Abstract
Background: Evidence of the cost-efficacy of ixekizumab for the treatment of moderate-to-severe plaque psoriasis (PsO) in the US is limited.
Objective: To estimate the number needed to treat (NNT) and monthly cost of achieving one additional Psoriasis Area and Severity Index (PASI) 75, 90, and 100 responder for ixekizumab and other Food and Drug Administration (FDA)-approved biologics in PsO.
Methods: A network meta-analysis estimated the probability of achieving PASI 75, 90, or 100 response during induction for each biologic. NNTs were calculated using response difference of each respective biologic vs placebo at the end of induction. Monthly costs per additional PASI responder were based on FDA-approved doses, wholesale acquisition costs, and induction NNTs.
Results: Induction NNTs for ixekizumab 80 mg once every 2 weeks (Q2W) relative to placebo were consistently lower across all levels of clearance compared with the other biologics. Monthly cost per additional responder was lowest for ustekinumab 45 mg at PASI 75 and for secukinumab 300 mg and ixekizumab 80 mg Q2W at PASI 90. Ixekizumab 80 mg Q2W had the lowest cost for PASI 100.
Conclusion: In this analysis, ixekizumab is the most cost-efficient biologic in the US when targeting complete resolution, as measured by PASI 100 in PsO.
Background
Psoriasis is a chronic inflammatory disorder that affects 2–3% of the adult US populationCitation1–3. Plaque psoriasis (PsO), which constitutes 79–90% of patients with psoriasis, is characterized by red, scaly skin plaques that are typically present on the scalp, knees, elbows, and/or lower back, and are often accompanied by bothersome symptoms such as itching and painCitation1,Citation3,Citation4. Approximately 20% of patients with PsO have moderate-to-severe disease, defined by the American Association of Dermatology as ≥5% body surface area (BSA) involvement or involvement of certain body areas such as the hands, feet, face, or genitals, which have a significant impact on patients’ health-related quality-of-life (HRQoL)Citation1.
Approved biologic agents for moderate-to-severe PsO have significantly shifted the treatment paradigm by presenting patients and healthcare providers with treatment options that have improved benefit-risk profiles compared to traditional systemic therapiesCitation5. Such biologics have allowed a high proportion of patients with PsO to achieve desired levels of skin clearance (i.e. almost clear to clear skin) that are associated with significant improvements in HRQoLCitation6,Citation7. In the US, several biologics have been approved for the treatment of moderate-to-severe PsO, including tumor necrosis factor (TNF)-alpha blockers (adalimumab, etanercept, and infliximab)Citation8–10, an interleukin (IL)-12/23 inhibitor (ustekinumab)Citation11, and an IL-17A inhibitor (secukinumab)Citation12. In 2016, the US Food and Drug Administration (FDA) approved ixekizumab, a novel IL-17A inhibitor, for the treatment of patients with moderate-to-severe PsOCitation13. Ixekizumab has demonstrated superior efficacy, as measured by Psoriasis Area and Severity Index (PASI), compared with placebo, etanercept, and ustekinumab, in a controlled clinical trial setting (UNCOVER-1 [NCT01474512], UNCOVER-2 [NCT01597245], UNCOVER-3 [NCT01646177], and IXORA-S [NCT02561806])Citation14–16.
Despite the strength of the direct evidence demonstrating the higher efficacy of ixekizumab as compared with both etanercept and ustekinumab in the UNCOVER and IXORA-S trials, respectively, evidence of the clinical efficacy of ixekizumab compared with other FDA-approved biologics indicated for the treatment of moderate-to-severe PsO remains limited. To address this gap, several published analyses have estimated the efficacy of ixekizumab relative to other biologics using robust statistical methods of adjusted indirect comparison or network meta-analysis (NMA), or have simply compared clinical efficacies across clinical trials using standardized estimates such as number needed to treat (NNT)Citation17–19. However, these methodologies have associated limitations that may be, at least partially, overcome by combining strategies to estimate NNTs using the relative efficacy findings of robust indirect comparisons or NMAs. This would not only provide more reliable NNT estimates to compare clinical efficacies across clinical trials, but may also help translate the complex findings of these methodologies to everyday clinical practice.
The NNT is an evidence-based tool often used in immunology to provide information on the relative benefit of systemic therapies within or across different indicationsCitation20–23. The NNT refers to the number of individuals that need to be treated by a given therapy to achieve the desired outcome in a single individual relative to a reference group such as placebo. The closer the value of the NNT is to 1, the more favorable the outcome. NNT values have also been used to calculate incremental costs, making it a useful measure for economic decision-making.
For the purpose of this analysis, an NMA was undertaken to evaluate the efficacy, relative to placebo, of ixekizumab 80 mg once every 2 weeks (Q2W, i.e. the FDA-approved induction dosing regimen of ixekizumab in PsOCitation24) compared with those of other FDA-approved biologics indicated for the treatment of moderate-to-severe PsO. This US-centric NMA was a sub-analysis of a more comprehensive NMA reported elsewhereCitation18. The relative efficacy findings generated from this NMA were used to estimate the NNT to achieve one additional PASI responder for ixekizumab and other FDA-approved biologics across different levels of clearance (PASI 75, 90, and 100) at the end of each respective biologic induction period. Using these NNT values, the cost of achieving one additional PASI 75, 90, or 100 responder was then calculated for each of the corresponding biologics.
Methods
Detailed methodology pertaining to the original comprehensive NMA and its associated systematic literature review (SLR) has been presented elsewhereCitation18. This NMA was a sub-analysis of the comprehensive NMA and, therefore, the same SLR criteria applied, except for the evaluated comparators, which were restricted to FDA-approved biologics prior to 2017Citation8,Citation9,Citation11,Citation12,Citation24, indicated for the treatment of moderate-to-severe PsO at their respective approved US prescribing information (PI) doses ()Citation25. Brodalumab was not included in the analyses as it was not FDA-approved at the time of completion of this NMA. Infliximab was also excluded given its different mode of administration (infusion) from the evaluated biologics included in this analysis, and its subsequent reimbursement under medical benefitCitation10.
Table 1. US PI dosing schedule, length of induction period based on the respective biologic clinical trials design, US WAC per dose, and calculated cost per induction period per patient.
In brief, input data for the comprehensive NMA were identified through an SLR of published and grey literature, following guidelines from the Cochrane Handbook of Systematic Reviews and InterventionsCitation26. This review covered publications in English language from January 1990 to November 2015 and included phase II, III, and IV randomized-controlled trials (RCTs) of relevant conventional systemic and biologic therapies in moderate-to-severe PsOCitation18. Baseline criteria (RCT design, patient characteristics, disease severity, etc.) of the studies included in the NMA were assessed for comparability. Cochran’s Q test was used to test for heterogeneity in the RCTs included in the evidence network. Consistency within the network was evaluated using a net heat mapCitation27.
The NMA used a random effects Bayesian model for multi-arm trials with a multinomial likelihood and probit link developed for simultaneous PASI response analyses comparing all possible comparators. All analyses were executed according to international guidelines for the conduct of NMAs and included standard consistency and heterogeneity testsCitation26,Citation28. The end of induction period PASI 75, 90, and 100 were the main outcomes of the NMA, defined as ≥75% improvement of PASI score from baseline, ≥90% improvement of PASI score from baseline, and complete resolution of skin plaque, respectively. Missing data were imputed using non-responder imputation.
For this analysis, the NNT to achieve an additional PASI 75, 90, or 100 responder was calculated for ixekizumab, and each of the other FDA-approved biologics using the response difference of each respective biologic vs placebo at the end of induction: NNT =1/(probability of response with biologic – probability of response with placebo). NNT point estimates and 95% credible intervals (Cr.Is) were based on PASI 75, 90, and 100 response rates at the end of week 12 for all evaluated biologics except adalimumab (week 16). The duration of induction was based on the length of induction phase, as described for each respective biologic in their clinical trials design.
Cost calculations
Induction period biologic cost calculations were based on the total number of doses administered during induction defined as a total of 12 weeks (except for adalimumab, which was 16 weeks). The total number of doses during induction was according to the FDA-approved US PI dosing schedule for the evaluated biologics (). Biologic costs were calculated with the assumption of 100% adherence to the indicated doses during the timeframe evaluated and were based on US wholesale acquisition costs (WAC, the list price of a drug product to wholesalers without inclusion of discounts, rebates, reductions, or other factors affecting price) per dose in US dollars ($US), as of March 14, 2017, derived from the Truven Health Analytics RED BOOK ()Citation25. Administration costs were not included in the analysis.
For comparison across the different biologics, cost calculations were standardized by calculating the monthly (i.e. 30-day) cost per additional PASI 75, 90, or 100 responder during the induction period for each biologic, which would be a helpful comparative metric for economic decision-making.
Results
In total, 24 studies were included in this NMA (). The estimated probability of achieving a PASI 75, 90, or 100 response (and corresponding response difference) was consistently highest with ixekizumab 80 mg Q2W vs placebo compared with the other biologics included in the NMA (). Similarly, the observed NNTs for ixekizumab 80 mg Q2W relative to placebo were consistently lower across all levels of clearance (PASI 75, 90, and 100) compared with all other biologics included in the NMA (, ). Etanercept 50 mg twice weekly (BIW) had the highest NNT across all levels of clearance.
Figure 1. Number needed to treat per additional PASI 75, 90, or 100 responder vs placebo for each of the evaluated biologics (non-responder imputation). Error bars represent 95% Cr.I. BIW, twice weekly; Cr.I, credible interval; EOW, every other week; PASI, Psoriasis Area and Severity Index; Q2W, every 2 weeks.
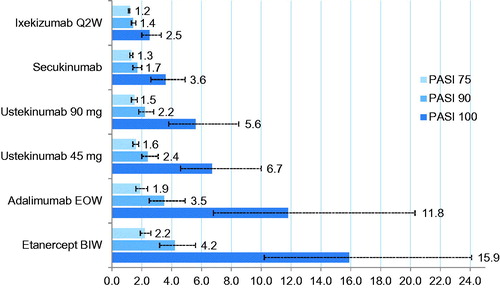
Table 2. Summary of studies included in FDA-approved US PI dose NMA.
Table 3. Conditional probabilities of achieving ≥75%, ≥ 90%, or 100% PASI response and response differences vs placebo for each of the evaluated biologics.
Table 4. Number needed to treat per additional PASI 75, 90, or 100 responder vs placebo for each of the evaluated biologics.
The monthly cost per additional PASI 75, 90, or 100 responder per biologic is shown in and . The monthly cost per additional PASI 75 responder was lowest for ustekinumab 45 mg, followed by adalimumab 40 mg, secukinumab 300 mg, and ixekizumab 80 mg Q2W, while it was higher for ustekinumab 90 mg and etanercept 50 mg BIW. For PASI 90, secukinumab 300 mg and ixekizumab 80 mg Q2W had the lowest monthly costs per additional responder followed by ustekinumab 45 mg, adalimumab 40 mg, ustekinumab 90 mg, and etanercept 50 mg BIW. Ixekizumab 80 mg Q2W had the lowest monthly cost per additional PASI 100 responder. Ustekinumab 90 mg and etanercept 50 mg BIW had the highest monthly cost per additional responder for PASI 100 as for all other evaluated levels of clearance.
Figure 2. Cost per additional PASI 75, 90, or 100 responder per month (30 days) for each of the evaluated biologics. All costs presented as US dollars. Error bars represent 95% Cr.I. BIW, twice weekly; Cr.I, credible interval; EOW, every other week; PASI, Psoriasis Area and Severity Index; Q2W, every 2 weeks.
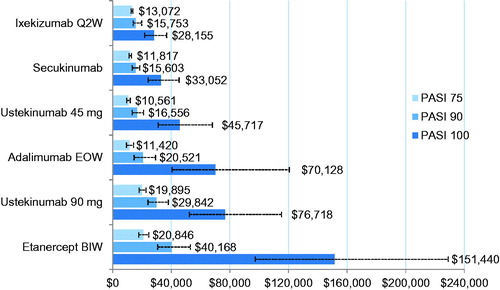
Table 5. Cost (US$) per additional PASI 75, 90, or 100 responder standardized per month (30 days) for each of the evaluated biologics.
Discussion
Advances in the management of PsO, including the development and approval of biologics, have brought with them the need for robust comparative analytics and creative evidence-based tools to facilitate the translation of comparative evidence for healthcare decision-makers. This study presents findings from an NMA evaluating the relative clinical efficacy and associated costs of biologics indicated for the treatment of moderate-to-severe PsO in the US. NMA is a particularly useful statistical approach when direct head-to-head pairwise comparisons are not available in the literature, as it combines direct and indirect evidence to provide a global estimate of treatment effects for a set of therapiesCitation46,Citation47. The findings of this NMA consistently demonstrated the higher efficacy, relative to placebo, of ixekizumab 80 mg Q2W as compared with secukinumab 300 mg, ustekinumab (90 mg, 45 mg), adalimumab 40 mg, and etanercept 50 mg BIW (in decreasing order of efficacy) across all levels of clearance (PASI 75, 90, or 100). These findings are consistent with those reported by Reich et al.Citation48. Although Reich et al.’s analysis included a different range of treatment options and PASI end-points, ustekinumab (90 mg, 45 mg), adalimumab, and etanercept were ranked in the same order in terms of predicted mean probability of response at PASI 50, 75, and 90.
Similarly, the lower NNT value for ixekizumab, calculated based on estimated response differences between ixekizumab vs placebo, showed a superior efficacy for ixekizumab to any of the other evaluated biologics at the different levels of clearance (PASI 75, PASI 90, PASI 100). The NNT results of this study are consistent with those reported by Poulin et al.Citation49, who estimated an average PASI 75 NNT of 1.5 for ustekinumab 90 mg, 1.6 for adalimumab and ustekinumab 45 mg, and 2.3 for etanercept, based on individual clinical studies, compared with our estimates of 1.5 for ustekinumab 90 mg, 1.9 for adalimumab 40 mg, 1.6 for ustekinumab 45 mg, and 2.2 for etanercept.
The results of the current study showed that, after translating the NNTs into cost per NNT based on the US WAC, the observed range of the monthly cost per additional responder among the evaluated biologics is relatively narrow for the PASI 75 response ($US10,561–$20,846), but the range widens at the higher levels of clearance, with the widest range in costs for achieving PASI 100 ($US28,155–$US151,440). For example, at the PASI 75 level, the monthly cost per additional responder ranged from $US10,561 for ustekinumab 45 mg to $US13,072 for ixekizumab 80 mg Q2W and $US20,846 for etanercept 50 mg BIW. For PASI 90, secukinumab 300 mg and ixekizumab 80 mg Q2W had the lower monthly costs per additional responder estimated at $US15,603 and $US15,753, respectively, while etanercept 50 mg BIW had the highest cost ($US40,168). As for the PASI 100 level, the monthly cost per additional responder was lowest for ixekizumab ($US28,155) and highest for etanercept 50 mg BIW ($US151,440). The results from this study suggest that, as management goals in the treatment of moderate-to-severe PsO evolve to target the highest possible levels of clearance, there is a clear separation in costs per NNT that favors the biologics with improved efficacy at the PASI 90 or 100 levels, such as the case with ixekizumab. This study showed that ixekizumab provided significantly lower monthly costs per additional PASI 100 responder compared with all other evaluated biologics (i.e. $US28,155 for ixekizumab compared to $US33,052 for secukinumab, $US45,717 for ustekinumab 45 mg, $US70,128 for adalimumab, $US76,718 for ustekinumab 90 mg, and $US151,440 for etanercept). There is increasing evidence demonstrating that patients who achieve complete skin clearance reported no psoriasis symptoms and no impairment in HRQoL within an acceptable safety profileCitation5,Citation50,Citation51.
The monthly costs per responder results from this study are comparable with a previously reported cost-efficacy analysis of US FDA-approved systemic treatments for moderate-to-severe PsO by D’Souza and PayetteCitation22, which estimated monthly costs per NNT to achieve PASI 75 as $US3,974.61–$US7,678.78 for adalimumab, $US7,177.89–$US7,263.99 for ustekinumab 45 mg, and $US8,284.71–$US10,674.89 for etanercept. Even though biologics prices had increased from the time their analyses were conducted, the rank of cost per NNT stayed the same for the evaluated biologics in both studies.
Strengths and limitations
The NMA on which the current NNT data are based has been developed in line with current health technology assessment methods guidance and has provided robust results, which have been confirmed in a wide range of sensitivity analysesCitation18. One limitation is that the heterogeneity of RCT designs across the included comparators beyond the induction period did not allow assessment of the relative efficacy of ixekizumab over longer time periods.
This analysis was based on efficacy results from clinical trials and not on real-world data, which may be affected by factors such as levels of compliance and persistence with treatment, or dose escalations or reductions. Costs used in this analysis were based on US WAC as of March 2017 and do not take into account factors such as rebates, coinsurance, copayments, and other cost-containment factors. Costs associated with administration, laboratory services, and treatment-related adverse events have also been excluded.
Conclusions
Ixekizumab consistently demonstrated greater efficacy than other FDA-approved biologics in the treatment of moderate-to-severe PsO. This is evident from the superior efficacy of ixekizumab relative to etanercept and ustekinumab in head-to-head clinical trials, and the higher efficacy, relative to placebo, of ixekizumab compared to all other evaluated biologics in this and other published NMAs across all levels of skin clearance, including secukinumab, ustekinumab, adalimumab, and etanercept.
Based on this analysis, ustekinumab 45 mg had the lowest monthly cost per additional PASI 75 responder, secukinumab 300 mg and ixekizumab 80 mg Q2W had the lowest monthly cost per additional PASI 90 responder, while ixekizumab had the lowest monthly cost per additional responder for achieving the highest level of clearance (i.e. PASI 100), compared with the other evaluated FDA-approved biologics in the treatment of moderate-to-severe PsO. Overall, based on this analysis, ixekizumab is considered the most cost-efficient biologic in the US when targeting complete resolution as measured by PASI 100 in PsO.
Transparency
Declaration of funding
The study was funded by Eli Lilly and Company.
Declaration of financial/other interests
SAS, SAF, RB, DA, AS, BZ, and SH are all full-time employees and stock holders of Eli Lilly and Company. CL has consulted for and/or been an Advisory Board Member for Abbvie, Amgen, Boehringer-Ingelheim, Dermira, Eli Lilly and Company, Janssen, Leo, Pfizer, Sandoz, and UCB and Vitae; has been an Investigator for Actavis, Abbvie, Amgen, Boehringer-Ingelheim, Celgene, Coherus, Cellceutix, Corrona, Dermira, Eli Lilly and Company, Galderma, Glenmark, Janssen, Leo Pharma, Merck, Novartis, Novella, Pfizer, Sandoz, Stiefel, Wyeth; and attended a Speaker’s bureau for Abbvie, Celgene, Novartis and Eli Lilly and Company.
Acknowledgments
The authors thank Sue Williamson and Caroline Spencer (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this manuscript, which was funded by Eli Lilly and Company.
References
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008;58:826-50
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014;70:512-16
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014;70:871-81.e830
- Icen M, Crowson CS, McEvoy MT, et al. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol 2009;60:394-401
- Blauvelt A, Griffiths CE, Lebwohl M, et al. Reaching complete or near complete resolution of psoriasis: benefit and risk considerations. Br J Dermatol. Epub 16 Mar 2017; doi:10.1111/bjd.15463
- Farahnik B, Beroukhim K, Zhu TH, et al. Ixekizumab for the treatment of psoriasis: a review of phase III trials. Dermatol Ther (Heidelb) 2016;6:25-37
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015;73:400-9
- Humira® (adalimumab) Prescribing Information. North Chicago, IL: AbbVie Inc.; July 2016. Available at: http://www.rxabbvie.com/pdf/humira.pdf. [Last accessed 13 April 2017]
- Enbrel® (etanercept) Prescribing Information. Thousand Oaks, CA: Immunex Corporation; September 2013. Available at: http://pi.amgen.com/united_states/enbrel/derm/enbrel_pi.pdf. [Last accessed 13 April 2017]
- Remicade® (infliximab) Prescribing Information. Horsham, PA: Janssen Biotech, Inc.; October 2015. Available at: http://www.remicade.com/shared/product/remicade/prescribing-information.pdf. [Last accessed 13 April 2017]
- Stelara® (ustekinumab) Prescribing Information. Horsham, PA: Janssen Biotech, Inc.; March 2014. Available at: https://www.stelarainfo.com/pdf/prescribinginformation.pdf. [Last accessed 13 April 2017]
- Cosentyx® (secukinumab) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; January 2016. Available at: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/cosentyx.pdf. [Last accessed 13 April 2017]
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of moderate-to-severe plaque psoriasis [press release]. March 22, 2016. Available at: https://investor.lilly.com/releasedetail.cfm?ReleaseID=961838. [Last accessed 13 April 2017]
- Griffiths CEM, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386:541-51
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016;375:345-56
- Reich K, Lomaga M, Henneges C, et al. Efficacy and safety of ixekizumab compared to ustekinumab in patients with moderate-to-severe plaque psoriasis: a randomized head-to-head trial [oral presentation]. European Academy of Dermatology and Venereology 25th EADV Congress; September 28–October 2, 2016; Vienna, Austria.
- Strober B, Brnabic A, Schacht A, et al. Indirect comparison of ixekizumab and secukinumab using matched-adjusted indirect comparisons [poster FC03.06]. 25th European Academy of Dermatology and Venereology; September 28–October 2, 2016; Vienna, Austria
- Hartz S, Dutronc Y, Kiri SH, et al. Network meta-analysis to evaluate the efficacy of ixekizumab in the treatment of moderate-to-severe psoriasis [poster]. International Society for Pharmacoeconomics and Outcomes 19th Annual European Congress; October 29–November 2, 2016; Vienna, Austria
- Leonardi CL. Systemic treatment of psoriasis what’s new as of 1Q2015? [symposium session]. 73rd Annual Meeting of the American Academy of Dermatology; March 20–24, 2015; San Francisco, CA
- Citrome L. Show me the evidence: using number needed to treat. South Med J 2007;100:881-4
- Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand 2008;117:412-29
- D’Souza LS, Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol 2015;72:589-98
- Liu Y, Wu EQ, Bensimon AG, et al. Costs per responder associated with biologic therapies for Crohn’s Disease, Psoriasis and Rheumatoid Arthritis. Adv Ther 2012;29:620-34
- Taltz® (ixekizumab) Prescribing Information. Indianapolis, IN: Eli Lilly and Company; March 20, 2016. Available at: http://pi.lilly.com/us/taltz-uspi.pdf. [Last accessed 13 April 2017]
- Truven Health Analytics™ RED BOOK™. RED BOOK online®; 2017. Available at: http://www.micromedexsolutions.com/. [Last accessed 14 March 2017]
- Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: The Cochrane Collaboration; 2011
- Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods 2012;3:312-24
- Dias S, Welton NJ, Sutton AJ, et al. A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. Report by the Decision Support Unit. NICE DSU Technical Support Document No. 2. London: NICE; 2011
- Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008;158:558-66
- Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008;58:106-15
- Asahina A, Nakagawa H, Etoh T, et al. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol 2010;37:299-310
- Bissonnette R, Tardif JC, Harel F, et al. Effects of the tumor necrosis factor-alpha antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging 2013;6:83-90
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 2015;373:136-44
- Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010;362:118-28
- European Medicines Agency. EPAR Cosentyx (secukinumab). EMA/CHMP/665405/2015. October 22, 2015. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/003729/WC500199574.pdf. [Last accessed 13 April 2017]
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003;349:2014-22
- Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005;152:1304-12
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006;367:29-35
- Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386:552-61
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol 2012;67:86-92
- Zhu X, Zheng M, Song M, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol 2013;12:166-74
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci 2011;63:154-63
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371:1665-74
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008;371:1675-84
- Igarashi A, Kato T, Mato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol 2012;39:242-52
- Greco T, Biondi-Zoccai G, Saleh O, et al. The attractiveness of network meta-analysis: a comprehensive systematic and narrative review. Heart Lung Vessel 2015;7:133-42
- Greco T, Landoni G, Biondi-Zoccai G, et al. A Bayesian network meta-analysis for binary outcome: how to do it. Stat Methods Med Res 2016;25:1757-73
- Reich K, Burden AD, Eaton JN, et al. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. Br J Dermatol 2012;166:179-88
- Poulin Y, Langley RG, Teixeira HD, et al. Biologics in the treatment of psoriasis: clinical and economic overview. J Cutan Med Surg 2009;13(Suppl 2):S49-S57
- Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol 2016;75:77-82.e7
- Feldman SR, Bushnell DM, Klekotka PA, et al. Differences in psoriasis signs and symptom severity between patients with clear and almost clear skin in clinical practice. J Dermatol Treat 2016;27:224-7
