Abstract
Background: In Argentina, varicella vaccination was included in the national schedule for mandatory immunizations in 2015. The vaccine has been shown to substantially reduce the morbidity and mortality associated with the virus. The purpose of this study was to evaluate the clinical and economic burden associated with varicella in Argentina prior to vaccine introduction.
Methods: This was a multi-center, retrospective chart review study among patients aged 1–12 years with a primary varicella diagnosis in 2009–2014 in Argentina. Healthcare resource utilization (HCRU) associated with varicella and its complications, unit costs, and work loss were used to estimate direct and indirect costs. All costs are presented in 2015 United States dollars (USD).
Results: One hundred and fifty children with varicella were included (75 outpatients, 75 inpatients), with a mean age of 3.8 (SD = 2.4) and 2.9 (SD = 2.2) years, respectively. One or more complications were experienced by 28.0% of outpatients and 98.7% of inpatients, the most common being skin and soft tissue infections, pneumonia, sepsis, cerebellitis, and febrile seizure. HCRU estimates included use of over-the-counter (OTC) medications (58.7% outpatients, 94.7% inpatients), prescription medications (26.7% outpatients, 77.3% inpatients), tests/procedures (13.3% outpatients, 70.7% inpatients), and consultation with allied health professionals (1.3% outpatients, 32.0% inpatients). The average duration of hospital stay was 4.9 (95% CI = 4.2–5.7) days, and the average duration of ICU stay was 4.8 (95% CI = 1.6–14.1) days. The total combined direct and indirect cost per varicella case was 2947.7 USD (inpatients) and 322.7 USD (outpatients). The overall annual cost of varicella in Argentina for children aged ≤14 years in 2015 was estimated at 40,054,378.0 USD.
Conclusion: The clinical burden of varicella in Argentina was associated with utilization of significant amounts of healthcare resources, resulting in substantial economic costs. These costs should be reduced with the recent implementation of routine vaccination of children.
Introduction
Varicella is caused by the varicella-zoster virus (VZV), and is a highly contagious infectious disease usually acquired in childhood. The incubation period is 10–21 days, at which point symptoms of the infection, including fever, malaise, headache, and abdominal pain, can emergeCitation1,Citation2. While most varicella cases are associated with a generalized pruritic vesicular rash, secondary effects, such as neurologic complications, skin and soft tissue complications, gastrointestinal or lower respiratory involvement, and pneumonia, can occasionally occurCitation3,Citation4.
While varicella incidence rates vary by region due to differences in age, immunosuppression, and climate, the estimated annual worldwide incidence of varicella is ∼2–16 cases for every 1,000 personsCitation5–7. In Argentina, the incidence of varicella between 2001 and 2010 was 3.1 cases per 1,000 personsCitation6, with 133,434 documented cases reported in 2015Citation8. However, many patients with uncomplicated, mild cases of varicella tend to forgo seeking medical care; consequently, the true annual number of cases may be under-estimated. As Argentinian estimates have found 97.6% varicella seroprevalence by the age of 15–19Citation9, one can assume that, without a vaccination program, the annual number of patients with varicella would be comparable to the birth cohort in Argentina in any given year (772,012 births in 2014)Citation10.
Varicella treatment guidelines in Argentina are designed primarily for at-risk individuals, and include the use of antiviral drugs, such as acyclovir, in immunocompromised patients, pregnant women, cases of secondary varicella infection, and, if able to treat in the first 24 hours of rash onset, any individual at risk of complicationsCitation11. The prophylactic varicella vaccine, initially only reimbursed for certain people who were either at risk from exposure, or at risk of transmissionCitation12, was recently added to the list of mandatory childhood immunizations, beginning in 2015, as part of a national strategy to reduce the overall varicella burden in the populationCitation11.
All varicella vaccines currently available are derivatives of the live attenuated Oka strainCitation13, with the exception of one vaccine, authorized for use in South Korea since 1993, and manufactured using the MAV/06 strain (SUDUVAX, Green Cross South Korea)Citation14. Oka-derived vaccines, which have been licensed since the mid-1980s, are generally well tolerated and effective, and are available as monovalent formulations or as quadrivalent combinations including measles, mumps, and rubella vaccines (MMRV)Citation15–20. Monovalent varicella vaccines that have been assessed for safety and efficacy in double-blind placebo controlled trials include VARIVAX (Merck & Co, Inc.)Citation15, VARILRIX (GlaxoSmithKline)Citation16, and OKAVAX (Biken)Citation17. Furthermore, in post-licensure settings, a recent meta-analysis found overall vaccine effectiveness to be 81% (95% CI = 78–84%) for one-dose vaccine schedules, and 92% (95% CI = 88–95%) for two dose schedulesCitation21. No vaccine effectiveness data is available for the MAV/06 strain vaccine. All varicella-containing vaccines are licensed for use among persons aged ≥12 months; in certain countries, one monovalent and two combined vaccines are licensed for use from 9 months of ageCitation22.
With respect to immunogenicity, a systematic review assessing the duration of protection conferred by varicella vaccines reported no waning of immunity for up to 14 years in a setting where VARIVAX was the only vaccine in routine use in children in the USCitation23. In settings where multiple varicella vaccines were in routine use, findings were mixed; some studies indicated no waning of protection for up to 7 yearsCitation24, while others showed that the risk of developing breakthrough varicella was higher starting 4–5 years after vaccinationCitation25,Citation26.
Several countries have included varicella vaccine as part of their immunization programs and have observed a dramatic decline in morbidity and mortality associated with varicella. In a recent review of the impact of varicella vaccine on hospitalization, reductions in hospitalizations rates ranged from 81.4–99.2% in the US, 95.2% in Spain, 94.0% in Uruguay, 93.0% in Canada, 84% in Italy, 77.6% in Germany, and 76.8% in AustraliaCitation27. In the US, the one dose childhood varicella vaccination program in 1996 lead to a 90% decrease in varicella incidence by 2005Citation28; following the implementation of a 2nd dose in 2006, by 2014 there was a further 84.6% decreaseCitation29—an overall decrease of 97.4% from the pre-vaccine period.
The primary objective of this study was to describe the burden of illness associated with varicella among children (1–12 years of age) in Argentina between 2009 and 2014 by assessing morbidity, healthcare resource utilization (HCRU), and the associated cost, separately for outpatients and inpatients, prior to the inclusion of the varicella vaccine in the mandatory immunization program. These results may also be used in future economic analyses for benchmarking VZV-related resource use and associated costs in real-world Argentinian settings.
Methods
Study design
This was a multi-center, retrospective chart review study evaluating the burden of illness associated with varicella. Study conduct followed the generally accepted standards of Good Pharmacoepidemiology PracticeCitation30, and ethics approvals were obtained for all sites from the respective local ethics committees as well as the provincial authorities when required. Since data were anonymously collected in a retrospective manner, identifiable only by an encrypted patient number, no patient consent was required.
Study population and case selection
Seven sites (six hospitals and one private practice) were selected to contribute 150 patient charts for inclusion and review in the current study. Of these, six sites were in urban centers [Buenos Aires (n = 2), Cordoba, San Salvador de Jujuy, Guaymallen, and San Justo] with one site considered rural (San Francisco; hospital-based setting).
For case selection, investigators were instructed to screen patient charts in their practices, starting from the most recent year and going back 5 years. Charts were considered eligible if a primary varicella diagnosis was identified between 2009 and 2014, and if the patient was 1–12 years of age at the time of diagnosis. For outpatients who revived a doctor’s note for absence from school, a second doctor’s note confirming that the child was fit to return, obtained at a follow-up visit, was required for inclusion; this practice is standard as per routine clinical care in Argentina. For all included patients, the date of primary varicella infection was defined as the index date, and each patient’s chart was reviewed from this date until the resolution of the disease or the last date of contact, if the resolution date was unavailable. All those patients who had a second case of varicella, those with varicella as a secondary diagnosis, or those who were previously vaccinated for varicella and had a diagnosis of breakthrough varicella, were excluded.
In addition, patients were classified into two separate populations: those who visited either a doctor’s office (family doctor, general practitioner, pediatrician, or infectious disease specialist), outpatient clinic/department of hospital, or emergency department (ER), without hospitalization for varicella, were listed as outpatients, and those who were admitted to a hospital were enrolled as inpatients. To allow generalizability of the study results to both populations, efforts were made during chart selection to obtain an approximate 1:1 ratio of outpatients to inpatients.
Outcome measures
Clinical complications of varicella were identified from patient charts; these included, but were not limited to: skin and soft tissue infection, meningitis, encephalitis, pneumonia, sepsis, acute osteomyelitis, septic arthritis, cerebellitis, keratoconjunctivitis, hepatitis, nephritis, febrile seizure, dehydration, severe pain, and coagulation disorder. The Medical Dictionary for Regulatory Activities (MedDRA; version 18.0) was used to code any other complications aside from those listed above, which were then reported by the preferred term. The number and proportion of patients using each resource, the frequency of use, and the duration of healthcare resource use for varicella and varicella-related complications was used to assess HCRU, which included the following resources: outpatient visits, doctor’s visits, allied healthcare contacts (defined as visits to a physiotherapist, social worker, or specialized physician), tests/procedures performed (primarily imaging modalities, cultures, confirmatory VZV assessments), prescription medications prescribed (antibiotics, antivirals, immunoglobulins, and others), over-the-counter (OTC) medications (acetaminophen, ibuprofen, “other” anti-inflammatory medication, topical anti-itch creams, oral antihistamines, and “other” OTC medication), hospitalizations, ER visits/stays, and intensive care unit (ICU) stays.
To calculate the direct costs, a “bottom up” cost estimate approach was adopted. Summarized in , unit costs for prescriptionCitation31,Citation32 and OTC medication useCitation31,Citation32, doctor’s visitsCitation33–36, allied healthcare contactsCitation37–39, tests/proceduresCitation34–36,Citation39–41, hospitalization stay (per day)Citation33,Citation35,Citation36,Citation41,Citation42,Citation43, ER visits/staysCitation41, and ICU stays (per day)Citation41,Citation44 were derived from the literature and from Argentinian and international data sources. Average unit costs were used for parameters with multiple sources. Unit costs were then multiplied by the amount of resources used per patient to derive per patient direct costs. For prescription and OTC medications, each medication type was costed individually; individual costs were then summed to derive total costs within the respective medication parameter. A similar costing method was used for tests/procedures and allied healthcare professional contacts. Conversion to 2015 prices was based on the Consumer Price Index (CPI) for ArgentinaCitation45.
Table 1. Key unit costs (2015 USD)Table Footnote† for healthcare resources.
The indirect costs were calculated as the loss of revenue incurred by caregivers as a result of caring for varicella-infected children, using the number of work days the caregiver missed, along with the national average income statistics reported by the Organization for Economic Co-Operation (OECD)Citation46. The number of days spent in the hospital/ICU was used to estimate the number of work days missed for inpatients, and the duration of time elapsed between issuance of a doctor’s note to stay home and the return to school (based on estimates derived from the current study population) was used to estimate work days lost for outpatients.
Annual costs (direct, indirect, and total), were then extrapolated to children ≤14 years of age. This extrapolation was based on the calculated cost per varicella case, the number of varicella cases reported for 2015 in Argentina (n = 133,434)Citation8 (only cases who sought medical care would be captured in the surveillance system), the proportion of varicella cases in Argentina attributed to patients ≤14 years of age (91.82%)Citation12, and the percentage of cases in this age group hospitalized reported for Brazil based on the Brazilian Unified Health System Information Department (DATASUS) (0.16%)Citation47. Taking into consideration known under-reporting of hospitalizations to DATASUSCitation48, as well as expert opinion which found the Brazilian hospitalization rate due to varicella low in the context of an Argentinian clinical setting, a sensitivity analysis of the extrapolated costs was also conducted. This sensitivity analysis took into consideration two scenarios: (1) a VZV-related hospitalization rate of 2.5% of all cases, based on expert opinion, and (2) a VZV-hospitalization rate of 4.0% of all cases, based on a published abstract that reviewed pediatric charts in two different hospitals in ArgentinaCitation49.
All costs are presented in 2015 USD [1 Argentine Peso (ARS) = 0.11 USD at the time of data analysis]Citation50.
Statistical methods
All enrolled patients were included in the statistical analysis, and sub-group analyses were performed for outpatients and inpatients. All study objectives were addressed using descriptive statistics which, for continuous variables, included the mean and SD or 95% CI, and, for categorical variables, included frequency distributions. Logarithmic transformation was used for the calculation of 95% CIs for any outcome measure that had a low number of cases. SAS software version 9.4 (SAS Institute Inc., Cary, NC) was used to perform all analyses.
Results
summarizes the patient and disease characteristics of the study cohort at varicella diagnosis. A total of 75 outpatients and 75 inpatients were included in this study, for a total of 150 patients. The mean age of outpatients was 3.8 (SD = 2.4) years, and for inpatients it was 2.9 (SD = 2.2) years, with slightly more male patients than females enrolled in each group (53.3% and 61.3%, respectively). In both groups, patients predominantly (outpatients = 48.0%; inpatients = 66.7%) presented with 50–249 lesions during their rash outbreak. The outpatient group had a higher number of patients presenting with <50 skin lesions (26.7% vs 4.0%), whereas the inpatient group had more patients presenting with >500 lesions (2.7% vs 10.7%). All but one inpatient (98.7%) experienced at least one varicella-related complication, compared to 28.0% of outpatients (). Of those experiencing complications, 4.8% of outpatients had more than one complication, compared to 23.0% of inpatients (). Of note, only one (1.3%) patient in each group was considered immunocompromised.
Figure 1. Types of complications associated with varicella. *Proportions based on the total number of patients. †Proportions based on the total number of complications. ‡Inpatients: Other includes encephalitis (3.1%), keratoconjunctivitis (2.1%), dehydration (2.1%), hemorrhagic varicella (2.1%), arthritis (1.0%), gingivostomatitis (1.0%), myositis (1.0%), pancytopenia (1.0%), preseptal cellulitis left eye (1.0%), septic arthritis (1.0%), scarlatiniform rash (1.0%).
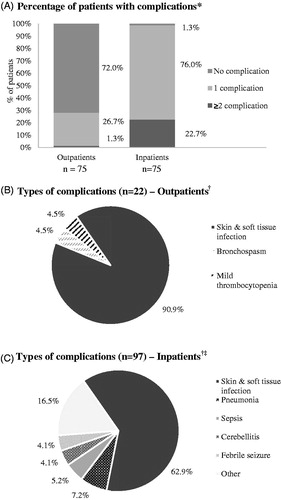
Table 2. Patient and disease characteristics at varicella diagnosis.
presents the varicella-related complications reported for outpatients and inpatients, respectively. The most common complications for outpatients were skin and soft tissue infection (90.9% of all complications), bronchospasm (4.5%), and mild thrombocytopenia (4.5%), whereas, for inpatients, frequent complications included skin and soft tissue infection (62.9%), pneumonia (7.2%), sepsis (5.2%), cerebellitis (4.1%), and febrile seizure (4.1%). The inpatient group also experienced additional complications, such as encephalitis (3.1%), keratoconjunctivitis (2.1%), dehydration (2.1%), hemorrhagic varicella (2.1%), arthritis (1.0%), gingivostomatitis (1.0%), myositis (1.0%), pancytopenia (1.0%), preseptal cellulitis of the left eye (1.0%), septic arthritis (1.0%), and scarlatiniform rash (1.0%).
reports the varicella-associated HCRU by patient group. Among outpatients, hospital outpatient clinics were more frequently visited (86.7% of patients; 81.3% visited more than once) compared to doctor’s offices (12.0%; 12.0% visited more than once) or ERs (10.7%; 1.3% visited more than once); patients may have reported more than one outpatient healthcare contact. Among outpatients using these resources, the average number of times visiting a doctor’s office or hospital outpatient clinic was slightly more than two [2.2 (95% CI = 1.4–3.3) and 2.1 (95% CI = 1.8–2.5), whereas the ER was visited, on average, once per person [1.1 (95% CI = 0.5–2.0)]. Following hospital outpatient clinic visits, medications were the most commonly used resource among outpatients, with 58.7% using OTC medications at a rate of 1.4 (95% CI = 1.1–1.8) per patient among users (most commonly antihistamine medications), and 26.7% using prescription medications (predominantly antibiotics), with an average use per patient (among users) of 1.8 (95% CI = 1.3–2.5). Tests/procedures and allied health professional consultations were used by 13.3% and 1.3% of outpatients, with a mean use per patient of 2.4 (95% CI = 1.6–3.5) and 1.0, respectively.
Table 3. Varicella associated healthcare resource utilization.
Among the inpatient group, the mean number of days spent in the hospital was 4.9 (95% CI = 4.2–5.7) with 8.0% of patients requiring admittance to the ICU for an average of 4.8 (95% CI = 1.6–14.1) days among users. The resource used most often by inpatients was prescription medications [77.3% of patients; mean number per patient among users: 2.3 (95% CI = 2.0–2.7)], most commonly antibiotics, and OTC [94.7%; 2.6 (95% CI = 2.3–3.0) per patient among users], mainly anti-inflammatory medications. In terms of outpatient services, 4.0% of inpatients had visited a doctor’s office [3.0 (95% CI = 1.4–5.4) visits per patient among users], 26.7% had visited an ER [1.1 (95% CI = 0.7–1.6) per patient among users] and 17.3% visited a hospital outpatient clinic [1.5 (95% CI = 0.9–2.2) per patient among users] prior to hospital admission. Tests/procedures and allied health professional consultations were used among 70.7% [mean number per patient = 6.8 (95% CI = 6.1–7.5)] and 32.0% [5.4 (95% CI = 4.5–6.4)] of inpatients, among users, respectively.
Actual direct and indirect associated costs per varicella case for outpatients and inpatients by type of resource utilized is summarized in . The overall mean direct cost per patient for outpatients was 115.7 (95% CI = 105.7–125.7) USD and for inpatients it was 2804.6 (95% CI = 2085.0–3524.1) USD. For outpatients, visits to hospital outpatient clinics accounted for most of the overall direct cost [mean = 93.0 (95% CI = 82.4–103.6) USD], whereas hospitalization cost [mean = 2464.6 (95% CI = 1949.7–2979.5) USD] made up the principal portion of the overall direct cost for inpatients. The indirect cost of varicella was similar between outpatients and inpatients, with mean costs per case of 207.0 (95% CI = 179.8–234.2) USD and 143.2 (95% CI = 113.6–172.8) USD, respectively.
Table 4. Cost (USD) per pediatric case of varicella.
presents the extrapolated annual costs (direct, indirect, and total) associated with varicella among all children ≤14 years of age in Argentina. These estimates were based on the cost per varicella case reported above, the number of varicella cases reported for 2015 in Argentina (n = 133,434)Citation8, the proportion of varicella cases in Argentina attributed to patients ≤14 years of age (91.82%)Citation12, and the percentage of cases in this age group hospitalized reported for Brazil (hospitalization rate = 0.16%)Citation47. Based on an estimated annual incidence of 122,492 pediatric (≤14 years of age) varicella cases who sought care, consisting of 195 inpatients and 122,296 outpatients, the total estimated annual direct and indirect costs associated with varicella in pediatric patients in Argentina for 2015 are 14,699,250.8 USD and 25,355,127.2 USD, respectively, for a total cost of 40,054,378.0 USD.
Table 5. Estimated annual (2015) costs (USD) for children with varicella in Argentina.Table Footnote†
Results of the sensitivity analysis of the extrapolated costs, which varied rates of hospitalization due to VZV in two scenarios, demonstrated that the total annual expenditure associated with varicella in Argentina may range between 40,054,378.0 (Scenario 1; 2.5% hospitalization rate; Supplementary Table 1) and 52,407,863.3 USD (Scenario 2: 4.0% hospitalization rate; Supplementary Table 2).
Discussion
This study aimed to assess the burden of varicella in the pediatric population of Argentina, more specifically the HCRU and associated costs in patients who sought care as either outpatients or as inpatients, in addition to the frequency and types of complications experienced. Although limited, an assessment of indirect cost due to work loss was determined; no out-of-pocket expenses were investigated. Overall, the study demonstrated that varicella is associated with substantial clinical and healthcare burden among pediatrics in Argentina.
A high clinical burden was shown, with 28.0% of outpatients and 98.7% of inpatients experiencing one or more complications. Limited data exist regarding complication rates among children with varicella in Latin America; however, the proportion of outpatients experiencing complications was higher than that reported for varicella pediatric outpatients throughout Europe, contrasted to Italy (3.5%), Germany (5.9%), and Switzerland (12.0%)Citation51–53. The rate of varicella-related complications was also much higher than for pediatric inpatients in Germany, Turkey, and Belgium who experienced complications (65.0%, 79.0%, and 79.6%, respectively)Citation54–56. However, the most common types of complications reported in this study included skin and soft tissue infection, pneumonia, sepsis, cerebellitis, and febrile seizure; these are in agreement with those reported throughout other Latin American countriesCitation6,Citation49,Citation57,Citation58. The higher rate of inpatient and outpatient complications, compared to European estimates, may be attributable to regional variations in disease severity and/or care-seeking behavior, further highlighting the need for Latin-American specific estimates related to disease burden.
With respect to healthcare resource use, 86.7% of inpatients and 17.3% of outpatients had visited an outpatient clinic at least once for varicella during their illness, with average visits per patient of 2.1- and 1.5-times, respectively. Among inpatients, a mean of 4.9 days of hospital stay was reported in our study, which is consistent with the range of 4.6–4.8 days, depending on age category, previously reported for BrazilCitation55,Citation59–62.
Our cost estimates are reasonably aligned with those for other countries in the Americas, specifically Canada and the US. A Canadian study estimated the total direct cost for both complicated and uncomplicated cases at 23,954,617 CAD, in 1999Citation63, equivalent to 25,260,042 USD in 2015Citation64, or 69.13 CAD per varicella case in 1999 (74.01 USD in 2015), compared to 119.98 USD per varicella case in our current study (weighted average for direct costs based on proportion of pediatric inpatient and outpatients cases); the higher cost in the present study is probably associated with the fact that only care-seekers were included and that the Argentinean outpatients included were those that presented to a second visit to the doctor for a return-to-school note. This is compared to the Canadian population where significant portions of outpatients either did not seek care or had only a telephone consultation. In addition, uncomplicated cases reported only one healthcare contact, if at all. As such, differences in the care-seeking behavior of the study population included in the current study, versus those of the patients included in the Canadian study mentioned above, may not allow for strict comparison of costs, permitting instead a simple evaluation of the current estimates in the context of other published work.
Conversely, a US study estimated the cost per inpatient visit as ranging between 3,654–19,537 USD in 2009, which amounts to 4,345–23,236.22 USD in 2015 adjusted for inflationCitation65. This is significantly higher than our estimate for direct hospitalization costs in Argentina that range between 2,085.0–3,524.1 USD, despite the US estimates accounting for potentially milder forms of the disease due to high vaccination coverageCitation66. However, for outpatients we estimated a direct cost per varicella case between 105.7–125.7 USD, compared to 86.8–266.4 USD (converted from 73–224 USD in 2009, as reported) from the US study. These numbers are more comparable, likely linked to the higher healthcare seeking behavior in Argentinian outpatients because of the requirement to have physician notes for sick leave.
In 2015, the varicella vaccine was added to the list of mandatory immunizations for Argentinean children; it is, therefore, expected that a significant reduction in the number of cases, and associated clinical and healthcare burden, will be observed over the next few years. This is based on reports from other countries who have already implemented a vaccination program. Uruguay, as of 1999, implemented a free and mandatory vaccination program among children aged 12 months and older, with no catch-up schedule. This resulted in coverage rates of 88–96% among those born between 1999–2004, and the incidence of varicella was reduced by 62–97% among Uruguayan pediatric patients within 6 years after the introduction of the vaccineCitation67. Furthermore, during the same time period, varicella related hospitalizations declined by 87%Citation67. In addition, cost-effectiveness analyses have been performed for both BrazilCitation47 and ColombiaCitation68, demonstrating that varicella vaccination would not only be cost-effective, but also substantially reduce the healthcare resource utilization and clinical burden associated with varicella, supporting the hypothesis that reductions in these varicella-associated costs and clinical burden should be observed in Argentina within a few years following routine vaccine introduction.
One limitation of the current study is related to the retrospective chart review design; this may have resulted in the under-estimation of the HCRU associated with varicella in Argentina, as only a cross-section of patient care may have been captured, with an inherent risk for information related to healthcare use not completely documented in the patient charts. With respect to unit cost estimates, which, due to a lack of comprehensive Argentinian data, were derived from various sources, accuracy of the costs cited for each specific parameter cannot be verified, and may have resulted in an incomplete picture of the true heathcare costs due to varicella. Under-estimation of rates of varicella incidence may also have impacted the study results; as the Argentinian clinical surveillance system is subject to under-reporting, the actual overall incidence of VZV, and resultant HCRU and costs, may be higher than reported herein. Taken together, the current study may, as a result, present a conservative cost estimate related to VZV infection in Argentina, and should be interpreted with caution.
Conversely, over-estimation of healthcare use and costs, and a resultant over-statement of varicella-related burden, may have been caused due to the inherent selection bias of the current study, whereby only patients seeking medical care in inpatient or outpatient settings were captured. In addition, calculation of indirect costs was carried out under the assumption that all patients were employed and missed work for the entirety of their child’s absence from school. However, the requirement of outpatients to have a doctor’s note permitting their return allowed for an exact duration of days missed to be ascertained, and consequently inherently limited over-/under-estimation of work loss in parents who missed days from their full-time job for the entire duration of their child’s absence from school.
Finally, the relatively small sample size of the study and the small number of participating sites may have an impact on the external validity of our findings. There were, however, several strengths with respect to generalizability, including multiple sites (both urban and rural), thus enhancing the external validity of our findings. In addition, a sensitivity analysis was performed, using Argentinian-specific estimates of inpatient admissions, in order to supplement the main study results.
Despite risk of bias and uncertainty, with limitations potentially contributing to a simultaneous over- and under-estimation of certain parameters and assumptions, this study is the first to report on the burden of varicella infection in Argentina, and provides important insight into the Argentinian experience with respect to varicella-related complications, HRCU, and costs in a pre-vaccination real world clinical setting.
Conclusion
Varicella is associated with considerable economic and clinical burden in Argentina, leading to substantial HCRU and societal costs. Set prior to the introduction of a vaccine program, this study found that the estimated cost of treating varicella annually in Argentina was comparable to North American countries, such as Canada and the US. Under the current routine vaccination plan, these costs are expected to lessen over time; as such, further investigation regarding the impact of the varicella vaccine in Argentina will be required. Considering this, the results of this study provide valuable data which may be input into health economic analyses assessing the evolution of VZV-related HCRU and costs from pre- to post-vaccine eras.
Transparency
Declaration of funding
The study was funded by Merck & Co., Inc.
Declaration of financial/other relationships
Financial arrangements of the authors with companies whose products may be related to the present report are listed as follows, as declared by the authors. NG reports consultancy fees from Merck Sharp & Dohme Corp., Sanofi Pasteur, Pfizer, and Novartis. HKY, BJK, and LJW are employees of Merck & Co., Inc., Kenilworth, NJ. ER is an employee of JSS Medical Research, and a paid consultant of Merck & Co., Inc., Kenilworth, NJ. HM is an employee of Merck Sharpe & Dohme Corp. IA LLC, Carolina, Puerto Rico. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
Results from this study have been presented at the 5th ISPOR Latin America Conference in Santiago, Chile, September 6–8, 2015.
Supplementary_Table_2_R1.docx
Download MS Word (14.4 KB)Supplementary_Table_1_R1.docx
Download MS Word (13.5 KB)Acknowledgments
Jenaya Rickard from JSS Medical Research provided support for medical writing. Kyung Min Song, Changia Shao, and Alexandra Altland from Merck & Co., Inc., Kenilworth, NJ, provided support for study management. Viviana Romanin was involved with data collection in collaboration with Dr Giglio. Fabiana Garcia, Hector J. Abate, Macarena Uranga, and Veronica Pepino were principal investigators at individual study sites.
References
- Arvin AM. Varicella-zoster virus. Clin Microbiol Rev 1996;9:361–81
- Whitley RJ. Varicella-zoster virus infections. In: Galasso GJ, Whitley RJ, Merigan TC, editors. Antiviral agents and viral diseases of man. New York, NY: Raven Press; 1990. p 235-63
- Manfredi R, Chiodo F, Titone L, et al. Chickenpox complications among immunocompetent hospitalized children in Italy. Pediatr Med Chir 1997;19:99-104
- Ozdemir H, Candir MO, Karbuz A. Chickenpox complications, incidence and financial burden in previously healthy children and those with an underlying disease in Ankara in the pre-vaccination period. Turk J Pediatr 2011;53:614-25
- Bardach A, Cafferata ML, Klein K, et al. Incidence and use of resources for chickenpox and herpes zoster in Latin America and the Caribbean—a systematic review and meta-analysis. Pediatr Infect Dis J 2012;31:1263-8
- Heininger U, Seward JF. Varicella. Lancet 2006;68:1365-76
- Sadzot-Delvaux C, Rentier B, Wutzler P, et al. Varicella vaccination in Japan, South Korea, and Europe. J Infect Dis 2008;197(Suppl2):S185-S190
- Ministerio de Salud. Boletín integrado de vigilancia: Secretaría de Promoción y programas sanitarios (N° 296 - SE 5). Ministerio de Salud; 2016. República Argentina. Available from: http://www.msal.gob.ar/images/stories/boletines/Boletin-Integrado-De-Vigilancia-N296-SE5.pdf
- Dayan GH, Panero MS, Debbag R, et al. Varicella seroprevalence and molecular epidemiology of varicella-zoster virus in Argentina, 2002. J Clin Microbiol 2004;42:5698-704
- Ministerio de Salud. Estadisticas Vitales: Information Basica - ANO 2014 (Serie 5 - Número 58). Ministerio de Salud; 2015. Buenos Aires, República Argentina. http://deis.msal.gov.ar/wp-content/uploads/2016/01/Serie5Nro58.pdf
- Ministerio de Salud. Fundamentos de la introducción de la vacuna contra varicela al Calendario Nacional de Inmunizaciones 2015. Ministerio de Salud; 2014. Argentina. https://www.santafe.gov.ar/index.php/web/content/download/218645/1137054/file/Lineamientos%20varicela%202015.pdf
- Ministerio de Salud. Recomendaciones sobre control de la varicela para equipos de salud. Ministerio de Salud; 2011. República Argentina. http://www.msal.gob.ar/images/stories/epidemiologia/inmunizaciones/equipos-de-salud/recoemndaciones-varicela-es-2011.pdf
- Takahashi M, Okuno Y, Otsuka T, et al. Development of a live attenuated varicella vaccine. Biken J 1975;18:25-33
- Oh SH, Choi EH, Shin SH, et al. Varicella and varicella vaccination in South Korea. Clin Vaccine Immunol 2014;21:762-8
- White CJ, Kuter BJ, Hildebrand CS, et al. Varicella vaccine (VARIVAX) in healthy children and adolescents: results from clinical trials, 1987 to 1989. Pediatrics 1991;87:604-10
- Just M, Berger R, Luescher D. Live varicella vaccine in healthy individuals. Postgrad Med J 1985;61(Suppl4):129-32
- Arbeter AM, Starr SE, Plotkin SA. Varicella vaccine studies in healthy children and adults. Pediatrics 1986;78:748-56
- Gatchalian S, Leboulleux D, Desauziers E, et al. Immunogenicity and safety of a varicella vaccine, Okavax, and a trivalent measles, mumps and rubella vaccine, MMR-II, administered concomitantly in healthy Filipino children aged 12–24 months. Southeast Asian J Trop Med Public Health 2003;34:589-97
- Knuf M, Habermehl P, Zepp F, et al. Immunogenicity and safety of two doses of tetravalent measles-mumps-rubella-varicella vaccine in healthy children. Pediatr Infect Dis J 2006;25:12-18
- Kuter BJ, Brown ML, Hartzel J, et al. Safety and immunogenicity of a combination measles, mumps, rubella and varicella vaccine (ProQuad). Hum Vaccin 2006;2:205-14
- Marin M, Marti M, Kambhampati A, et al. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics 2016;137:e20153741
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Weekly Epidemiological Record (WER) 2014;25:265-88
- World Health Organization. Systematic review of available evidence on effectiveness and duration of protection of varicella vaccines. WHO; 2014. Geneva. http://www.who.int/immunization/sage/meetings/2014/april/presentations_background_docs/en/
- Fu C, Wang M, Liang J, et al. The effectiveness of varicella vaccine in China. Pediatr Infect Dis J 2010;29:690-3
- Kurugol Z, Halicioglu O, Koc F, et al. Varicella rates among unvaccinated and one-dose vaccinated healthy children in Izmir, Turkey. Int J Infect Dis 2011;15:e475-80
- Huang WC, Huang LM, Chang IS, et al. Varicella breakthrough infection and vaccine effectiveness in Taiwan. Vaccine 2011;29:2756-60
- Hirose M, Gilio AE, Ferronato AE, et al. The impact of varicella vaccination on varicella-related hospitalization rates: global data review. Rev Paul Pediatr 2016;34:359-66
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites—United States, 1995–2005. J Infect Dis 2008;197(Suppl2):S71-S5
- Lopez AS, Zhang J, Marin M. Epidemiology of varicella during the 2-dose varicella vaccination program – United States, 2005–2014. MMWR Morb Mortal Wkly Rep 2016;65:902-5
- The International Society for Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practices (GPP). ISP; 2015. Bethesda. https://www.pharmacoepi.org/resources/guidelines_08027.cfm
- Alfabeta.net. Manual Farmacéutico On Line; 2014 version. http://www.alfabeta.net/productos/mfonline-ar.jsp
- Alfabeta.net. Manual Farmacéutico On Line; 2016 version. http://www.alfabeta.net/productos/mfonline-ar.jsp
- Rojas G, Bartoloni L, Dillon C, et al. Clinical and economic characteristics associated with direct costs of Alzheimer’s, frontotemporal and vascular dementia in Argentina. Int Psychogeriatr 2011;23:554-61
- Embassy of the United States of America in Argentina. Instrucciones Para El Examen Medico. Embassy of the United States of America in Argentina; 2014. Buenos Aires, Argentina. http://photos.state.gov/libraries/48675/CONS-IV/Med%20Exam%20Instructions%20_1014_.pdf
- International Federation of Health Plans. Comparative price report: medical and hospital fees by country. International Federation of Health Plans; 2011. London, UK. http://www.indiana.edu/∼uhrs/UFCBC/pubs/2011iFHPPriceReportGraphs_version3.pdf
- International Federation of Health Plans. Comparative price report: variation in medical and hospital prices by country. International Federation of Health Plans; 2012. London, UK. http://hushp.harvard.edu/sites/default/files/downloadable_files/IFHP%202012%20Comparative%20Price%20Report.pdf
- No author. Cuánto cuesta una consulta a un especialista. Minuto Uno [newpaper on the internet]; 2012. https://www.minutouno.com/notas/260223-cuanto-cuesta-una-consulta-un-especialista
- Raggio J. Cuánto se paga hoy una sesión de terapia. La Nacion [Newspaper on the internet]; 2012. http://www.lanacion.com.ar/1497761-cuanto-se-paga-hoy-una-sesion-de-terapia
- Gobierno de la Ciudad Autónoma de Buenos Aires, Ministero de Salud. Nomenclador de Prestaciones de Salud. 2014. Buenos Aires. http://www.buenosaires.gob.ar/salud/sigehos
- Confederacion Unoficada Bioquima de la republica Argentina. Nomenclador Bioqumico Unico (NBU). PMO y Practicas Especiales; 2012. República Argentina.
- Gentile A, Salgueiro AL, García Bournissen F, et al. Cost of Bordetella pertussis illness in tertiary hospitals in Argentina. Arch Argent Pediatr 2013;111:295-302
- International Federation of Health Plans. Comparative price report: variation in medical and hospital prices by country. IFHP; 2013. London, UK. http://static.squarespace.com/static/518a3cfee4b0a77d03a62c98/t/534fc9ebe4b05a88e5fbab70/1397737963288/2013%20iFHP%20FINAL%204%2014%2014.pdf
- Miravitlles M, Jardim JR, Zitto T, et al. [Pharmacoeconomic study of antibiotic therapy for exacerbations of chronic bronchitis and chronic obstructive pulmonary disease in Latin America]. Arch Bronconeumol 2003;39:549-53
- Rosenthal VD, Guzman S, Migone O, et al. The attributable cost and length of hospital stay because of nosocomial pneumonia in intensive care units in 3 hospitals in Argentina: a prospective, matched analysis. Am J Infect Control 2005;33:157-61
- The World Bank. Inflation, GDP deflator (annual %). 2015. Argentina. http://data.worldbank.org/indicator/NY.GDP.DEFL.KD.ZG?end=2016&locations=AR-CA&start =1961&view=chart
- OECD.stat. 2015. https://stats.oecd.org/
- Valentim J, Sartori AM, de Soárez PC, et al. Cost-effectiveness analysis of universal childhood vaccination against varicella in Brazil. Vaccine 2008;26:6281-91
- de Campos C, Romao M, Ferraz A, et al. Hospitalizations by the Brazilian Health System due to traffic accidents. XVIII Congreso Panamericano de Ingeniería de Tránsito, Transporte y Logística (PANAM 2014). Procedia – Social and Behavioral Sciences 2014;162:282-9
- Morínigo S, Chiarelli G, Corallo T, et al. Hospitalizaciones por varicela. Abstract RPD 84. Presented at 7° Congreso Argentino de Infectología Pediátrica, 1° jornadas de enfermería en infectología Pediátrica; 2014
- OECD. OECD Data. Exchange Rates, Argentina. OECD; 2015. https://data.oecd.org/conversion/exchange-rates.htm
- Fornaro P, Gandini F, Marin M, et al. Epidemiology and cost analysis of varicella in Italy: results of a sentinel study in the pediatric practice. Pediatr Infect Dis J 1999;18:414-19
- Iseli A, Aebi C, Banz K, et al. Prospective surveillance of varicella-zoster virus infections in an out-patient setting in Switzerland. Hum Vaccin 2009;5:843-6
- Wagenpfeil S, Neiss A, Banz K, et al. Empirical data on the varicella situation in Germany for vaccination decisions. Clin Microbiol Infect 2004;10:425-30
- Blumental S, Sabbe M, Lepage P, et al. Varicella paediatric hospitalisations in Belgium: a 1-year national survey. Arch Dis Child 2016;101:16-22
- Liese JG, Grote V, Rosenfeld E, et al. The burden of varicella complications before the introduction of routine varicella vaccination in Germany. Pediatr Infect Dis J 2008;27:119-24
- Turel O, Bakir M, et al. Children hospitalized for Varicella: complications and cost burden. ViHRI 2013;2:226-30
- Peuchot A, Peuchot J, Corazza R, et al. Hospitalizaciones por complicaciones en varicela. Abstract RPD 6: presented at 7° Congreso Argentino de Infectología Pediátrica, 1° jornadas de enfermería en infectología Pediátrica. Ciudad de Córdoba, Provincia de Córdoba, Argentina. April 3–5, 2014
- Melonari P, Rodriguez Saá C, Olguín M, et al. Complicaciones y costos por varicela en ninos de un hospital pediatrico de Argentina. Abstract PO 166: presented at 7° Congreso Argentino de Infectología Pediátrica, 1° jornadas de enfermería en infectología Pediátrica. Ciudad de Córdoba, Provincia de Córdoba, Argentina. April 3–5, 2014.
- Mallet E, Maitre M, Delalande-Dutilleul L, et al. [Evaluation of varicella complications through a retrospective hospital survey in a paediatric center over 16 years in France]. Arch Pediatr 2004;11:1145-51
- Marchetto S, de Benedictis FM, de Martino M, et al. Epidemiology of hospital admissions for chickenpox in children: an Italian multicentre study in the pre-vaccine era. Acta Paediatr 2007;96:1490-3
- Bonhoeffer J, Baer G, Muehleisen B, et al. Prospective surveillance of hospitalisations associated with varicella-zoster virus infections in children and adolescents. Eur J Pediatr 2005;164:366-70
- Brisson M, Edmunds WJ. Epidemiology of varicella-zoster virus in England and Wales. J Med Virol 2003;70(Suppl1):S9-S14
- Law B, Fitzsimon C, Ford-Jones L, The Immunization Monitoring Program-Active (IMPACT). et al. Cost of chickenpox in Canada: part II. Cost of complicated cases and total economic impact. Pediatrics 1999;104:7-14
- OANDA Corporation; 2016. https://www.oanda.com/currency/average
- United States Department of Labor. Bureau of Labor Statistics Data Tools. US DoL; 2016. Washington, DC, United States. http://data.bls.gov/pdq/SurveyOutputServlet
- Zhou F, Shefer A, Wenger J, et al. Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics 2014;133:577-85
- Quian J, Rüttimann R, Romero C, et al. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997–2005. Arch Dis Child 2008;93:845-50
- Paternina-Caicedo A, De la Hoz-Restrepo F, Gamboa-Garay O, et al. How cost effective is universal varicella vaccination in developing countries? A case-study from Colombia. Vaccine 2013;31:402-9
