Abstract
Aims: The aim in this study is to evaluate economic value for leuprorelin acetate 6-month depot compared with leuprorelin acetate 3-month depot in Japanese pre-menopausal breast cancer patients from a societal perspective.
Methods: The cost analysis was conducted by estimating direct and indirect cost, and intangible costs associated with one 6-month injection compared with two 3-month injections. Claims data were used for the analyses of direct and indirect cost and Medical Fee Schedule Table for direct cost. Discrete choice experiments were conducted by web-based survey to determine the intangible costs. Another web-based survey was also conducted on premenopausal breast cancer patients with injections of leuprorelin acetate, to calibrate the results of discrete choice experiments.
Results: The medical costs saved for having one less injection in pre-menopausal breast cancer patients with leuprorelin acetate injection were JPY 6,183. The productivity loss saving was JPY 1,419. An estimation of intangible costs saved for having one less injection of leuprorelin acetate was JPY 58,430, which included the disbenefit due to pain (JPY 8,535), injection site reactions (JPY 44,051), waiting time (JPY 9,595), and subtracting value in medical consultation (JPY 3,751). The total cost saved for having one less injection was JPY 66,032.
Limitations: The respondents from the internet panel provided by a survey company do not necessarily reflect a population of Japanese society.
Conclusions: Leuprorelin acetate 6-month depot demonstrates a higher value than leuprorelin acetate 3-month depot through saving medical costs and loss of productivity, as well as intangible costs saved for having one less injection when treating pre-menopausal breast cancer patients. In the costs for treating with leuprorelin acetate, the percentage of intangible costs might not be negligible. The intangible costs will probably be actively evaluated to proceed to patient-centered healthcare in society.
Introduction
Breast cancer is the most commonly diagnosed cancer, and one of the leading causes of death in femalesCitation1. It represents ∼25% of all cancers in females, and nearly 1.7 million patients were newly-diagnosed with breast cancer worldwide in 2012Citation2. Approximately 7% of breast cancer patients are diagnosed under the age of 40, which accounts for more than 40% of all cancers in females in this age groupCitation3. For the patients at pre-menopausal ages with hormone receptor (HR)-positive breast cancer, it is important to reduce endogenous estrogen levels to suppress cancerCitation4. Luteinizing hormone-releasing hormone (LH-RH) agonists are commonly used for this purpose, in combination with tamoxifen or aromatase inhibitorsCitation5. About 60% of patients with breast cancer are positive for HR and candidates for endocrine therapyCitation5. One of the LH-RH agonists, leuprorelin acetate, has been prescribed by various depot formulations with different acting durationsCitation6. In Japan, 1-, 3- and 6-month depot formulations are currently available. Compared with the 3-month depot formulation, the 6-month depot formulation has been proven to be non-inferior for the suppression of serum estradiol to menopausal levelsCitation7. No significant differences in the safety profiles and tolerability were found between the two treatmentsCitation7. Consequently, we considered that both efficacy and safety are comparable between the 3- and 6-month depot formulations. For one 6-month injection compared with two 3-month injections, leuprorelin acetate 6-month depot formulation is expected to decrease the costs, such as direct costs, indirect costs, and intangible costs, which represent the monetary value of the patients’ burden. For example, as for direct costs, the medical costs associated with follow-up examination or injection fee can decrease due to fewer injections. As for indirect costs, there is less productivity loss due to fewer visits to hospital. In addition, intangible costs decrease because patients decrease their burden, such as pain or injection site reaction, based on fewer injections. On the other hand, skipping one injection could be perceived as less beneficial for the patients, because patients may think there is value in seeing a doctor during the consultation. Thus, we assumed that the cost for efficacy and safety is equivalent between leuprorelin acetate 6-month depot and 3-month depot, and accordingly we compared these two protocols in Japanese pre-menopausal breast cancer patients by estimating direct costs, indirect costs and intangible costs from the societal perspective.
Methods
Study design
In this study, we compared the costs for the 6-month depot formulation with the 3-month depot formulation by estimating the costs associated with having one less injection of leuprorelin acetate in pre-menopausal breast cancer patients. We used the same methods as previously described in our study for prostate cancerCitation8. This analysis included direct and indirect cost and intangible cost. Direct costs were defined as direct medical costs and did not include long-term care costs. Indirect costs defined the loss of productivity and did not include transportation fee or the loss of productivity for a carer. As for loss of productivity for a carer, since there were few respondents who required a carer, we did not include the costs. As for transportation fee, since the difference in the distance from patients’ home to a hospital varies, we did not include this cost. Intangible costs defined the monetary value for patients’ emotional value, such as pain and injection site reactions. For the analysis of direct cost, we used claims data and a Medical Fee Schedule TableCitation9. In Japan, the Medical Fee Schedule Table is standardized under a social health-insurance system that covers all of its citizens. For the analysis of indirect cost, we used claims data and the Japanese Basic Survey on Wage Structure in 2016Citation10. We conducted a discrete choice experiment to estimate monetary value in the intangible costs for a general population. Another survey on pre-menopausal breast cancer patients with injections of leuprorelin acetate was carried out to calibrate the results of the discrete choice experiment. We estimated the intangible costs for one injection of leuprorelin acetate by fitting the results of emotional value for patients with injections of leuprorelin acetate in a calibration survey to the results of the monetary value of the emotional value due to injection in the discrete choice experiment.
The study was approved by the Ethics Committee, Research Institute of Healthcare Data Science (RI2016004) and registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN000024760).
Data source and participants
The web-based survey for the discrete choice experiments and the calibration were carried out by using an online panel managed by ANTERIO Inc. (Tokyo, Japan). The panels included individuals who had agreed to participate in web-based surveys in Japan. The respondents for discrete choice experiments were Japanese women aged between 20–49 years old, because the age range is similar to that of pre-menopausal breast cancer patients, who had received more than one injection in a hospital in the past year, in order to perform discrete choice experiments in a general population. The injection includes for vaccination, medication, anesthesia, blood donation, or for other reasons in a hospital in the past year. In the discrete choice experiments’ survey, we sampled 309 women of the general population (respondents without breast cancer) selected randomly from an internet panel which included 7.5 million people. We also sampled 107 breast cancer patients, including those with hormone therapy, preferentially from an internet panel. The response rate was 72.3% in the discrete choice experiment. In the calibration survey, we sampled breast cancer patients with injections of leuprorelin acetate from an internet panel for breast cancer patients. The survey was conducted between November 2016 and January 2017.
A claims database from April 2008 to November 2016 provided by Medical Data Vision (Tokyo, Japan) was used to calculate the percentage of patients who did or did not receive medical service 3 months after the leuprorelin acetate injection to the total patients receiving a leuprorelin acetate 6-month depot injection to analyze direct and indirect cost. Breast cancer patients were identified from individuals who had been diagnosed as C50 according to the diagnosis under the International Classification of Diseases 10th RevisionCitation11.
Discrete choice experiments and survey for calibration
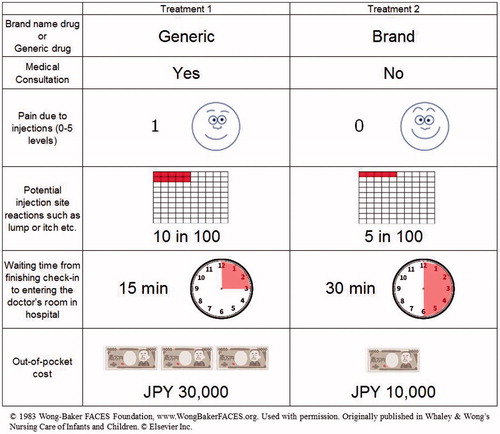
A discrete choice experiment was conducted to estimate the intangible costs for one injection. Attributes and their levels for discrete choice experiments were selected by a physician, as shown in . shows the attributes: drugs (brand-name or generic), medical consultation by doctors, pain due to injection, frequency of injection site reactions, and waiting time from finishing check-in to entering the doctor’s room in a hospital, and the level for each attribute. The Wong-Baker FACES Pain Rating Scale was used to evaluate the pain due to injection. Out-of-pocket cost was also included as an attribute to calculate the intangible costs for one injection. The orthogonal design resulted in the creation of 27 choice tasks from a total of 648 possible combinations. Respondents in the web-based survey answered 20 choice task questions from 27 choice task versions selected through orthogonal design. In choice task questions, respondents were required to choose one preferable treatment among two options (see a representative example of choice task questions in ). Each respondent completed 20 choice tasks. The survey for choice task questions was performed via the internet.
Table 1. Attributes and attribute levels in discrete choice experiments.
To estimate the intangible costs saved by having one less injection of leuprorelin acetate, a calibration survey was conducted for pre-menopausal breast cancer patients who received treatment with leuprorelin acetate injections. They were given four questions, including the frequency of consulting a doctor, level of pain due to injections, frequency of injection site reaction, and length of waiting from finishing check-in to entering the doctor’s room in the hospital. They had to answer by selecting one option from a list for questions 1 and 2, and particular numbers for questions 3 and 4. Questions and possible answers included:
Question 1: Have you consulted a doctor? (possible answers: every time, almost every time (∼80%), one in two, few (∼20%), never);
Question 2: How did you feel your pain due to injection? (0, 1, 2, 3, 4, 5 level of Wong-Baker FACES Pain Rating Scale);
Question 3: How many times did you have injection site reactions in 100 injections?; and
Question 4: How long did you wait from finishing check-in to entering the doctor’s room in the hospital?
From this survey, we calculated the average value for four attributes in patients who received treatment with leuprorelin acetate injections.
Direct and indirect cost estimation
Direct costs included a follow-up consultation fee, medical costs related to diagnosis, and medical costs related to injection. All the costs will be reduced for breast cancer patients who do not go to the hospital at 3 months after injection of leuprorelin acetate 6-month depot formulation, while only the medical costs related to injection will be reduced for those patients who go to the hospital at 3 months after injection. These medical costs, as described in , were estimated by the following steps: (1) identified medical care shown on guidelines for breast cancerCitation12; (2) assumed a claim corresponding to the medical activities in step 1; (3) calculated medical costs in the claim from the Medical Fee Schedule Table; and (4) evaluated the medical cost by using the weighted average value in both the monetary values of patients who did or did not receive medical service 3 months after the leuprorelin acetate injection. The patients who received medical service 3 months after injections of leuprorelin acetate 6-month depot formulation were identified with having medical act codes such as medication, medical examination and diagnostic imaging on the claims database. The patients receiving leuprorelin acetate 6-month depot formulation and 3-month depot formulation were identified by a claim receipt termed LEUPLIN PRO for injection kit 22.5 mg and LEUPLIN SR for injection kit 11.25 mg in the claims database.
Table 2. Medical cost saved for breast cancer patients with injections of leuprorelin acetate.
We analyzed productivity loss in the patients as indirect costs according to the Japanese Guideline for Cost-Effectiveness EvaluationCitation13. The average monthly wage for pre-menopausal breast cancer patients prescribed leuprorelin acetate 3-month depot formulation was assumed to be JPY 304,000. This value is the average wage across all industries, all ages, and for both genders obtained from the Japanese Basic Survey on Wage Structure in 2016Citation10. We assumed that the estimated time for productivity loss was a half day in 30 days. We evaluated the indirect cost saved by using the weighted average value from the monetary values for patients who did or did not receive medical service 3 months after the leuprorelin acetate injection.
Statistical analysis
A conditional logit model was applied to analyze data of choice task questions in discrete choice experiments. The model reveals the hidden utility behind each level of each attribute by the inverse estimation, based on each choice of the two choice task cards. The utility is assumed to be the same among people in the conditional logit model. The ratio of the coefficient of each attribute to the coefficient of out-of-pocket attribute is the monetary value of each level of each attribute. This ratio is marginal rate of substation (MRS) in economics and reflects willingness-to-pay (WTP) for the change of an attributeCitation14. From the results of the survey for calibration, the average value of each attribute in one injection of leuprorelin acetate was calculated and, then, the intangible costs saved for having one less injection of leuprorelin acetate were estimated.
Sensitivity analyses were conducted on the intangible costs saved to evaluate the difference between patient sub-groups, which were the respondents with or without breast cancer, those with cancer receiving hormone therapy, and those aged 20–29, 30–39, and 40–49 years. Sensitivity analyses were conducted to estimate both the total cost saved for having one less injection of leuprorelin acetate by changing the percentage of patients with medical service 3 months after the leuprorelin acetate injection and the total cost saved for having one less injection of leuprorelin acetate. We conducted cross-sectional analysis to analyze the medical costs saved and productivity loss savings.
Results
Patient identification
The number of respondents for discrete choice experiments was 416: 309 of the respondents did not have breast cancer, 107 were breast cancer patients, and 41 of the breast cancer patients were receiving injections for hormone therapy. The number of respondents for the calibrations was 31. The mean (standard deviation, SD) age of respondents without breast cancer patients in discrete choice experiments was 38.4 (7.2). The mean age of respondents for the calibrations was 42.5 (4.0).
We found 5,169 and 387 patients prescribed leuprorelin acetate 3-month depot and 6-month depot, respectively, in the Medical Data Vision database. The mean (SD) age of patients with leuprorelin acetate 3-month depot and 6-month depot was 43.3 (5.5) and 43.8 (5.3), respectively.
Intangible costs saved for one injection by discrete choice experiments
Intangible costs saved for each attribute with discrete choice experiments were estimated from Logit model’s coefficients (Supplementary Table 1). The amount of intangible costs saved for reducing pain due to injection from score 5 to 0 was the highest among all the attributes, indicating that respondents were willing to pay the most for out-of-pocket costs for reducing pain due to injection ().
Table 3. Intangible costs saved for one injection by discrete choice experiments.
The average value of each attribute for one injection of leuprorelin acetate among the patients who had treatment with leuprorelin acetate is shown in . Intangible costs saved for having one less injection of leuprorelin acetate were calculated by the values in pain (JPY 8,535), injection site reactions (JPY 44,051), waiting time (JPY 9,595), and subtracting value in medical consultation (JPY 3,751). The intangible costs saved for having one less injection were JPY 58,430 ().
Table 4. Intangible costs saved for one injection in breast cancer patients with injections of leuprorelin acetate: Note that intangible costs for one injection were calculated based on the results of discrete choice experiments in respondents without breast cancer.
To estimate the costs from the society perspective in primary results, we selected the respondents without breast cancer as a general population. We next performed sensitivity analysis to evaluate the difference in intangible costs between patients’ and societal perspective. The amount of intangible costs saved for having one less injection in respondents with and without breast cancer was JPY 61,024 and JPY 58,430, respectively ().
Table 5. Sensitivity analysis of intangible costs saved for one injection in breast cancer patients with injections of leuprorelin acetate.
Total costs saved for having one less injection of leuprorelin acetate
To evaluate the economic value for leuprorelin acetate 6-month depot compared with leuprorelin acetate 3-month depot, we estimated the costs associated with having one less injection of leuprorelin acetate in breast cancer patients. Direct medical costs saved by having one less injection in breast cancer patients receiving treatment with injections of leuprorelin acetate 6-month depot were analyzed. The medical cost saved for 28% of breast cancer patients who did not receive medical service at 3 months after injections was JPY 14,060 (). The cost saved for 72% of breast cancer patients who received medical service at 3 months was JPY 3,120. Thus, the medical costs saved for having one less injection in breast cancer patients receiving treatment with injections of leuprorelin acetate was JPY 6,183.
The productivity loss savings for breast cancer patients with and without medical service at 3 months after injections was JPY 0 and JPY 5,067, respectively. Thus, the productivity loss savings for breast cancer patients receiving treatment with injections of leuprorelin acetate was JPY 1,419.
Total costs saved for breast cancer patients with and without medical service at 3 months after injections were JPY 61,550 and JPY 77,557, respectively. Thus, the total costs saved for breast cancer patients treated with leuprorelin acetate 6-month depot injections were JPY 66,032, which included medical costs saved (JPY 6,183), productivity loss savings (JPY 1,419), and intangible costs saved (JPY 58,430) ().
Table 6. Total costs saved for one injection in breast cancer patients with injections of leuprorelin acetate.
Discussion
A cost analysis was conducted to evaluate economic value in leuprorelin acetate 6-month depot by comparison with leuprorelin acetate 3-month depot. The drug price of leuprorelin acetate 6-month depot (JPY 102,414) is lower than twice the drug price of leuprorelin acetate 3-month depot (JPY 133,782)Citation15. Thus, the drug cost saved for pre-menopausal breast cancer patients receiving treatment with injection of leuprorelin acetate 6-month depot was JPY 62,736 in 1 year. In addition, total costs saved for having one less injection of leuprorelin acetate in pre-menopausal breast cancer patients are estimated to be JPY 66,032 in our results. In 1 year, the total cost saved for the patients was JPY 132,064. Thus, savings in total costs including drug costs per 1 year was JPY 194,800. These results suggested that this was not a negligible value for both the payers and society. Thus, leuprorelin acetate 6-month depot has an advantage in lower drug price and also in the costs saved for having one less injection in pre-menopausal breast cancer patients compared with leuprorelin acetate 3-month depot.
In this study, intangible costs saved by reducing pain due to injection from a score of 1 to 0 out of 5 in women were JPY 2,498. We also demonstrated that intangible costs saved by reducing pain due to injection from a score of 1 to 0 in men were JPY 261Citation8. There have been some reports that pain sensitivity due to injection in women was higher than that in menCitation16. The review reported that women showed higher levels of pain after intramuscular injection of algesic substances than menCitation16. There is no report about gender difference in monetary value for pain reduction due to injections. We showed that the value in reduction of pain due to injection for women tended to be higher than that for men, even if patients of both genders had the same level of pain reduction.
Total costs saved for having one less injection in pre-menopausal breast cancer patients receiving treatment with injections of leuprorelin acetate were JPY 66,032. We also demonstrated that the total costs saved for having one less injection in prostate cancer patients with injections of leuprorelin acetate were JPY 27,265Citation8. The value in pre-menopausal breast cancer patients was JPY 38,767 more expensive than that in prostate cancer patients. The majority of the value differentials was between the value in injection site reactions in prostate cancer patients (JPY 11,545) and in pre-menopausal breast cancer patients (JPY 44,051). The frequency of injection site reactions in pre-menopausal breast cancer patients (41%) was more than that in prostate cancer patients (13%). Therefore, the high frequency of injection site reaction in cancer patients receiving treatment with leuprorelin acetate injections may represent high total costs saved for having one less injection.
We estimated the cost savings in medical costs and productivity loss for having one less injection of leuprorelin acetate from the percentage of breast cancer patients with medical service 3 months after the injection. In our results, 28% of the breast cancer patients did not receive medical service at 3 months after injections, and the total cost saved was JPY 66,032. If 10% and 50% of breast cancer patients did not receive medical service at 3 months after injections, the total cost saved was JPY 63,151 and JPY 69,554, respectively. These results indicated that there was no big difference in the total cost saved for having one less injection of leuprorelin acetate to change the percentage of patients with medical service at 3 months after the injection.
In this study, we estimated productivity loss for having one less injection of leuprorelin acetate according to the Japanese Guideline for Cost-Effectiveness EvaluationCitation13. The average monthly wage for pre-menopausal breast cancer patients prescribed leuprorelin acetate was assumed to be JPY 304,000, which is the average wage across all industries, for all ages, and for both genders, which was obtained from the Japanese Basic Survey on Wage Structure in 2016Citation10. In our results, the cost saving in productivity loss is JPY 1,419, and the total cost saved, including medical and intangible costs, was JPY 66,032. The average age of breast cancer patients with leuprorelin acetate is ∼40 years. If we assume the average wage for pre-menopausal breast cancer patients as the average wage in women aged 40–45 years, the cost saving in productivity loss and the total cost saved is JPY 1,225 and JPY 65,838. These results suggested that there is little difference in the total cost saved for having one less injection of leuprorelin acetate between the estimation from the average wage across all industries, for all ages, and for both genders and the average wage in women aged 40–45 years.
There is a potential bias in the respondents with pre-menopausal breast cancer because they were recruited from a web-based panel survey company in the discrete choice experiments and calibrations. In previous reports, the incidence of injection site induration in pre-menopausal breast cancer patients receiving treatment with leuprorelin acetate 6-month depot was 43.4%Citation7. In this study, the frequency of injection site reactions in respondents with pre-menopausal breast cancer was 41%, which is in agreement with the frequency of injection site reactions in previous reports.
This study has several limitations. The respondents from the internet panel provided by a survey company do not necessarily reflect a population of the Japanese society. The data integrity depends on veracity of the responses received. In the calibration survey, the pre-menopausal breast cancer patients were recruited from an internet panel. They were required to use a computer for accessing and answering questionnaires. Thus, there might be selection bias for the population of patients with pre-menopausal breast cancer.
Conclusions
This study demonstrates that leuprorelin acetate 6-month depot has an advantage value, which is JPY 66,032, savings in medical costs, productivity loss, and intangible costs by requiring one less injection in pre-menopausal breast cancer patients compared with leuprorelin acetate 3-month depot. Among the costs reduced, intangible costs saved represent the highest value. In the costs for leuprorelin acetate, the percentage of intangible costs was not a negligible value. The intangible costs will probably be actively evaluated to proceed to patient-centered healthcare in society.
Transparency
Declaration of funding
This study was funded by Takeda Pharmaceutical Company Limited.
Declaration of financial/other relationships
RG received advisory/consultation fees and research funding from Bayer Yakuhin, Ltd. RG received research funding from Pfizer Inc. JK received advisory/consultation fees and research funding from Takeda Pharmaceutical Co., Limited. JK received research funding from Takeda Pharmaceutical Co., Esai Co., Chugai Co. and AstraZeneca Pharmaceuticals. KI and KT are employees of Milliman, which has received consultancy fees from Takeda Pharmaceutical Company Limited. SH and AU are employees of Takeda Pharmaceutical Company Limited. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Supplemental material
Download JPEG Image (279.4 KB)References
- Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev 2015;25:1-12
- Park W. Breast cancer role of ovarian function suppression in premenopausal women with early breast cancer. J Breast Cancer 2016;19:341-8
- Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol 2009;36:237-49
- Goel S, Sharma R, Hamilton A, et al. LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women. Cochrane Database of Systematic Reviews 2009;4:CD004562
- Del Mastro L, Levaggi A, Giraudi S, et al. Luteinising hormone releasing hormone agonists (LH-RHa) in premenopausal early breast cancer patients: Current role and future perspectives. Cancer Treat Rev 2011;37:208-11
- Sethi R, Sanfilippo N. Six-month depot formulation of leuprorelin acetate in the treatment of prostate cancer. Clin Interv Aging 2009;4:259-67
- Kurebayashi J, Toyama T, Sumino S, et al. Efficacy and safety of leuprorelin acetate 6-month depot, TAP-144-SR (6M), in combination with tamoxifen in postoperative, premenopausal patients with hormone receptor-positive breast cancer: a phase III, randomized, open-label, parallel-group comparative. Breast Cancer 2017;24:161-70
- Goto R, Uda A, Hiroi S, et al. Cost analysis of leuprorelin acetate in Japanese prostate cancer patients: Comparison between 6-month and 3-month depot formulations. J Med Econ 2017;20:1155-62
- Social Insurance Laboratory. Interpretation of Medical Fee Schedule Table. Tokyo (Japan): Social Insurance Laboratory Co. Ltd; 2016 (Japanese)
- Japanese basic survey on wage structure on 2016 (In Japanese) [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; [cited 2017 Feb 24]. Available from: http://www.mhlw.go.jp/toukei/itiran/roudou/chingin/kouzou/z2015/dl/13.pdf/
- Statistical classification of diseases and cause of death (In Japanese) [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; [cited 2017 Feb 24]. Available from: http://www.mhlw.go.jp/toukei/sippei/
- The Japanese Breast Cancer Society. Practice guideline for breast cancer in 2015 [In Japanese]. Japan: Kanehara & Co., Ltd.; 2015
- Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council [Internet]. Tokyo (Japan): Central Social Insurance Medical Council; [cited 2017 Feb 24]. Available from: http://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000109789.pdf
- Ryan M., Gerard K., Amaya-Amaya M. Using discrete choice experiments to value health and health care. Berlin (Germany): Springer Science & Business Media; 2007
- National Health Insurance drug price list on 2017 Feb 15 [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; [cited 2017 Feb 24]. Available from: http://www.mhlw.go.jp/topics/2016/04/tp20160401-01.html
- Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447-85