Abstract
Objective: A large, pivotal, phase 3 trial in patients with newly diagnosed multiple myeloma (MM) demonstrated that denosumab, compared with zoledronic acid, was non-inferior for the prevention of skeletal-related events (SREs), extended the observed median progression-free survival (PFS) by 10.7 months, and showed significantly less renal toxicity. The cost-effectiveness of denosumab vs zoledronic acid in MM in the US was assessed from societal and payer perspectives.
Methods: The XGEVA Global Economic Model was developed by integrating data from the phase 3 trial comparing the efficacy of denosumab with zoledronic acid for the prevention of SREs in MM. SRE rates were adjusted to reflect the real-world incidence. The model included utility decrements for SREs, administration, serious adverse events (SAEs), and disease progression. Drug, administration, SRE management, SAEs, and anti-MM treatment costs were based on data from published studies. For the societal perspective, the model additionally included SRE-related direct non-medical costs and indirect costs. The net monetary benefit (NMB) was calculated using a willingness-to-pay threshold of US$150,000. One-way deterministic and probabilistic sensitivity analyses were conducted.
Results: From a societal perspective, compared with zoledronic acid, the use of denosumab resulted in an incremental cost of US$26,329 and an incremental quality-adjusted life-year (QALY) of 0.2439, translating into a cost per QALY gained of US$107,939 and a NMB of US$10,259 in favor of denosumab. Results were sensitive to SRE rates and PFS parameters.
Limitations: Costs were estimated from multiple sources, which varied by tumor type, patient population, country, and other parameters. PFS and overall survival were extrapolated beyond the follow-up of the primary analysis using fitted parametric curves.
Conclusion: Denosumab’s efficacy in delaying or preventing SREs, potential to improve PFS, and lack of renal toxicity make it a cost-effective option for the prevention of SREs in MM compared with zoledronic acid.
Introduction
Multiple myeloma (MM) is a rare, incurable, and aggressive cancer of plasma cell origin that accounts for 1.8% of all cancers in the US, and is the second most common hematological malignancyCitation1,Citation2. It is characterized by a chronic pattern of remission and relapse and features increased osteoclast activation, resulting from an imbalance between receptor activator of nuclear factor kappa B ligand (RANKL) and osteoprotegerinCitation1,Citation3. As a consequence of this disturbed equilibrium, ∼ 80–90% of patients with MM develop osteolytic bone lesions, or soft spots that appear as ‘holes’ on a standard bone X-ray during the course of the diseaseCitation4,Citation5. These lesions never heal spontaneously, weaken the bone, increase the risk of fractures, and are the primary cause of bone pain in patients with MMCitation4,Citation5. During the course of the disease, nearly all patients with MM are at risk of a broad range of bone complications, known as skeletal-related events (SREs)Citation2,Citation6. These include: (1) pathologic fractures; (2) spinal cord compression; (3) surgery to bone; and (4) radiation to boneCitation7. SREs are associated with a substantial decrease in the patients’ health-related quality of life, largely due to bone pain and forced immobility, as well as poor prognosis and increased risk of death associated with MMCitation7–9.
SREs are burdensome, not only for the patient, but also to the healthcare system. They lead to incrementally higher costs and healthcare resource use including significantly more hospitalizations, outpatient clinic visits and emergency department (ED) visits for patients with MM who have SREs vs those who do notCitation10,Citation11. Furthermore, healthcare resource use also increases with SRE burdenCitation10,Citation11. A retrospective analysis found that, overall, all-cause total costs were ∼ $80,000 (2016 US$) per patient per year (PPPY) higher for those with SREs than those without, and ∼ $50,000 PPPY higher for those with multiple vs single SREsCitation10.
The prevention or reduction of bone complications is considered a major goal of supportive care for patients with MMCitation12. International guidelines recommend initiating therapy to prevent SREs along with antimyeloma therapy for patients with active disease, even if osteolytic bone lesions are not apparentCitation13,Citation14. Until recently, treatment options for the prevention of SREs in MM in the US were limited to bisphosphonates, with zoledronic acid being the standard of care (4 mg intravenously [IV] every 3–4 weeks). Despite the predominance of zoledronic acid in this setting, it is not without limitations, including safety warnings for renal toxicityCitation15, risk of acute-phase reactionsCitation16,Citation17, and requiring intravenous administrationCitation15, all of which contribute to the healthcare resource burden in these patientsCitation18.
Denosumab was recently approved in the US for the prevention of SREs in patients with MMCitation19. It is a fully human monoclonal antibody of the immunoglobulin G2 isotype that binds to and neutralizes RANKL, thereby inhibiting osteoclast activation and functionCitation19. In contrast to zoledronic acid, denosumab is not excreted via the kidneys and, consequently, can be used regardless of the patient's renal function, with no renal monitoring or dose adjustments requiredCitation15,Citation19. RANKL inhibition with denosumab is a novel approach to prevent SREs in patients with MM, as it exerts potent antiresorptive activity and may have additional anti-tumor effectsCitation20. Denosumab is administered every 4 weeks (Q4W) as a subcutaneous injection (SC)Citation19. Denosumab was approved in the US and Europe for the prevention of SREs in patients with solid tumors in 2010; real-world data indicate that denosumab and zoledronic acid are the main agents used to prevent SREs in patients with solid tumorsCitation19,Citation21,Citation22.
The safety and efficacy of denosumab, compared with zoledronic acid, for the prevention of SREs in patients with MM were recently evaluated in the 20090482 study (NCT01345019), one of the largest clinical trials in MM to date. In this phase 3, double-blind, multi-center study, 1,718 patients with newly diagnosed MM were randomized 1:1 to receive denosumab (120 mg, SC, Q4W) or zoledronic acid (4 mg, intravenously [adjusted], Q4W)Citation20. The study met its primary end-point; denosumab was non-inferior to zoledronic acid in delaying time to first on-study SRE (hazard ratio [HR] = 0.98; 95% confidence interval [CI] = 0.85–1.14; p = .01). Owing to a high number of SREs (60%) during the first 3 months of the study, a post hoc landmark analysis at 15 months was performed for time to first SRE. This showed that, compared with zoledronic acid, denosumab demonstrated better efficacy in delaying time to first SRE (HR = 0.66; 95% CI = 0.44–0.98; p = .039)Citation20. Median progression-free survival (PFS), assessed as an exploratory end-point, was 46.1 months with denosumab vs 35.4 months with zoledronic acid (HR = 0.82; 95% CI = 0.68–0.99; descriptive p = .036), leading to an observed 10.7 month difference in median PFS between the two treatmentsCitation20. Adverse events (AEs) in this phase 3 study reflected the known safety profile of each agent and were similar between treatment armsCitation20.
Integrated analysis of the data from three identically designed, randomized, double-blind, phase 3 trials of patients with bone metastases and breast cancer, prostate cancer, other solid tumors or MM demonstrated that denosumab was superior to zoledronic acid in delaying the time to first SRECitation23, yet comparisons of the cost-effectiveness of denosumab and zoledronic acid have yielded variable conclusionsCitation24–30. This variability may have been caused by different perspectives of value. There is no single answer to the question of the value of an innovative drug. Traditionally, cost-effectiveness analyses have focused on direct medical costs, such as drug acquisition costs, and their short-term budget impactCitation31. More recently, additional factors such as the comprehensive clinical, humanistic, and downstream economic benefits of drugs have been incorporated into economic models in order to provide a societal perspective on drug valueCitation31. In the current analysis, which aimed to assess the value of denosumab for the prevention of SREs in patients with MM, we have taken into account this societal perspective, which places the patient at the center of the analysis. We have also provided an analysis from the payer perspective.
Given that denosumab has been shown to prevent or delay SREs, may extend PFS compared with zoledronic acid, and does not impact on renal functionCitation19, it was important to assess its economic value in patients with MM in the US. The objective of the analysis was to estimate the incremental cost-effectiveness ratio (ICER) and the net monetary benefit (NMB) of denosumab vs zoledronic acid in patients with MM, from the perspectives of both society and payers.
Methods
The XGEVA Global Economic Model (X-GEM) used for this analysis builds on the model published in Stopeck et al.Citation28 that evaluated the cost-effectiveness of denosumab vs zoledronic acid for the prevention of SREs in patients with solid tumors in the US. The model incorporates outcomes of the 20090482 studyCitation20.
Model design
A partitioned survival model was constructed to assess the cost-effectiveness of denosumab vs zoledronic acid in patients with newly diagnosed MM by integrating the treatment and outcomes of a cohort of patients who are at risk of experiencing SREs. The model structure was the same for both treatment arms.
Five health states were included, according to whether patients were on or off treatment with zoledronic acid or denosumab, had MM progression or not, or had died (). Patients could transfer among health states every 4 weeks. The total time horizon was set to the patient’s remaining lifetime to capture all of the future health and economic outcomes expected from the alternatives comparedCitation32.
Figure 1. Depiction of model health states. 1L, first line; 2L+, second line or later; Abbreviations. MM, multiple myeloma; OFF SRE Prev Tx, patients not receiving treatment to prevent SREs; ON SRE Prev Tx, patients receiving treatment to prevent SREs; SRE, skeletal-related event; Tx, treatment.
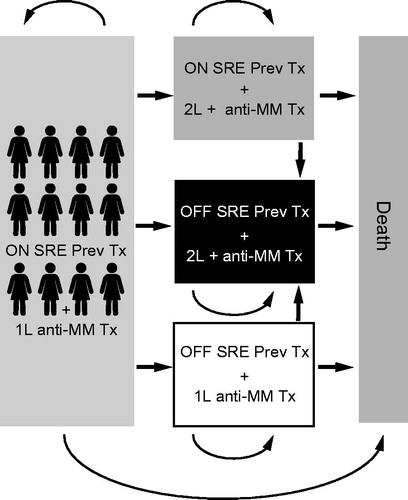
Four types of SRE can occur—radiation to bone, surgery to bone, spinal cord compression, and pathologic fracture—and each is associated with a different mean cost and impact on patient quality of life. To account for this, the model calculated the cost of an SRE based on the proportion of each type of SRE that occurred during the phase 3 study (Amgen data on file, 2017) () and the cost of each SRE.
Table 1. SRE counts and distributions from the 20090482 study.
Model population
Patients newly diagnosed with MM and with at least one osteolytic lesion were included in the model. In order to reflect real-world practice, 10% of the model population was not able to receive zoledronic acid owing to pre-existing severe renal impairmentCitation33; the model assumed that these patients would be treated with denosumab.
Model parameters
Clinical input was obtained from the 20090482 studyCitation20 and included the SRE rates, the SRE distribution, treatment compliance rates, treatment discontinuation rates, PFS, overall survival (OS) rates, and serious adverse event (SAE) rates. Treatment discontinuation rates, PFS, and mortality rates were derived from probability curves fitted to trial data and extrapolated beyond the follow-up of the primary analysis. The follow-up for the primary analysis was up to 45 months; the median time on study was similar between the two arms (17.3 months for denosumab vs 17.6 months for zoledronic acid)Citation20. Additionally, a real-world adjustment for SRE rates was applied to the clinical trial results. The rates of SREs, SAEs, PFS, OS, and treatment discontinuation were modeled independently of each other.
Skeletal-related event rates
To calculate the trial-based annual SRE rate, using data from the 20090482 studyCitation20, the number of SREs experienced by patients in each arm was divided by the number of patient-years in the respective arm ().
Table 2. SRE rates from the 20090482 study.
Consistent with other published cost-effectiveness analyses in this therapeutic areaCitation34, the model used constant rates for all SREs, the values of which depended on: (1) the specific treatment the patient was receiving to prevent SREs; and (2) whether the patient was on or off treatment for the prevention of SREs. Alternative SRE rate values, based on the results of a landmark analysis at 15 monthsCitation20, were also used. In this case, patients in each arm experienced differential rates before and 15 months after the start of SRE preventive treatment ().
Table 3. SRE rates from the 20090482 study (differential rates before and 15 months after the start of SRE preventive treatment).
The SRE rate for patients receiving no treatment (0.99 SRE/year) was estimated by dividing the SRE rate in the zoledronic acid arm by the rate ratio of SREs in patients with MM who were treated with zoledronic acid and patients with MM who did not receive treatment to prevent SREs; the rate ratio was taken from a managed care database study from the USCitation35.
Skeletal-related event real-world adjustment
Real-world studies have found that the SRE rate for zoledronic acid was higher than those reported from clinical trialsCitation36,Citation37. To account for this difference, a real-world adjustment relative rate ratio of 2.84 was applied to the clinical trial results (i.e. the trial SRE rates were multiplied by the rate ratio)Citation38. Although a real-world SRE adjustment factor lower than 2.84 has previously been reported for SREs in solid tumorsCitation36, the adoption of 2.84 in the base case for this analysis is consistent with the available (albeit limited) evidence of SRE rates in MMCitation37,Citation38.
Distribution of skeletal-related events
The model considered the SRE distribution as observed in the 20090482 study (Amgen data on file, 2017) without any further adjustment, as a proxy of real-world distribution of SREs ().
Treatment compliance rates
Treatment compliance was defined as the number of doses of zoledronic acid/denosumab received, divided by the number of scheduled doses up to the end of investigational product administration for each patient. These data were derived from the 20090482 studyCitation20. The treatment compliance rate was 0.881 for denosumab and 0.854 for zoledronic acid.
Treatment discontinuation rates
Treatment discontinuation rates in the 20090482 study were similar between the denosumab and zoledronic acid armsCitation20. The discontinuation of zoledronic acid and of denosumab was incorporated into the model based on treatment-specific data from the clinical trial, with long-term extrapolation based on parametric fitting of the pooled individual data from the trial armsCitation20. Treatment discontinuation rates were derived from the time-to-treatment-discontinuation probability distribution. Independent parametric fits using different distributions (exponential, Weibull, generalized gamma, log-logistic, log-normal) were performed. The Weibull and general gamma distributions were the two best-fitting distributions according to the Akaike Information Criterion (AIC). The generalized gamma and Weibull distributions had very similar visual fit and long-term extrapolation, with a mean time on treatment of 36.8 and 37.7 months, respectively. The generalized gamma probability distribution was deemed to be the most appropriate representation of discontinuation data and was, therefore, used in the base case. The same discontinuation rates were applied to both arms. After discontinuation, patients were assumed to experience the same SRE rates as patients who never received treatment to prevent SREs.
Progression-free survival and overall survival rates
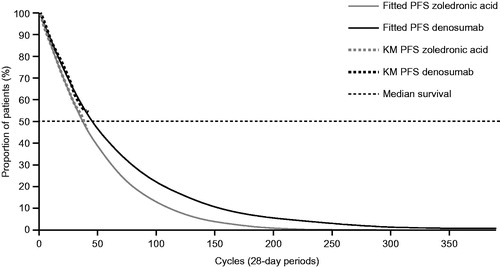
PFS was defined as the time interval from the randomization date to the date of first overall disease progression assessed and recorded by the investigator, or death during treatment phase from any cause, whichever comes earlier. Data from study 20090482 showed an increasing separation between the denosumab and zoledronic acid PFS Kaplan–Meier curves from month 1 onwardsCitation20. Because the observed survival distributions for PFS were limited by the time of follow-up at primary analysis, it was necessary to extrapolate them beyond the currently available follow-up time to obtain unbiased estimates of the gains in life expectancy and QALYs in both treatment arms. This is a common approach to survival curves in modelingCitation39. Extrapolation of PFS was performed by fitting parametric survival distributions to individual patient PFS data. The following exponential distributions were tested: exponential, Weibull, Gompertz, generalized gamma, log-logistic, and log-normal. The three best-fitting distributions in terms of AIC were the Weibull, the log-logistic, and the generalized gamma distributions. The three distributions were all associated with similar AICs and with relatively good visual fit to the trial data. Generalized gamma probability distributions () were deemed to be appropriate representations of PFS for both arms because they provided a good visual and statistical fit of PFS data; the log-logistic distribution provided an implausible long-term extrapolation of PFS (PFS curves crossed the OS curve after around 250 model cycles, at which time more than 10% of patients were still alive), and the median difference in PFS, estimated by the generalized gamma distribution, was closer to the results of study 20090482 than that estimated by the Weibull distribution. Furthermore, use of the generalized gamma distribution is consistent with the treatment discontinuation distribution, under the assumption of a constant relationship between time-to-treatment-discontinuation and treatment effect (which was also made for the SRE rates). Moreover, generalized gamma distributions of PFS have been used elsewhere to model PFS in patients with MMCitation40,Citation41, and their projections appear to be clinically plausible. Rates were derived from the PFS probability distributions.
Figure 2. Progression-free survival parametric model (unrestricted generalized gamma distributions compared with Kaplan–Meier curves). Abbreviations. KM, Kaplan–Meier; MM, multiple myeloma; PFS, progression-free survival.
Mortality rates were derived from the OS probability distribution, extrapolated by fitting survival data from the two pooled arms of the trial. In the 20090482 study, OS was similar for denosumab and zoledronic acid and, therefore, the model assumed the same mortality rate for both treatmentsCitation20. Parametric survival models with different probability distributions were fitted to the data (namely exponential, Weibull, generalized gamma, log-logistic, and log-normal distributions) and were used to inform the model. A correction was made using the US national life tablesCitation42 to ensure mortality rates predicted by the model would always be higher or equal to the general population mortality. The Weibull distribution was selected in the base case as it was the best-fitting distribution based on the AIC and provided good visual fit. Although the exponential, generalized gamma and log-logistic distribution had similar statistical goodness of fit, the long-term extrapolations were not plausible, with exponential and log-logistic distributions predicting implausibly high long-term survival, while the generalized gamma distribution crossed the PFS extrapolation before 10 years.
Serious adverse event rates
To reflect clinically and economically important events, SAEs reported in the 20090482 study were used to derive SAE rates in the model. Constant rates of SAEs were used. A SAE was defined as any untoward medical occurrence that resulted in death, was life-threatening, required prolonged hospitalization, or resulted in significant disability/incapacity, and which did not necessarily have to have a causal relationship with the administered treatment. Three SAEs were used to derive SAE rates included in the base case—hypocalcemia, osteonecrosis of the jaw (ONJ), and renal toxicity. SAE rates were calculated in a manner similar to the calculation of SRE rates, by using the total number of patients experiencing each SAE and dividing it by the person-time on study over which patients were followed for SAEs. SAE rates were based on the integrated summary of safety from the 20090482 studyCitation20. The rates of SAEs of interest for denosumab and zoledronic acid were as follows: hypocalcemia (0.9% vs 0.2%); positively adjudicated ONJ (0.7% vs 0.2%); and SAEs related to renal toxicity (2.7% vs 3.5%).
Model utilities
Utility decrements were a consequence of SREs, SAEs (such as ONJ, hypocalcemia, and renal toxicity), mode of drug administration (subcutaneous injections vs intravenous infusions), and MM disease progression ().
Table 4. Utility decrements.
Skeletal-related event utility decrements
The SRE utility values were derived from a sample of participants from the general population who participated in a utility studyCitation43, which was designed to establish the value that participants assigned to their quality of life, by hypothetically comparing varying life expectancies at different states of health. In this study, the utility decrements associated with the four types of SREs were assessed using eight health states—spinal cord compression (two health states: with and without paralysis), pathologic fracture (three health states: leg, rib, and arm), radiation (two health states: administered in two appointments and administered daily for 2 weeks), and surgery performed to stabilize bone (one health state)Citation43.
Drug administration utility decrements
A similar methodology was used for the assessment of the utility decrement associated with receiving a subcutaneous injection or intravenous infusion for SRE prevention (e.g. denosumab or zoledronic acid) in addition to anti-myeloma chemotherapyCitation43. The utility decrement for one subcutaneous injection compared with no injection was 0.0011, and that for one intravenous infusion compared with no infusion was 0.0021.
Serious adverse events utility decrements
The SAE utility decrements were based on analyses performed using a regression model by pooling data across solid tumor trials ()Citation28.
Multiple myeloma progression utility decrements
MM progression was modeled by applying a 19.5% decrement to the baseline utility of patients with non-progressive disease, as previously done by van Agthoven et al.Citation44, with a baseline utility of 0.80 for patients with non-progressive disease (typically such patients were on, or had recently completed, first-line anti-myeloma treatment).
Costs
The model inputs included direct medical costs for drug acquisition, drug administration, SAEs, SRE management (hospital, outpatient, long-term care and hospice, strong opioid, ED visits, physical therapy, and skilled nursing facility), direct non-medical costs (for caregiver time and driving/parking time to attend medical appointments), and indirect costs (short-term disability and productivity loss). All of the above costs were included in the societal perspective analysis. The payer perspective did not include direct non-medical costs or indirect costs.
Drug acquisition costs were based on average selling prices per dose (ASP; $1,928 and $45 for denosumab and zoledronic acid, respectively) from the Centers for Medicare & Medicaid Services (Q3 2017)Citation45. Wholesale drug acquisition costs per dose (WAC; $2,155 and $922, respectively) in 2017 were used for an alternative scenario analysis (). The doses given were: denosumab 120 mg subcutaneously every 4 weeks and zoledronic acid 4 mg intravenously every 4 weeks. Modifications to the zoledronic acid dose were performed according to the label.
Table 5. Summary of all cost inputs.
The costs of drug administration for subcutaneous injection and intravenous infusion (including an additional renal monitoring fee for each zoledronic acid administration) were taken from Stopeck et al.Citation28, and adjusted for inflation to 2017 prices. The costs of SAEs (such as ONJ, hypocalcemia, and renal toxicity) were also includedCitation46,Citation47 (). The monthly costs of anti-myeloma treatments in first-, second-, and third-line were based on WAC prices of regimens with at least 3% of market share (August 2017) weighted by their relative market shares and relative duration of use. The mean duration of each line of treatment was calculated as the mean duration of all the regimens with at least 3% of market share (August 2017) used for that line, weighted by their relative market shares.
The inpatient and outpatient costs associated with managing SREs, accounting for the proportion of patients admitted for inpatient hospitalization or treated in outpatient facilities, are summarized in .
The costs of SRE-related ED visits were calculated based on the study published by Nash Smyth et al.Citation11, evaluating the costs of treating patients with SREs vs treating those without SREs among patients with MM. Costs were reported as incremental cost per patient per month. Adjustments were made to account for the number of SREs during the follow-up period to estimate the cost per SRE. The SRE long-term care and hospice cost inputs were calculated based on a study by Jayasekera et al.Citation48 and long-term care, based on an internal analysis of the MarketScan claims database (Amgen data on file, 2017). The SRE physical therapy and devices costs were based on an internal analysis of the MarketScan claims database. Also, in this case, costs were reported as incremental cost per patient per month, and adjustments were made for the number of SREs per year. Other SRE-related costs, associated with the use of skilled nursing facilities, strong opioid usage, caregiver burden, and short-term disability and productivity loss, were calculated based on multiple sources, and are described in (Amgen data on file, 2017)Citation11,Citation48–52. All values were adjusted for inflation by multiplying the cost by the Medical Care consumer price index of March 2017.
Cost-effectiveness analysis
The cost-effectiveness of denosumab was calculated primarily in terms of the ICER by dividing the difference in total cost (ΔC) between denosumab (Cdmab) and zoledronic acid (Czol) by the difference in health outcomes (ΔE), measured in quality-adjusted life-years (QALYs) between denosumab (Edmab) and zoledronic acid (Ezol), thus: ICER = ΔC/ΔE. To help define the economic value of denosumab, we applied a threshold for health gains called the willingness-to-pay (WTP) threshold. This reflects the maximum amount that society is willing to pay for one additional QALY gainedCitation53. A constant 3% annual discount rate, to account for time preferences in health gains, was used in the model for both health and costs. For this analysis, the WTP threshold was assumed to be $150,000, which is consistent with the value previously used by several stakeholders in the US, and with World Health Organization (WHO) recommendationsCitation53. The value of denosumab was also estimated by the NMB, calculated as NMB = [(Edmab – Ezol) × WTP] − (Cdmab – Czol). Cost-effectiveness and NMB analyses were conducted from both societal and payer perspectives. For the societal analysis, based on demographic considerations, 35% of patients were assumed to be eligible for short-term disability and productivity loss. This was based on the proportion of patients who would be assumed to be employed full time while being treated. After first-line anti-myeloma treatment with a mean of nine cycles, patients who do not have disease progression incur significantly lower costs than those with progressive disease, due to the initiation of a new anti-myeloma treatment. To account for the uncertainties and complexities of the rapidly evolving MM treatment landscape, conservatively, only 50% of the potential savings associated with delaying initiation of second and subsequent lines of primary anti-myeloma treatment were included in the calculations.
We also conducted three scenario analyses to understand how changes in various inputs impacted on the cost-effectiveness of denosumab vs zoledronic acid. First, we changed the drug acquisition cost to use the WAC rather than the ASP. This was conducted to reflect the fact that the price for zoledronic acid varies by region (and may be higher than the ASP in some institutions) and to assess how much value is lost when comparing an innovative and a generic product. Second, we changed the efficacy of denosumab compared with zoledronic acid for the prevention of SREs by incorporating results from the pre-specified 15-month post-hoc analysis of the 20090482 study. In the primary analysis, used in the base case, most on-study SREs occurred within the first 3 months. Owing to the short duration of patient exposure to treatment, this may not have been long enough to detect treatment differences. The 15-month post-hoc analysis was conducted 1 year after most SRE events occurred, at a time point when the biological effect of each drug is likely to be measurable. Finally, we changed the proportion of patients with severe renal impairment from 10% to 25%. This was performed because real-world data suggest that the 10% used in the base case is probably a conservative estimate of the real-world proportion of patients with MM and severe renal impairmentCitation54.
The effect of parametric uncertainty on the ICER was evaluated via a one-way deterministic sensitivity analysis (this involved varying one parameter at a time to determine how model results were affected). The overall impact of the uncertainties affecting model parameters on the model output was evaluated using multivariate probabilistic sensitivity analyses (this involved varying multiple parameters simultaneously, the values of which were taken from their parameter-specific probability distributions, and then running the model 2,000 times).
Results
Base case: societal and payer perspectives
From a societal perspective, based on the ASPs, the use of denosumab instead of zoledronic acid resulted in an incremental cost of $26,329 (∼5% of the total lifetime SRE-related and primary anti-myeloma treatment costs; ) and an incremental benefit measured by QALYs of 0.2439, which translated into a cost of $107,939 per QALY gained () and an NMB difference of $10,259 in favor of denosumab (; ). When based on the WAC prices, the same analysis resulted in an ICER of $34,895 per QALY gained.
Figure 3. Net monetary benefit of denosumab vs zoledronic acid in patients with multiple myeloma from (a) the societal perspective and (b) the payer perspective. Societal perspective included SRE direct costs (hospital, outpatient, long-term care and hospice, strong opioid, emergency department visits, physical therapy, and skilled nursing facility), QALY monetization and direct non-medical (driving and parking, caregiver), and indirect costs (short-term disability and productivity loss). Payer perspective included SRE direct costs (hospital, outpatient, long-term care and hospice, strong opioid, emergency department visits, physical therapy, and skilled nursing facility) and QALY monetization. Assumed 50% MM treatment cost offsets and 35% patients eligible for short-term disability and productivity loss. Drug acquisition costs were based on average sales prices. Abbreviations. MM, multiple myeloma; NMB, net monetary benefit; QALY, quality-adjusted life year; SAE, serious adverse event; SRE, skeletal-related event; ZA, zoledronic acid.
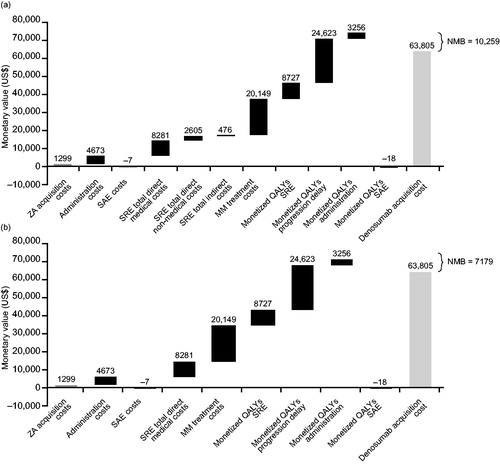
Table 6. Costs by category (discounted).
Table 7. Comparator incremental cost-effectiveness ratio results from the societal perspective.
From the payer perspective, based on the ASPs, the adoption of denosumab resulted in an incremental cost of $29,409 and an incremental benefit measured by a QALY of 0.2439, which translated into a cost of $120,569 per QALY gained and a NMB difference of $7,179 in favor of denosumab (; ). When based on the WAC prices, the same analysis resulted in an ICER of $47,525 per QALY gained.
Table 8. Comparator incremental cost-effectiveness ratio results from the payer perspective.
With ICERs below the WTP threshold of $150,000/QALY, denosumab was found to be cost-effective vs zoledronic acid, both from a societal and payer perspective, and regardless of whether drug acquisition costs were based on the ASP or WAC.
Additional scenarios: landmark analysis, patients with renal impairment, and real-world adjustment factor
When the results of the landmark analysisCitation20 were considered, the ICER from the societal perspective was $74,514 per QALY. If the proportion of patients with severe renal impairment was increased to 25%, the ICER was $64,068 per QALY from a societal perspective. If the SRE rate was adjusted with a lower factor (2.1)Citation36, and rates were based on the landmark analysis, the ICER was $102,585 per QALY from a societal perspective.
Sensitivity analyses
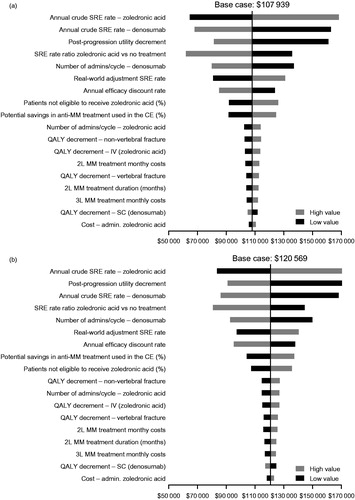
Univariate deterministic sensitivity analyses showed that the model results were relatively stable with respect to the values of key variables, and remained below the accepted threshold, with the exception of annual crude SRE rates and the post-progression utility decrement (see ).
Figure 4. One-way deterministic sensitivity analyses of key variables from (a) the societal perspective and (b) the payer perspective. Ranges for parameters used were as follows: annual efficacy discount rate = 0.00–0.05; percentage of patients not eligible to receive zoledronic acid = 0.05–0.15; annual crude SRE rate of denosumab = 0.55–0.64; annual crude SRE rate of zoledronic acid = 0.58–0.67; real world adjustment SRE rate = 2.01–4.01; SRE rate ratio for zoledronic acid vs no treatment = 0.42–0.82; zoledronic acid cost of administration = 189–231; denosumab number of cycles = 0.79–0.97; zoledronic acid number of cycles = 0.77–0.94; post-progression utility decrement = 0.57–0.72; QALY decrement SC = 0.0009–0.0014; QALY decrement IV = 0.0017–0.0025; QALY decrement vertebral fracture =0.05–0.15; QALY decrement non-vertebral fracture = 0.05–0.15; MM second-line treatment duration = 7.66–9.36; percentage of potential savings in anti-MM treatment used in the cost-effectiveness analysis = 0.40–0.60; second-line MM treatment monthly costs = 16,430–20,081; third-line MM treatment monthly costs = 16,530–20,204. Abbreviations. 2L, second line; 3L, third line; CE, cost-effectiveness analysis; IV, intravenous; MM, multiple myeloma; RR, risk ratio; SC, subcutaneous injection; SRE, skeletal-related event; QALY, quality-adjusted life-year.
Probabilistic multivariate sensitivity analyses showed that, at a WTP threshold of $150,000, the probability of denosumab being cost-effective vs zoledronic acid was more than 63% from the societal perspective and 60% from the payer perspective.
Discussion
To our knowledge, this is the first study to assess the cost-effectiveness of denosumab vs zoledronic acid in patients with MM. This analysis quantified the economic value delivered by denosumab to society and payers using the best available clinical and economic evidence to capture a wide range of costs and benefits accrued over a patient lifetime. The cost-effectiveness of denosumab compared with zoledronic acid in patients with MM holds for a WTP threshold of $150,000 per QALY gained, which is considerably lower than the $300,000 WTP threshold frequently used for cost-effectiveness analysis of novel drugsCitation55,Citation56. Denosumab also delivered a positive NMB to both society and payers. By quantifying the comparative value of treatmentsCitation57, we hope that this analysis will support patients, clinicians, and payers in making informed decisions on which agent to use for the prevention of SREs in patients with MM, which may ultimately help maximize patient outcomes. Given its substantial economic benefit, lack of renal toxicity, and expected impact on PFS, denosumab has the potential to provide value to patients, healthcare providers, payers, and society.
Although previous studies have assessed the cost-effectiveness of denosumab compared with zoledronic acid in patients with solid tumorsCitation24–30, it is not possible to directly compare the results of these previous analyses because of the different clinical settings. This is due to important differences in clinical outcomes reported from studies comparing the efficacy of these agents in solid tumors and in MM; denosumab has been shown to be superior compared with zoledronic acid in preventing SREs in patients with solid tumorsCitation23, whereas in MM denosumab has been shown to be non-inferior in preventing SREs and to extend PFS compared with zoledronic acidCitation20.
This economic evaluation has a number of strengths. In particular, the majority of clinical inputs were generated within a large, well-designed, double blind, randomized, controlled study comparing denosumab with zoledronic acid in patients with newly diagnosed MM. The model also accounts for direct non-medical costs and indirect costs that are usually neglected in other analyses (which typically focus solely on direct medical costs), and provides a more holistic view of the value of innovative drugs, such as denosumab. Moreover, the model bridges the gap between randomized controlled trials and real-world practice by accounting for important comorbidities (i.e. renal impairment), the route of drug administration, the extrapolation of key clinical outcomes beyond the clinical study follow-up, the impact on MM treatment, and the costs and burden for patients and caregivers.
There are, of course, several limitations, and the results presented should be interpreted within the context of the data inputs and modeling assumptions adopted. For instance, the costs of SREs in patients with MM were assumed to be similar to the costs of SREs from solid tumors, as cited in Stopeck et al.Citation28 However, because these costs are associated specifically with treating SREs, and not the primary tumor, it is reasonable to assume that any cost differences between treating SREs in solid tumors and MM would be minor. Other costs of healthcare services were estimated from multiple published sources that were not necessarily derived from patients with MM, and which varied by tumor type, patient population, country, and other parameters. PFS, OS, and time to discontinuation distributions were extrapolated beyond the follow-up of the primary analysis of the phase 3 study, and it should be noted that long-term (lifetime) extrapolations are affected by uncertainty, as they depend on multiple factors (some of which are unknown) and on complex dynamics. Additionally, for simplicity, a medical consumer price index (CPI) for inflation was applied to all costs, including non-medical costs. We anticipate that applying a non-medical CPI to non-medical costs would have minimal impact on the model. However, best modeling practices have been applied, and clinically and statistically motivated assumptions have been made whenever possibleCitation58. Finally, sensitivity analyses have been used to test the robustness of the results.
Conclusions
This analysis shows that denosumab is a cost-effective option for the prevention of SREs in patients with MM compared with the current standard of care, zoledronic acid. This is due to its combined impact on reducing SREs and the observed improvement of PFS compared with zoledronic acid, as well as its lack of impact on renal function. The available evidence points to the conclusion that denosumab would remain cost-effective under a variety of scenarios, providing value to patients, payers, and society.
Transparency
Declaration of funding
This study was funded by Amgen, Inc.
Declaration of financial/other relationships
NR has consulted for Amgen, Inc., BMS, Celgene, Merck, Novartis, Roche, and Takeda and received research funding from AstraZeneca. GDR has consulted for Amgen Inc. WW has participated in Steering Committees for Amgen; is an employee for Oncotyrol (20%); has participated in Advisory Boards and has consulted for Amgen, BMS, Celgene, cti, Gilead, Janssen, Novartis, Merck, Mundipharma, Pfizer, Roche, Sandoz, Takeda, and The Binding Site; has presented lectures for Amgen, Abbvie, BMS, Celgene, Gilead, Janssen, Mundipharma, Myelom- und Lymphomselbsthilfe Österreich, Novartis, Roche, Sandoz, Takeda, and The Binding Site; has participated in speaker bureau for Gilead; and has received research funding from Amgen, BMS, Celgene, Janssen, Novartis, Roche, Takeda, Oncotyrol, European Commission (FP7 - OPTATIO) and Bundesland Tirol Programm: ‘Translational research’. KS has consulted for Daiichi-Sankyo and Fujimoto Pharma; received research grants from Daiichi-Sankyo and honoraria from Daiichi-Sankyo and Fujimoto Pharma; and provided expert testimony for Amgen, Inc. RGS has received honoraria from Janssen, Takeda, and Pharmacyclics; received travel and accommodation expenses from Janssen, Takeda, and Celgene; and received research funding from Hospira. ET reports grants, personal fees, and non-financial support from Amgen, Celgene, and Janssen-Cilag; personal fees and non-financial support from Takeda; and personal fees from Novartis, GSK, Roche, and BMS, outside the submitted work. LK is a consultant for Amgen. LS, MI, and GH are employees of Amgen and hold Amgen stock. Peer reviewers on this manuscript have received an honorarium from JME for their review work. One reviewer discloses previous work on cost-effectiveness analyses sponsored by Novartis (maker of zoledronic acid) in which zoledronic acid was compared to denosumab; the results of these analyses have been reported in JME and elsewhere. This reviewer has not participated in work regarding denosumab or zoledronic acid or sponsored by Novartis since 2013. The remaining peer reviewers have no relevant financial relationships to disclose.
Acknowledgments
The authors would like to thank Jacqui Buchanan, Sumita Bhatta, Daniela Niepel, Nicolas Despiegel, and Aurelien Jamotte for their useful insights during the development of this manuscript. Medical writing support, funded by Amgen, Inc., was provided by Kelly Soady (PhD) from Oxford PharmaGenesis, Oxford, UK.
References
- Durie BGM. Concise review of the disease and treatment options. 2017. International Myeloma Foundation North Hollywood, CA, USA. Available at https://www.myeloma.org/sites/default/files/images/publications/UnderstandingPDF/concisereview.pdf
- Roodman GD. Pathogenesis of myeloma bone disease. Blood Cells Mol Dis 2004;32:290-2
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011;364:1046-60
- Hameed A, Brady JJ, Dowling P, et al. Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastasis 2014;7:33-42
- Multiple Myeloma Research Foundation. Bone lesions and damage. Available at https://www.themmrf.org/multiple-myeloma/symptoms/bone-lesions/
- von Moos R, Strasser F, Gillessen S, et al. Metastatic bone pain: treatment options with an emphasis on bisphosphonates. Support Care Cancer 2008;16:1105-15
- Coleman R, Body JJ, Aapro M, et al. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2014;25(Suppl 3):iii124-37
- Yong M, Jensen AO, Jacobsen JB, et al. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007). Breast Cancer Res Treat 2011;129:495-503
- Tosi P. Diagnosis and treatment of bone disease in multiple myeloma: spotlight on spinal involvement. Scientifica (Cairo) 2013;2013:104546
- Bhowmik D, Hines DM, Intorcia M, et al., editors. Healthcare resource utilization and costs of skeletal-related events in patients with multiple myeloma. ISPOR 22nd Annual International Meeting; May 20–24, 2017; Boston, MA.
- Nash Smyth E, Conti I, Wooldridge JE, et al. Frequency of skeletal-related events and associated healthcare resource use and costs in US patients with multiple myeloma. J Med Econ 2016;19:477-86
- Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc 2016;91:101-19
- Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia 2014;28:981-92
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Multiple myeloma. NCCN; 2017. Fort Washington, PA, USA. Available at https://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf
- Novartis. Zometa. Prescribing information. Novartis; 2013. East Hanover, NJ, USA. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021223s028lbl.pdf
- Diel IJ. Effectiveness of bisphosphonates on bone pain and quality of life in breast cancer patients with metastatic bone disease: a review. Support Care Cancer 2007;15:1243-9
- Reid IR, Gamble GD, Mesenbrink P, et al. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 2010;95:4380-7
- Barrett-Lee P, Bloomfield D, Dougherty L, et al. An audit to determine the time taken to administer intravenous bisphosphonate infusions in patients diagnosed with metastatic breast cancer to bone in a hospital setting. Curr Med Res Opin 2007;23:1575-82
- Amgen. XGEVA prescribing information. Amgen; 2018. Thousand Oaks, CA, USA. Available at http://pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/xgeva/xgeva_pi.pdf
- Raje N, Terpos E, Willenbacher E, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol 2018;19:370-81
- von Moos R, Body J-J, Rider A, et al. Bone-targeted agent treatment patterns and the impact of bone metastases on patients with advanced breast cancer in real-world practice in six European countries. J Bone Oncol 2018;11:1-9
- Hernandez RK, Adhia A, Wade SW, et al. Prevalence of bone metastases and bone-targeting agent use among solid tumor patients in the United States. Clin Epidemiol 2015;7:335-45
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012;48:3082-92
- Koo K, Lam K, Mittmann N, et al. Comparing cost-effectiveness analyses of denosumab versus zoledronic acid for the treatment of bone metastases. Support Care Cancer 2013;21:1785-91
- Ford J, Cummins E, Sharma P, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess 2013;17:1-386
- Dellis A, Papatsoris A. Cost-effectiveness of denosumab as a bone protective agent for patients with castration resistant prostate cancer. Expert Rev Pharmacoecon Outcomes Res 2016;16:5-10
- Shapiro CL, Moriarty JP, Dusetzina S, et al. Cost-effectiveness analysis of monthly zoledronic acid, zoledronic acid every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases: CALGB 70604 (Alliance). J Clin Oncol 2017;JCO2017737437
- Stopeck A, Rader M, Henry D, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ 2012;15:712-23
- Snedecor SJ, Carter JA, Kaura S, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J Med Econ 2013;16:19-29
- Snedecor SJ, Carter JA, Kaura S, et al. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther 2012;34:1334-49
- Garrison LPJ, Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health 2017;20:213-16
- Rochau U, Jahn B, Qerimi V, et al. Decision-analytic modeling studies: an overview for clinicians using multiple myeloma as an example. Crit Rev Oncol Hematol 2015;94:164-78
- Gonsalves WI, Leung N, Rajkumar SV, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J 2015;5:e296
- McKeage K, Plosker GL. Zoledronic acid: a pharmacoeconomic review of its use in the management of bone metastases. Pharmacoeconomics 2008;26:251-68
- Henk HJ, Teitelbaum A, Perez JR, et al. Persistency with zoledronic acid is associated with clinical benefit in patients with multiple myeloma. Am J Hematol 2012;87:490-5
- Hatoum HT, Lin SJ, Smith MR, et al. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer 2008;113:1438-45
- Ashcroft J, Duran I, Hoefeler H, et al. Healthcare resource utilisation associated with skeletal-related events in European patients with multiple myeloma: results from a prospective, multinational observational study. Eur J Haematol 2018. doi:10.1111/ejh.13044
- Hechmati G, Cure S, Gouepo A, et al. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ 2013;16:691-700
- Williams C, Lewsey JD, Mackay DF, et al. Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and markov decision-analytic modeling. Med Decis Making 2017;37:427-39
- Sabatelli L, Jamotte A, Giannopoulou C, et al. PCN19 - Estimation of the health benefit associated with a potential denosumab-induced extension of progression free survival in multiple myeloma patients. Value Health 2017;20:A414-A415
- Büyükkaramikli NC, de Groot S, Fayter D, et al. Pomalidomide with dexamethasone for treating relapsed and refractory multiple myeloma previously treated with lenalidomide and bortezomib: an evidence review group perspective of an NICE single technology appraisal. PharmacoEconomics 2017;36:145-59
- Center for Disease Control. National vital statistic reports: United States life tables 2012. CDC; 2016. Hyattsville, MD, USA. Available at https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_08.pdf
- Matza LS, Chung K, Van Brunt K, et al. Health state utilities for skeletal-related events secondary to bone metastases. Eur J Health Econ 2014;15:7-18
- van Agthoven M, Segeren CM, Buijt I, et al. A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma; a prospective randomised phase III study. Eur J Cancer 2004;40:1159-69
- Centers for Medicare & Medicaid Services. Payment allowance limits for medicare Part B Drugs. CMMS; 2017. Baltimore, MD, USA. Available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2017ASPFiles.html
- Bell MJ, Miller JD, Namjoshi M, editors. Comparative budget impact of formulary inclusion of zoledronic acid and denosumab for prevention of skeletal-related events in patients with bone metastases. International Society for Pharmacoeconomics and Outcomes Research 16th Annual Meeting; May 21–25, 2011; Baltimore, MD.
- Xie J, Namjoshi M, Wu EQ, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm 2011;17:621-43
- Jayasekera J, Onukwugha E, Bikov K, et al. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics 2014;32:173-91
- Qian DW, Bhowmik D, Kachru N. Utilization patterns of bone-targeting agents among patients with multiple myeloma: analysis of real-world data. Blood 2015;126:4501
- von Moos R, Body JJ, Egerdie B, et al. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support Care Cancer 2016;24:1327-37
- Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology 2004;67:390-6
- Gridelli C, Ferrara C, Guerriero C, et al. Informal caregiving burden in advanced non-small cell lung cancer: the HABIT study. J Thorac Oncol 2007;2:475-80
- Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med 2003;163:1637-41
- Qian Y, Bhowmik D, Bond C, et al. Renal impairment and use of nephrotoxic agents in patients with multiple myeloma in the clinical practice setting in the United States. Cancer Med 2017;6:1523-30
- Braithwaite RS, Meltzer DO, King JT Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care 2008;46:349-56
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796-7
- Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 1996;276:1253-8
- Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72
- Barlev A, Song X, Ivanov B, et al. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm 2010;16:693-702
