Abstract
Background: Parkinson’s disease (PD) is an incurable, progressive neurological condition, with symptoms impacting movement, walking, and posture that eventually become severely disabling. Advanced PD (aPD) has a significant impact on quality-of-life (QoL) for patients and their caregivers/families. Levodopa/carbidopa intestinal gel (LCIG) is indicated for the treatment of advanced levodopa-responsive PD with severe motor fluctuations and hyper-/dyskinesia when available combinations of therapy have not given satisfactory results.
Aims: To determine the cost-effectiveness of LCIG vs standard of care (SoC) for the treatment of aPD patients.
Methods: A Markov model was used to evaluate LCIG vs SoC in a hypothetical cohort of 100 aPD patients with severe motor fluctuations from an Irish healthcare perspective. Model health states were defined by Hoehn & Yahr (H&Y) scale—combined with amount of time in OFF-time—and death. SoC comprised of standard oral therapy ± subcutaneous apomorphine infusion and standard follow-up visits. Clinical efficacy, utilities, and transition probabilities were derived from published studies. Resource use was estimated from individual patient-level data from Adelphi 2012 UK dataset, using Irish costs, where possible. Time horizon was 20 years. Costs and outcomes were discounted at 4%. Both one-way and probabilistic sensitivity analyses were conducted.
Results: The incremental cost-effectiveness ratio for LCIG vs SOC was €26,944/quality adjusted life year (QALY) (total costs and QALYs for LCIG vs SoC: €537,687 vs €514,037 and 4.37 vs 3.49, respectively). LCIG is cost-effective at a payer threshold of €45,000. The model was most sensitive to health state costs.
Conclusion: LCIG is a cost-effective treatment option compared with SoC in patients with aPD.
Introduction
Parkinson’s Disease (PD) is a progressive neurodegenerative disorder with motor and non-motor symptoms that negatively impact health, functional status, and quality-of-life (QoL) for patients and their caregivers, as well as pose a challenge to healthcare providers and societyCitation1,Citation2. The burden of PD to the payer and patient is high in terms of both increased disability and decreased quality-of-life and increased use of health and social care resourcesCitation3. Previous work suggests that sub-optimal management can lead to higher healthcare costsCitation4. A recent paper estimated the annual cost burden of PD to the UK in the region of €2.4 billion, ranking it in the top 20 of brain disorders annual costs while per patient costs were estimated as exceeding €20,000, ranking PD in the top six per patient spend brain disordersCitation5. Direct non-medical costs accounted for most of the PD cost reportedCitation5. With limited resources, effective treatment to reduce costs and better manage disease progression is needed.
During early disease stage, motor symptoms such as tremor, rigidity, and brady/hypokinesiaCitation6 are well-controlled with oral levodopaCitation7. However, in advanced PD (aPD) stages, fluctuations in plasma levodopa levels are observed following oral administration, due to oral drug pharmacokinetics, erratic gastric emptying, and levodopa absorption, leading to reduced control of motor complications. Alternative adjunctive oral therapies may initially reduce the duration of motor symptoms (i.e. decrease “off” time), but, over time, this effect wanes. Currently, additional aPD treatment options include device-aided treatment options such as apomorphine infusion, levodopa/carbidopa intestinal gel (LCIG) infusion, and brain surgery such as deep brain stimulation (DBS) techniques. For those patients who have failed on or are unsuitable for apomorphine, or are inappropriate candidates for DBS treatment, treatment options are limited.
LCIG (Duodopa) provides continuous dopaminergic stimulation via infusion into the small intestine, bypassing the stomach in order to maintain stable levodopa plasma concentrations within a narrow therapeutic window. LCIG is licensed for treatment of advanced levodopa-responsive PD with severe motor fluctuations and hyper/dyskinesia when available combinations of medicinal products have not given satisfactory results. LCIG represents an efficacious therapy optionCitation7–9 in aPD who are not inadequately controlled on oral PD medications. It has been shown to significantly impact OFF time (defined here as waking time experiencing a loss of treatment effect), and improve quality-of-lifeCitation8,Citation10,Citation11 with continued positive impact on OFF time duration and ON time without troublesome dyskinesia over timeCitation7,Citation9,Citation11,Citation12. LCIG provides faster absorption of levodopa and less inter-subject variability of levodopa concentrations than oral administrationsCitation13, representing a viable treatment alternative for aPD patients.
LCIG has an orphan drug status in the EU, US, Australia, and Japan due to the small number of aPD patients and the severity of the conditionCitation6,Citation14. LCIG has recently been approved for funding by NHS EnglandCitation15. The objective of this analysis was to explore the cost-effectiveness of LCIG relative to standard care in an Irish setting.
Methods
Model structure
A simple Markov model was constructed to estimate the cost-effectiveness of LCIG relative to standard care in the treatment of aPD. The analysis was based on a previous model developed to estimate the cost-effectiveness of LCIG in a UK settingCitation10, with updated clinical data reflecting the final 12-month results of a large international prospective open-label trialCitation7. While other Markov models for PD have applied similar methodology, it was felt that making discrete adjustments (e.g. the time horizon without truncating treatment) to the model by Lowin et al.Citation10 was most suitable for this studyCitation16–18. This model incorporates the Hoehn and Yahr (HY) stage structure and off-time stages which are the most commonly used measures in clinical trials and clinical practice to describe Parkinson’s disease progression. Incorporating HY-off states is most appropriate for representing the chronic progressive nature of the disease. Standard care was defined as best available oral treatment and was deemed an appropriate comparator for the modeled patient population. The analysis was conducted from the perspective of the Irish health payer and presented in terms of the incremental cost per quality adjusted life year (QALY) gained through use of LCIG over standard care.
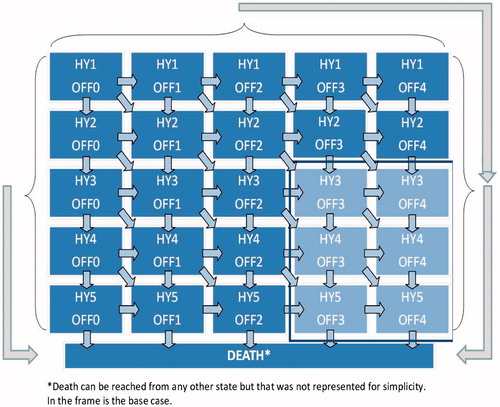
Model motor health states were defined by HY disease stage categories (stages I–V) and the amount of OFF-time experienced (expressed as the percentage of the waking day spent in an OFF state: 0–25% time in OFF = OFF I, 26–50% = OFF II, 51–75% = OFF III, 76–100% = OFF IV). The model simulated patient progression through health states over a lifetime time horizon, with patients accumulating costs and benefits while in each health state. Each OFF state allowed for a fluctuation of ±12.5% OFF time within a given cycle (reflecting the 25% band width of each cycle). The starting cohort was defined as patients with aPD with H&Y status III, IV, or V, experiencing severe motor fluctuations (greater than 50% of waking time in OFF state), and mean age of 64 yearsCitation19. Transition probabilities were estimated directly from trial data for the first year of the analysis (data on file, Abbvie) with subsequent transitions estimated by applying the expected LCIG treatment effect to long-term data on progression associated with standard care. During the first year of treatment patients could experience treatment benefit and/or progress (defined according to the trial data). In subsequent years it was assumed that the patients could either maintain their health states or progress according to natural history of disease (no long-term data is available to support improvement over time). This is an established approach to modeling PD progressionCitation20–22. The model incorporated all-cause mortality from the Irish Central Statistics OfficeCitation23 and PD-specific mortalityCitation24. The model structure is illustrated in with events modeled according to 6-month cycles.
Figure 1. Schematic overview of model structure and health states. Model schematic represents the Markov period of the model, not initial treatment benefit (up to 12 months). The diagram represents the long-term Markov model comprised of 25 PD health states and death. The lighter sub-section represents Duodopa’s current indications and the health states over which patient cohorts entering the model are distributed.
Clinical inputs
Initial treatment benefit for LCIG was defined based on patient-level data from the LCIG multi-center clinical trial (AbbVie, data on file), the main findings of which have been publishedCitation7. These data were mapped to a transition matrix in order to assess uncertainty around progression over the trial period. represents initial health state and health state movement over the 1-year trial period. In the base case, those patients who improved HY state to HY1 or HY2 during the observational period were corrected to HY3 in the first cycle of the Markov model so that initial benefit did not skew long-term results. LCIG long-term (post-12-month) treatment benefit was modeled through an assumption of sustained impact on OFF state, but no disease modification (that is, no long-term impact on HY progression). This was implemented in the model by applying a long-term treatment effect to standard care progression. Standard care progression was modeled by estimating HY and OFF progression over time. Progression through HY stages was estimated based on published transition probabilitiesCitation24. Reported monthly transition probabilities were adjusted using an exponential function to obtain the per cycle (6 month) probability of progression. As moving more than one HY stage is clinically unlikely within a 6-month period, transitions were capped so that patients could only progress one stage within a given cycle. Patients’ progression through OFF states was based on a regression of published literatureCitation21,Citation22. The two sets of transitions are reported in . Assumptions relating to the long-term treatment impact for LCIG patients on active treatment were based on published data demonstrating a sustained OFF benefit over timeCitation12,Citation25 and a review of the active treatment data (suggesting no deterioration in OFF state while on active treatment). Note that zero deterioration in OFF state would allow fluctuations in time spent in OFF of ±12.5% due to the bandwidth of the OFF state (25%). In the base case, the model conservatively assumes a 50% reduction in risk of progression while on active treatment rather than zero deterioration (the assumption is explored in sensitivity analysis). This is consistent with the previously published modelCitation10. Treatment discontinuation is defined based on trial values for the first year and then, according to published data for subsequent yearsCitation12. Long-term efficacy assumptions (including expected treatment effect and discontinuation) are reported in . The proportion of patients remaining on treatment after 5 years is assumed, in the absence of strong empirical evidence, to be 50%. This assumption is tested in sensitivity analysis.
Table 1. Patient distribution across health states at baseline and end of 6 and 12 months according to patient-level data from study S187-3-004Citation19.
Table 2. Transition probabilities (per 6-month cycle): natural history data.
Table 3. Duodopa-specific long-term efficacy and discontinuation inputs.
Resource use and costing
The cost associated with LCIG and non-LCIG use was estimated based on multiple cost sourcesCitation26–28. The direct cost of LCIG was based on expected cassette use (90% of patients were expected to be maintained on one cassette per day) and reported rates of device complications (). After 5 years on active treatment we assume that a proportion of patients will switch to oral medications. This is set to 50% in the base case and based on the expectation that a substantial proportion of patients will develop dementia over time, and that LCIG is unlikely to be continued in these patients. This assumption was based on expert opinion and tested in scenario analysis. Additional treatment-related resource use was estimated based on practice patterns defined for a UK analysisCitation10. These resources were approved by local clinicians as representative of best practice in an Irish setting. Details of the resource use are provided in .
Table 4. Resource use for LCIG and SoC.
Health state costs and concomitant medication use for LCIG and non-LCIG patients were estimated based on a large scale resource survey, the Adelphi Disease-Specific Program (DSP), previously reported for a UK analysis of the datasetCitation4. Medication costs for non-LCIG patients were further split by regimens containing apomorphine vs those based on oral medications only, with costs assigned based on the latest published MIMSCitation26. Costs are reported in . Direct medical (hospitalizations, consultations, tests) and non-medical resource (professional caregiver consultations) associated with the 25 health states was estimated based on dataset observations, with costs applied from the perspective of the Irish health payer. Where local data were unavailable, appropriate assumptions were applied. Within the Adelphi DSP dataset, observations were heavily weighted toward less severe health states. In order to estimate resource use costs across all health states, a series of regression models were developed to estimate the relationship between health states and direct medical and non-medical costs. Indirect costs such as those relating to informal care by friends and family were not included in the health state costs. Estimated health state costs are reported in .
Table 5. Medical resource unit cost.
Table 6. Health state costs (predicted vs actual).
Health state utility
Health state utilities were estimated from a pooled dataset of EQ5D data (AbbVie data on file) derived from four key PD studies the Adelphi DSP (n = 1,410), patient-level data (n = 321) of published clinical studyCitation7, DAPHNE (Duodopa in Advanced Parkinson’s: Health Outcomes & Net Impact, CTgov ref NCT00141518) (n = 77), and GLORIA (n = 354)Citation29. The relationship between H&Y stage, OFF state, and utility was estimated from a regression equation obtained from a GEE (generalized estimating equation) analysis of the pooled dataset. Combining the EQ5D data from these studies allowed an increase in the sample size for more severe health states, improving the precision of the estimation of the utility in these states. Regression outputs indicated a robust relationship between H&Y status, OFF state, and EQ5D, with the utility impact of worsening a single H&Y state estimated at –0.110 and of worsening a single OFF state estimated at –0.030. Additional detail relating to the utility analysis is available on request. Estimated health state utilities are reported in . Scenario analysis assesses the impact of use of alternate utilities for more advanced states.
Table 7. Estimated utility used in model analyses.
Treatment of uncertainty
Uncertainty around key model inputs was assessed through exploration of plausible variations in input parameters through univariate sensitivity analysis and application of probabilistic sensitivity analysis (PSA). Upper and lower limits for the univariate analyses and PSA distributions were set according to best practice. In addition, scenario analyses explored those parameters associated with the greatest level of uncertainty, namely (1) the impact of different patient distributions after the first 12 months of treatment, i.e. the patients who moved to HY1 or HY2 over the trial period were allowed to progress according to expected natural history, (2) increased/reduced long-term impact of progression through OFF states, (3) cross-reference to an alternate utility set for advanced health states where health state utilities less than zero were applied (as reported in Lowin et al.Citation10, HY1 and HY2 not reported and so utility not adjusted, OFF 0 not reported, OFF 0 instead mapped to reported OFFI utility), (4) alternate assumptions relating to the proportion of patients maintained on two cassettes (95% of patients maintained on one cassette), and (5) alternate assumptions relating to the proportion of patients remaining on treatment after 5 years (in the absence of strong empirical evidence, the 50% base case assumption is varied by ±20%, assuming between 30–70% of patients on active treatment at 5 years switch back to standard care medications).
Results
The analysis cohort comprised patients with aPD and severe motor fluctuations (defined as patients stage H&Y 3, 4 or 5 with >50% of waking time spent in OFF). In the base-case analysis, per patient costs over the lifetime of the patient were estimated at €537,687 for LCIG compared to €514,037 for standard care. Expected life years per patient were 10.10 for LCIG and 9.76 for standard care, and the expected per patient QALYs were estimated at 4.37 and 3.49, respectively. The model estimated an incremental cost per life years gained (LYG) for LCIG relative to standard care of €70,480, and a cost per QALY gained of €26,944.
The expected distribution of patients over time differed according to the treatment arm (). LCIG patients spent less time in higher OFF states compared to patients maintained on standard care. In addition, initial improvement in HY state (as modeled from trial data) means that short-term improvements in HY are realized. The results of the core sensitivity analyses are summarized in . The model was most sensitive to variations in health state costs and assumptions relating to long-term benefit and discontinuation, with outputs varying from cost savings to incremental cost-effectiveness ratios (ICERs) estimated in the region of €60,000 per QALY gained (at extreme values of health state costs). The model was not sensitive to assumptions related to cost of treatment-related visits, AE rates for LCIG, or the discount rate selected, and demonstrated limited sensitivity to changes in short-term (up to 12 month) drop-out rate. Scenario analyses are reported in . Outputs from these analyses indicate that the model was sensitive to assumptions around the application of utility in advanced health states; assumptions relating to disease modification; and assumptions relating to time on treatment; where the assumption of treatment truncation at 5 years was modified, the ICER range was estimated between €14,000–€51,000 per QALY gained.
Figure 2. Distribution of patients over time for Duodopa and SoC. Each color shade represents a different health state. From the outside working inwards: Tan/beige shades = death; Blue shades = HY5; Red shades = HY4; Green shades = HY3; Purple shades = HY2; Turquoise shades = HY1.
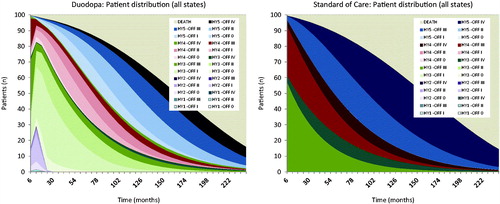
Figure 3. One-way sensitivity analyses. Uncertainty around key model inputs was assessed through exploration of plausible variations in input parameters. Long-term efficacy and health states costs were key drivers of the ICER.
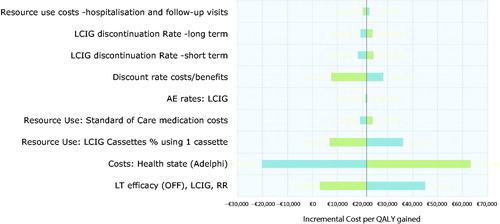
Table 8. Scenario outputs.
Discussion
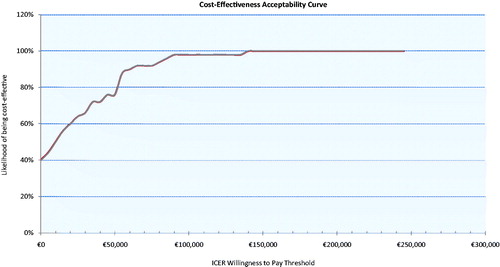
This paper reports a robust previously published model adapted to an Irish setting and updated with results from long-term data from an international open label clinical trial with a large number of patients. Based on current model assumptions, we found that LCIG was associated with substantial QALY gains in aPD patients when compared against standard care alongside limited increased lifetime costs, resulting in an estimated ICER of €26,944 per QALY gained. This estimate is well within the current stated cost-effectiveness threshold of €45,000 for drug interventions in Ireland. The trial on which this analysis is based represents the largest sample of LCIG patients studied and reported to date and, while there remains uncertainty around expected effectiveness, sensitivity analyses show that the model was stable to plausible changes in input values with the CEAC, indicating 76% probability of cost-effectiveness at a willingness-to-pay threshold of €45,000 per QALY gained ().
Figure 4. Cost-effectiveness acceptability curve. At a WTP threshold of €45,000, LCIG has a 76% likelihood of being cost-effective.
Standard of care was selected as the relevant comparator for a number of reasons. LCIG and DBS patients comprise different cohorts, with different demographics and clinical characteristics. For example, DBS is typically not recommended for patients >70 years of age, whereas LCIG could be used in all ages. In addition to age, cognitive decline, level of dyskinesia, motor fluctuations, patient preferences, and levodopa responsiveness can also influence the choice of therapy. Recent consensus studies further corroborate the differences in patient population who would be considered as appropriate candidates for advanced therapies such as LCIG and DBSCitation30,Citation31. Due to these differences as well as the challenge in randomization, blinding, and the ethical concerns around a sham surgery, LCIG and DBS are never directly compared in clinical trials. Thus, it was not considered appropriate to include DBS as a relevant comparator.
A 20-year lifetime time horizon was considered appropriate for capturing the outcome of all patients in this model based on the time horizon assumptions applied in other models for patients with aPDCitation16–18. While the patients included in the model might be those with most advanced disease, we believe a 20-year time horizon is appropriate. It is possible that, despite their advanced age and severity of the disease, some patients will remain on treatment for longer than 5–10 years. Additionally, while Lowin et al.Citation10 truncated all LCIG treatment after 5 years, in this model, this was adapted whereby only 50% of patients were assumed to discontinue LCIG and switch to oral medication after 5 years due to the development of dementia and subsequent unsuitability of treatmentCitation10. This was based on expert clinical opinion of the use of LCIG in real-world clinical practice.
Sensitivity analyses indicated that the model was most sensitive to health state costs, use of more than one LCIG cassette, the expected long-term efficacy of LCIG, and time on treatment. The approach to health state costing follows previous work through application of costs to a large scale resource study conducted in the UK in 2010Citation4. The costing is subject to uncertainty through limited availability of resource use data from the Adelphi study, for the more advanced health states and through lack of inclusion of Irish patients. Nonetheless, the regression applied is an established methodology for filling these missing dataCitation32, and the approach to costing follows best practice guidelines in that, where possible, local costs were applied. In the absence of an alternate large-scale dataset, the approach taken represents an established methodology but lack of sufficient numbers of observed PD subjects in higher states is a limitation of the analysis. In addition, the complexities of the Irish costing system are in the process of update, and it would be interesting to revisit this analysis when additional costs become available.
Parameters relating directly to LCIG have most impact on model outcomes and, despite an increasing evidence base, remain the parameters associated with the most uncertainty. The high impact of assumptions relating to LCIG cassette use is not unexpected given the high cost of cassette; in the base case we apply a reasonably conservative approach, assuming 10% of patients may require two cassettes per day, an assumption which is broadly in line with an ad-hoc assessment of LCIG supply in Ireland (where use of two cassettes is found in less than 10% of patients, personal communication by AbbVie). However, the long-term effectiveness of interventions for aPD and expected time on treatment before switch back to oral medication remains uncertain. Lack of robust long-term data has been noted as a limitation in another model; however, LCIG has had a sustained effect because patients continued with it for years, in spite of the inconvenience of a PEGCitation6. In this analysis, we assumed a 50% decrease in risk of progression to higher OFF states when on LCIG relative to standard care medication. This assumption follows previous methodologyCitation10, and may be conservative, with longer term data suggesting sustained effects over timeCitation12,Citation25,Citation33–35. Uncertainty around the patient pathway of aPD patients means that it is difficult to predict long-term outcomes for individual cohorts of patients. In the absence of robust empirical evidence to estimate time on treatment, we apply a modifier in the model which assumed that, after 5 years on active treatment, a substantial proportion of patients switch back to standard medication; this assumption is based on the opinion of the clinical author. Although it requires further exploration, it is thought to be consistent with the likely cognitive decline of aPD patients and consequent truncation of LCIG. The assumption was explored in sensitivity analysis, where patients were assumed to switch to standard medication after 10 years of active treatment and the model was reasonably sensitive to this. Further research is key to support the ICERs reported in the current analyses and given current uncertainty, the expected cost-effectiveness of LCIG may better be considered as within a range of the values presented here.
Despite the remaining uncertainty around core model inputs, in particular the long-term expectations around treatment effect and duration, the current model represents a robust structure for assessment of the expected benefits of LCIG use in an Irish setting. The model updates previously reported treatment effects based on a robust international trial conducted in a large cohort of aPD patientsCitation19. The model generated QALY gains in line with previously published studiesCitation10,Citation36, empirically supported by the outcomes of the 12-month analysisCitation19, and in contrast to other previously published cost-effectiveness studiesCitation37. Although associated with a higher cost than standard care, LCIG was associated with greater QALYs. In the current study, the ICER was estimated in the region of ∼€27,000 per QALY gained with a plausible range reasonably defined within the limits set by the scenario analyses (reported range of €14,000–€53,000 per QALY gained).
Our study is based on current model assumptions and includes trial data from the largest sample of LCIG patients studied, including 12-months follow-up of LCIG-naïve patients. While there is a lack of consensus around accepted cost-effectiveness thresholds for orphan drugsCitation38,Citation39, the ICERs reported are indicative of value for money from a payer perspective and within established thresholds for standard cost-effectiveness evaluations.
Conclusions
LCIG can be considered a cost-effective treatment for advanced PD. Despite limitations, this study adds effectively to the evidence base on use of LCIG in patients with aPD, and supports initiation of a dialog to consider increased funding in a population currently constrained by limited treatment options and availability. This analysis adds substantially to the existing literature in the aPD area and models new data showing improved outcomes at 12 months in a large international cohort of LCIG-treated patients. Despite increasing interest and research in the area of PD, long-term data remain limited, and there is a clear unmet need for publication of real world evidence relating to long-term outcomes of PD. Once these data become available, an update of the current model is strongly recommended.
Transparency
Declaration of funding
This study and manuscript were funded by AbbVie. The design, study conduct, and financial support for the study were provided by AbbVie. AbbVie participated in the study design, research, interpretation of data, writing, review, and approval of the manuscript.
Declaration of financial/other relationships
JL and HK were/are salaried employees of QuintilesIMS, who received consultancy fees from AbbVie. KS, TM, YJJ, and RB are employees of AbbVie, and may own AbbVie stock or stock options; they contributed to study design, data interpretation, manuscript development, and review. KRC has received educational funding from UCB and honoraria for sponsored symposiums from UCB, AbbVie, Britannia, US Worldmeds, Otsuka, Medtronic, and Zambon. He has also acted as a consultant for AbbVie, UCB, Britannia, Medtronic, and Mundipharama. KRC supported the study with his clinical expertise and contributed to the manuscript and review. The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
We would like to acknowledge Professor Michael Hutchinson, Department of Neurology, Saint Vincent’s Hospital Dublin, Ireland, for his input on the clinical practice in Ireland.
References
- Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 2011;26:399-406
- Chaudhuri KR, Healy DG, Schapira AH, et al. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 2006;5:235-45
- Findley LJ. The economic impact of Parkinson’s disease. Parkinsonism Relat Disord 2007;13(Suppl):S8-S12
- Findley LJ, Wood E, Lowin J, et al. The economic burden of advanced Parkinson’s disease: an analysis of a UK patient dataset. J Med Econ 2011;14:130-9
- Fineberg NA, Haddad PM, Carpenter L, et al. The size, burden and cost of disorders of the brain in the UK. J Psychopharmacol 2013;27:761-70
- Kristiansen IS, Bingefors K, Nyholm D, et al. Short-term cost and health consequences of duodenal levodopa infusion in advanced Parkinson’s disease in Sweden: an exploratory study. Appl Health Econ Health Policy 2009;7:167-80
- Fernandez HH, Standaert DG, Hauser RA, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord 2015;30:500-9
- Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 2014;13:141-9
- Slevin JT, Fernandez HH, Zadikoff C, et al. Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis 2015;5:165-74
- Lowin J, Bergman A, Chaudhuri KR, et al. A cost-effectiveness analysis of levodopa/carbidopa intestinal gel compared to standard care in late stage Parkinson’s disease in the UK. J Med Econ 2011;14:584-93
- Puente V, De FO, Oliveras C, et al. Eighteen month study of continuous intraduodenal levodopa infusion in patients with advanced Parkinson’s disease: impact on control of fluctuations and quality of life. Parkinsonism Relat Disord 2009;16:218-221
- Nyholm D, Klangemo K, Johansson A. Levodopa/carbidopa intestinal gel infusion long-term therapy in advanced Parkinson’s disease. Eur J Neurol 2012;19:1079-85
- Othman AA, Dutta S. Population pharmacokinetics of levodopa in subjects with advanced Parkinson’s disease: levodopa-carbidopa intestinal gel infusion vs. oral tablets. Br J Clin Pharmacol 2014;78:94-105
- Willis M, Persson U, Zoellner Y, et al. Reducing uncertainty in value-based pricing using evidence development agreements: the case of continuous intraduodenal infusion of levodopa/carbidopa (Duodopa(R)) in Sweden. Appl Health Econ Health Policy 2010;8:377-86
- NHS England. NHS England announces annual investment decisions for certain specialised services 2015; December 2015. Available at: https://www.england.nhs.uk/2015/07/annual-investment-decisions/
- Dams J, Balzer-Geldsetzer M, Siebert U, et al. Cost-effectiveness of neurostimulation in Parkinson’s disease with early motor complications. Mov Disord 2016;31:1183-91
- Eggington S, Valldeoriola F, Chaudhuri KR, et al. The cost-effectiveness of deep brain stimulation in combination with best medical therapy, versus best medical therapy alone, in advanced Parkinson’s disease. J Neurol 2014;261:106-16
- Kawamoto Y, Mouri M, Taira T, et al. Cost-effectiveness analysis of deep brain stimulation in patients with Parkinson’s disease in Japan. World Neurosurg 2016;89:628-35 e1
- Fernandez HH, Vanagunas A, Odin P, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease open-label study: interim results. Parkinsonism Relat Disord 2013;19:339-45
- Davey P, Rajan N, Lees M, et al. Cost-effectiveness of pergolide compared to bromocriptine in the treatment of Parkinson’s disease: a decision-analytic model. Value Health 2001;4:308-15
- Palmer C, Schmier J, Snyder E, et al. Patient preferences and utilities for ‘off-time’ outcomes in the treatment of Parkinson’s disease. Quality Life Res 2000;9:819-27
- Palmer C, Nuijten MJ, Schmier J, et al. Cost effectiveness of treatment of Parkinson’s disease with entacapone in the United States. Pharmacoeconomics 2002;20:617-28
- Irish Central Statistics Office. Irish Life Tables 2010–2012. 2012. Available online at http://www.cso.ie/en/releasesandpublications/er/ilt/irishlifetablesno162010-2012/
- Johnson SJ, Diener MD, Kaltenboeck A, et al. An economic model of Parkinson’s disease: implications for slowing progression in the United States. Mov Disord 2013;28:319-26
- Caceres-Redondo MT, Carrillo F, Lama MJ, et al. Long-term levodopa/carbidopa intestinal gel in advanced Parkinson’s disease. J Neurol 2014;261:561-9
- MIMS Ireland September 2015; 2015. https://www.imt.ie/mims/
- Health Service Executive. National Casemix Programme. Ready Reckoner of Acute Hospital inpatient and day case activity and costs (summarised by DRG) relating to 2011 costs and activity; 2013. Ireland: Health Service Executive. Report No
- NHS reference costs 2014–2015; 2015. Available online at https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015
- Antonini A, Yegin A, Preda C, et al. Global long-term study on motor and non-motor symptoms and safety of levodopa-carbidopa intestinal gel in routine care of advanced Parkinson’s disease patients; 12-month interim outcomes. Parkinsonism Relat Disord 2015;21:231-5
- Antonini A, Tolosa E. Apomorphine and levodopa infusion therapies for advanced Parkinson’s disease: selection criteria and patient management. Expert Rev Neurotherapeut 2009;9:859-67
- Odin P, Ray Chaudhuri K, Slevin JT, et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord 2015;21:1133-44
- Harkanen T, Maljanen T, Lindfors O, et al. Confounding and missing data in cost-effectiveness analysis: comparing different methods. Health Econ Rev 2013;3:8
- Antonini A, Odin P, Opiano L, et al. Effect and safety of duodenal levodopa infusion in advanced Parkinson’s disease: a retrospective multicenter outcome assessment in patient routine care. J Neural Transm (Vienna) 2013;120:1553-8
- Zibetti M, Merola A, Artusi CA, et al. Levodopa/carbidopa intestinal gel infusion in advanced Parkinson’s disease: a 7-year experience. Eur J Neurol 2014;21:312-18
- Buongiorno M, Antonelli F, Camara A, et al. Long-term response to continuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: the Barcelona registry. Parkinsonism Relat Disord 2015;21:871-6
- Walter E, Odin P. Cost-effectiveness of continuous subcutaneous apomorphine in the treatment of Parkinson’s disease in the UK and Germany. J Med Econ 2015;18:155-65
- Lundqvist C, Beiske AG, Reiertsen O, et al. Real life cost and quality of life associated with continuous intraduodenal levodopa infusion compared with oral treatment in Parkinson patients. J Neurol 2014;261:2438-45
- Drummond MF. Challenges in the economic evaluation of orphan drugs. Eurohealth 2008;14:16-7
- Simoens S. Pricing and reimbursement of orphan drugs: the need for more transparency. Orphanet J Rare Dis 2011;6:42
