Medical costs for consultation and injection were expected to decrease once patients change from leuprorelin acetate 3-month depot to 6-month depot injection; but, 66% of the prostate cancer patients with injections of leuprorelin acetate 6-month depot received medical service 3 months after the injection. However, 84% of the prostate cancer patients were ≥70 years old, and therefore they might have need for medical consultation due to other disease(s). Another possibility is that they have to visit the hospital as part of their routine for cancer screening every 3 months.
This study has several limitations. The respondents from the internet panel provided by a survey company are not necessarily a representative population of the Japanese society. The integrity of these data depends on the veracity of the responses received. The group of the prostate cancer patients used for calibration in the survey were recruited from an internet panel and were required to use a computer for accessing and answering the questionnaire. Thus, there might be selection bias for the population of patients with prostate cancer.
Conclusions
This study demonstrates that leuprorelin acetate 6-month depot has an advantage monetary value, as shown by a reduction of JPY 27,265 in medical costs, productivity loss, and intangible costs for having one less injection in prostate cancer patients compared with leuprorelin acetate 3-month depot. The highest value of intangible costs was the reduced frequency of injection site reactions. In the costs for treating prostate cancer patients with leuprorelin acetate, the percentage of intangible costs might not be negligible. The intangible costs will probably be actively evaluated when proceeding to patient-centered healthcare in the society.
Transparency
Declaration of funding
This study was funded by Takeda Pharmaceutical Company Limited.
Declaration of financial/other relationships
RG received advisory/consultation fees and research funding from Bayer Yakuhin, Ltd. RG received research funding from Pfizer Inc. MO received advisory/consultation fees and research funding from Astellas Pharmaceuticals. MO received honoraria from Takeda Pharmaceutical Co., Astellas Pharmaceuticals, Sanofi Co., Janssen Pharmaceuticals Co., and AstraZeneca Pharmaceuticals. KI and KT are employees of Milliman, which has received consultancy fees from Takeda Pharmaceutical Company Limited. SH and AU are employees of Takeda Pharmaceutical Company Limited.
Supplemental table 1
Download JPEG Image (385.3 KB)Acknowledgments
The authors wish to thank Dr Takehiko Segawa at Kyoto City Hospital for helpful discussions.
References
- Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2015;25:1-12
- GLOBOCAN2012 Estimated cancer incidence, mortality and prevalence worldwide in 2012 [Internet]. Lyon (France): International Agency for Research on Cancer; [cited 2017 Feb 24]. Available from: http://globocan.iarc.fr/Pages/fact_sheets.cancer.aspx
- The surveillance, epidemiology, and end results (SEER) program of the National Cancer Institute [Internet]. Rockville (MD): National Cancer Institute; [cited 2017 Feb 24]. Available from: https://seer.cancer.gov/statfacts/html/prost.html
- Ukawa S, Nakamura K, Okada E, et al. Clinical and histopathological characteristics of patients with prostate cancer in the BioBank Japan project. J Epidemiol 2017;27(3S):65-70
- Mariotto AB, Robin Yabroff K, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011;103:117-28
- Kitazawa T, Matsumoto K, Fujita S, et al. Cost of illness of the prostate cancer in Japan—a time-trend analysis and future projections. BMC Health Serv Res 2015;15:453
- Akaza H. Advanced prostate cancer treatment guidelines: a global perspective; trends of hormone therapy in Japan. BJU Int 2004;94(Suppl3):5
- The Japanese Urological Association. Practice guideline for prostate cancer in 2016. Tokyo (Japan): Kanehara & Co., Ltd; 2016 (Japanese)
- Sethi R, Sanfilippo N. Six-month depot formulation of leuprorelin acetate in the treatment of prostate cancer. Clin Interv Aging 2009;4:259-67
- Suzuki K, Namiki M, Fujimoto T, et al. Efficacy and safety of leuprorelin acetate 6-month depot in prostate cancer patients: A Phase III, randomized, open-label, parallel-group, comparative study in Japan. Jpn J Clin Oncol 2015;45:1168-74
- Morris S, Devlin N, Parkin D, et al. Economic analysis in healthcare, 2nd Edition. Hoboken (NJ): Wiley;2012
- Robinson R. Cost-benefit analysis. Brit Med J 1993;307:924-6
- Eisenberg JM. Clinical economics. A guide to the economic analysis of clinical practices. JAMA 1989;262:2879-86
- Shanahan M, Ritter A. Cost benefit analysis of two policy options for cannabis: Status quo and legalisation. PLoS One 2014;9:e95569
- Anders B, Ommen O, Pfaff H, et al. Direct, indirect, and intangible costs after severe trauma up to occupational reintegration – an empirical analysis of 113 seriously injured patients [Direkte, indirekte und intangible kosten nach einem schweren trauma]. GMS Psychosoc Med 2013;10:1-15
- Social Insurance Laboratory. Interpretation of Medical Fee Schedule Table. Tokyo (Japan): Social Insurance Laboratory Co. Ltd; 2016 (Japanese)
- Guideline for preparing cost-effectiveness evaluation to the central social insurance medical council [Internet]. Tokyo (Japan): Central Social Insurance Medical Council; [cited 2017 Feb 24]. Available from: http://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000109789.pdf
- Statistical classification of diseases and cause of death (In Japanese) [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; [cited 2017 Feb 24]. Available from: http://www.mhlw.go.jp/toukei/sippei/
- Japanese Basic Survey on Wage Structure on 2016 (In Japanese) [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; [cited 2017 Feb 24]. Available from: http://www.mhlw.go.jp/toukei/itiran/roudou/chingin/kouzou/z2015/dl/13.pdf/
- Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care. Berlin (Germany): Springer Science & Business Media; 2007
- Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Clin Oncol 2016;34:2925-33
- National Health Insurance drug price list on 2017 Feb 15 [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; [cited 2017 Feb 24]. Available from: http://www.mhlw.go.jp/topics/2016/04/tp20160401-01.html
- Hauber AB, González JM, Groothuis-Oudshoorn CGM, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health 2016;19:300-15
- Brett Hauber A, Nguyen H, Posner J, et al. A discrete-choice experiment to quantify patient preferences for frequency of glucagon-like peptide-1 receptor agonist injections in the treatment of type 2 diabetes. Curr Med Res Opin 2016;32:251-62
- Qin L, Chen S, Flood E, et al. Glucagon-like peptide-1 receptor agonist treatment attributes important to injection-naïve patients with type 2 diabetes mellitus: a multinational preference study. Diabetes Ther 2017;8:321-334