Abstract
Background: Lung cancer is one of the most prevalent cancers in the US. This study was designed to evaluate the actual drug wastage and cost to the healthcare system using patient-level retrospective observational electronic medical record (EMR) data from a cohort of lung cancer patients in the US.
Methods: Data from the Flatiron Health advanced non-small cell lung cancer (NSCLC) cohort was used for this study. Drug administered amount (in mg) was used to determine an optimal set of available vial sizes to minimize waste. Drug wastage was defined as the difference between the drug amount in the optimal set of vials and the administered amount. Wholesale acquisition costs were used to value the cost of drugs, with and without vial sharing assumptions. The amount and cost of waste were quantified over the 2-year study period (January 2015–December 2016).
Results: There were 8,467 eligible patients included in this study, providing data from 103,826 unique drug administrations across multiple lines of therapy. Overall wastage was 4.37% of the total medication used to care for patients. While costs per administration were low, the total cost of wastage for the study population represented $16,630,112 across the 2-year study period. Assuming that vial sharing occurred at the site level slightly reduced waste to 3.74% (reducing costs to $15,953,212 over 2 years).
Conclusions: Drug wastage is an important concern and has implications on healthcare costs in NSCLC. Evaluation of these real-world data suggest that pharmacists and physicians are able to reduce drug wastage by optimizing vial combinations and sharing vials among patients. Even small amounts of reduction in wastage could be useful in reducing healthcare costs in the US; however, caution is needed with drug rounding efforts to ensure patients do not receive a sub-optimal dose of medication.
Introduction
Lung cancer is the second most common cancer, and the leading cause of cancer deaths in the US. Approximately 234,030 new cases of lung cancer are diagnosed each year (121,680 in men and 112,350 in women), with 154,050 deaths (83,550 in men and 70,500 in women)Citation1. Lung cancer is categorized into two histological sub-groups: non-small cell lung cancer (NSCLC) and small cell lung cancer, representing 85% and 15% of all lung cancers, respectivelyCitation2. More than half (53.2%) of all lung cancers are diagnosed when the disease has already metastasizedCitation3. The care of patients with advanced/metastatic NSCLC has improved in recent years and, as of 2018, is supported by high-quality scientific evidence for more than 16 different treatment regimens in the National Comprehensive Cancer Network (NCCN) GuidelinesCitation4. This has led to considerable heterogeneity of treatmentCitation5, while allowing for tailoring treatment (e.g. regimen, dose) to the individual patient.
Cost considerations become more important in the choice between treatments with similar efficacy and toxicity profiles. In the past decade, multiple agents within the same drug class (e.g. immune checkpoint inhibitors, anti-angiogenic agents) have been approved by the US Food and Drug Administration (FDA) for the care of patients with NSCLC and have become part of the armamentarium of the treating oncologist. As these new, higher-priced medications are increasingly used to care for patients with NSCLC, the need to minimize waste becomes a greater concern to ensure that resources used in the delivery of care are actually benefitting the patient.
Drug wastage is an important public health concern, and may contribute to the escalating costs of cancer care—although the exact amount is yet unknownCitation6. Drug that is disposed of (e.g. waste) unnecessarily escalates the cost of care. Drug wastage can occur due to vial sizes that do not match the drug needed to treat the wide range of actual patient body sizes (i.e. oncology drugs are generally dosed by body weight or by body surface area). When a mismatch between the dose needed and vial size occurs, the provider may end up with excess unused drug in a vial (or must increase the dose to the patient to use it). On the other hand, this can also occur when there is insufficient drug in a set of vials—the provider may use a small amount from a new vial to accurately dose the patient (leaving the majority of the next vial unused as waste) or risk under-dosing the patient. Most institutions allow for a 5% rounding to occur, which is considered to be within appropriate dosing guidelines and is expected to reduce wastageCitation7. This rounding is generally considered appropriate to minimize waste when patient dose–vial size mismatching occurs.
Prior research has estimated the costs of wastage to the US healthcare system and found waste to be substantial in oncologyCitation6. However, prior research has not been able to measure actual drug used, the application of dose rounding procedures, and could not evaluate if over- or under-dosing occurs to mitigate the costs associated with drug waste, which remains a gap in knowledge. This study was designed to quantify the wastage of intravenous cancer drugs for the treatment of NSCLC using patient-level data from an electronic medical record (EMR) database to address this gap. The overarching goal of this work is to build on prior modeling work to more accurately quantify the actual drug waste in a NSCLC cohort in the US using real-world data.
Methods
Data source
The Flatiron Health Advanced NSCLC cohort includes a geographically diverse population of patients with advanced NSCLC at Flatiron network of community and academic cancer centers across the US. The database includes patients whose advanced NSCLC diagnoses occurred on or after January 1, 2011 and who have two or more visits documented in the EMR during that same time period. The database is refreshed monthly and includes both structured and some unstructured EMR data elements, such as patient demographics (gender, race, birth year, and state of residence), type of cancer facility visited (community vs academic), clinical diagnoses, laboratory data, biomarker tests, and results, medications ordered, and/or administered, dose in milligrams of each drug administered, line of therapy (derived), month and year of death, and other patient clinical characteristics including cancer stage at diagnosis, tumor histology, and performance status.
Eligibility criteria
Eligible patients in the study cohort were those that received therapy with cisplatin, carboplatin, paclitaxel, nab-paclitaxel, ramucirumab, pemetrexed, necitumumab, bevacizumab, pembrolizumab, or nivolumab between January 1, 2015 and December 31, 2016 (2-year study period). Patients must have had at least one body weight measurement if they received ramucirumab, bevacizumab, pembrolizumab, or nivolumab (which are dosed in mg/kg). Patients must have had body surface area or at least one height and one weight measurement if they received cisplatin, paclitaxel, nab-paclitaxel, or pemetrexed (which are dosed in mg/m2). Patients receiving carboplatin must have recorded body weight, serum creatinine, age, and gender data to be included (this drug is dosed as area under the curve)Citation8. Patients under 18 years of age were excluded. These data are exempt from human subjects reviews as they are de-identified in accordance with the standards set by the Heath Insurance Portability and Accountability Act (HIPAA) and do not meet the criteria for human subjects according to the Code of Federal Regulations [45 CFR 46.102(f)].
Drug used and waste measurements
The number of milligrams (mg) administered to each patient at each administration was used to estimate the optimal set of vials needed to treat the patient. The optimal set of vials minimizes the difference between the total amount of drug in the vials and the amount of drug administered. The differences between the actual administered amounts and the quantity of drug in the optimal set of vials was used to estimate the total waste. The number of vials was capped at six per patient per infusion, given what would be reasonable for a pharmacy to implement in practice to achieve efficiencies. Drugs were evaluated overall, categorized into branded (bevacizumab, necitumumab, nivolumab, nab-paclitaxel, pembrolizumab, pemetrexed, and ramucirumab) and generic agents (carboplatin, cisplatin, and paclitaxel) and were evaluated individually.
Sensitivity analyses were conducted under a vial sharing assumption scenario. If two or more patients receive the same agent within ±1 day at the same practice site (e.g. “patient stacking”), the milligrams used were combined to estimate the optimal number of vials used between patients. This was explored by clinic site, as there are observable differences between clinics that treat more patients and, therefore, have more opportunities for vial sharing than may be observed at clinics that treat fewer patients.
Drug dosing
The FDA-approved doses of each agent in NSCLC were used to determine the recommended dose per patient (). If the patient received a dose less than the labeled dose at any single infusion, it was assumed that reduced dosing had not occurred if whole vials were used and the total dose was not more than 5% lower than the approved dose. Similarly, if the patient received a dose higher than the labeled dose, it was assumed that increased dosing had not occurred if whole vials were used and the total dose was not 5% higher than the approved dose. Given that reduced dosing is not uncommon in cancer (e.g. due to adverse events or toxicity prevention), higher doses are of greater concern if used to minimize waste. Sensitivity analyses were conducted using a 10% rounding assumptionCitation9. Factors associated with reduced dosing and increased dosing were evaluated, including, but not limited to, geographic region, patient age, gender, body weight, body surface area, clinical site, site volume, line of therapy (e.g. maintenance, 1L, 2L), number of administrations of agent (1L therapy only), agent, academic vs community practices, and number of drugs in the regimen.
Table 1. Dosing and vial sizes of intravenous agents for the treatment of patients with non-small cell lung cancer.
Cost of wastage
Analyses were conducted using wholesale acquisition costs (WAC). As the true “price” of drugs is unknown due to multiple contracting and negotiated amounts, these values were used to estimate the cost per milligram of wasted drug. Drug costs were obtained from Truven RedBook data in March 2017Citation10. Cost analyses were also conducted using a vial sharing assumption as a sensitivity analysis.
Results
A total of 8,490 patients met eligibility criteria, representing 103,826 unique drug administrations across multiple lines of therapy during the 2-year study period. The cohort was 52.7% male, with a median age of 69.0 years; 64.2% were white, and the cohort was representative of the major geographic regions in the US (South 36.8%; Northeast 24.6%; Midwest 21.6%; West 15.1; Missing 2%). All practices were community-based; no academic institutions were represented in the eligible Flatiron cohort.
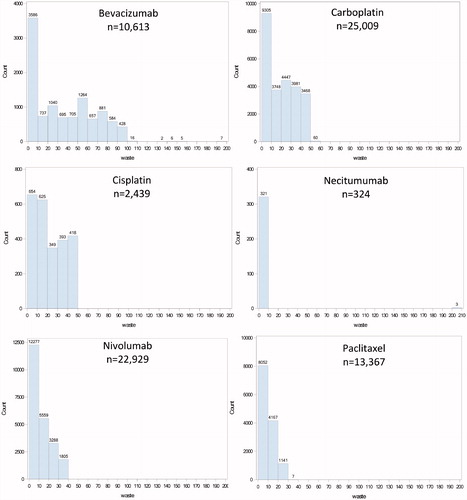
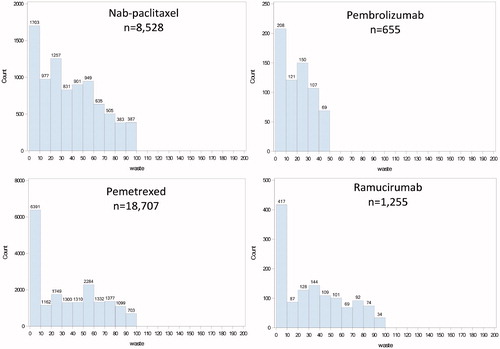
The number of patients, infusions, milligrams of drug available and administered, and estimates of waste overall and by drug are displayed in . Across the cohort, a total of 46,695,995 mg of drug was administered to patients. Assuming the most efficient use of available vial sizes, there would be 48,830,810 mg of drug available for patient care. The difference results in 2,134,815 mg of drug wasted over the 2-year study period (4.4% of available drug). The distribution of drug waste by drug is presented in . The proportion of available drug wasted varied considerably by drug, ranging from 0.2% (although this is in a small number of patients using necitumumab) to 16.9% for nab-paclitaxel and cisplatin. Each of these three drugs at each end of the range has only a single vial size available. The sensitivity analysis of the vial sharing assumption only slightly reduced drug waste to 1,815,775 mg (3.74% of available drug).
Figure 1. Histograms of wastage (mg/administration) by drug (n, number of administrations).
Table 2. Drug use and wastage, overall and by individual drug.
Assuming dose rounding within 5% is consistent with the recommended dosage for NSCLC, 46.9% of all drug administrations were consistent with the labeled dose (). The remaining administrations were primarily due to reduced doses (46.1% of administrations), and fewer were higher-than-recommended doses (7% of all administrations) (). Sub-groups had similar proportions; of note, branded agents were used within the range of labelled dose in 70.6% of all administrations, whereas generic agents were used within the labelled range in 10.4% of all administrations. This difference is largely attributable to the lower doses used in generic vs branded agents (85.8% of administrations of generic agents were reduced doses vs 20.4% of branded drug administrations). The sensitivity analysis, allowing up to 10% rounding, only slightly decreased the overall proportion of doses with reduced dose (41.1% of all administrations) and of those with higher doses (3.2% of all administrations). This was consistent across all sub-groups.
Table 3. Drug dosing (rounding assumption at 5% and 10% tolerance).
While the overall rate of drug wasted was relatively low (4.4%), this was associated with a total cost of $16,630,112 for the 8,467 patients in this study over a 2-year study period (). This were ∼$1,964 in costs due to wastage per patient with NSCLC. Vial sharing assumptions only slightly reduced the costs due to wastage to $15,953,212 total and $1,884 per patient.
Table 4. Cost due to wastage, without and with vial sharing assumptions.
Discussion
In this study, an average of 4.4% of drug was wasted in the form of unused medication. However, this was not consistent across all drugs, with several demonstrating >15% of waste due to unused drug (e.g. cisplatin, nab-paclitaxel, and pembrolizumab). These data are based on actual amounts of drug used to care for individual patients and the use of an optimal set of vials to minimize waste, regardless of the dosage.
When considering doses on the FDA label of each drug, the data suggest there may be an additional 7% of drug (9.1% of branded agents and 3.8% of generic agents, respectively) that could be considered “waste” in the form of higher-than-recommended doses administered to patients in this study. It is not clear from the available data if these higher doses had clinical rationale or if they were used simply to ensure an entire vial was used. This remains to be investigated. Importantly, the cost of this used, but potentially unnecessary, drug that could be considered waste was not included in these cost calculations, but would more than double the unnecessary cost of anti-cancer medication treatment to the healthcare system.
While the percentage of unused medication waste was relatively low, it was associated with an average cost of $1,964 per patient. This per-patient average cost, as expected, was substantially higher for the branded vs generic agents ($2,351 vs $14 per patient over the 2-year period). This study included 8,467 patients over a 2-year period, whereas ∼105,828 patients are diagnosed with metastatic NSCLC each year in the US. Therefore, the total costs observed in this study ($16,630,112 over 2 years) are lower than what might be expected at the national level, they are not nearly as high as the cost estimates of prior workCitation6. In this study of real-world patient data, drug wastage was found to be less than that estimated in other publications. While efforts are certainly needed to limit this further, a 4.4% average drug waste rate suggests that pharmacists and providers are addressing this issue by treating patients in a way that optimizes drug use. Their efforts could be supported by responsible manufacturing of oncology drug products through the production of appropriate vial sizes, and research to understand the impact of modified dosing of medication.
Although our study was limited to patients in this EMR dataset, the data obtained from patient-level data in a real world setting could be used as one data input to better estimate national healthcare costs of anti-cancer drug therapy for advanced or metastatic NSCLC. Future work should use the actual values from patient-level data as obtained in this study to estimate the cost of wastage in the US in this population. This may overcome some limitations of prior modeling work that was based on estimates of real-world drug usage.
Over-spending has been associated with large, single-dose vials in prior studies in oncologyCitation6; however, many of these use flat dosing, not based on patient weight. This previous study modeled patient estimates rather than using patient-level data. However, there was one drug in lung cancer (pemetrexed) that was included in both studies. Both the current study and the prior work demonstrated consistent findings with regard to pemetrexed for lung cancer (4% waste). However, measured unused waste was 16.9% for nab-paclitaxel in the current study (vs 9% for this agent in breast cancer in the prior study), and 11.9% for pembrolizumab (vs 24% for melanoma in the prior study)Citation6. There are likely differences that could be explained for drugs such as nab-paclitaxel, which is dosed at 260 mg/m2 in breast cancer vs 100 mg/m2 in lung cancer, which could lead to different amounts of drug left in a set of vials. However, the discrepancy in pembrolizumab suggests that the modeled estimates of waste may be too high as compared to what was observed in the current study, as pembrolizumab has the same recommended dose in NSCLC as it does in melanoma (200 mg). Importantly, dose was not necessary for the measurement of waste in this study as the actual amount of drug administered to the patient in milligrams was available in the EMR dataset.
A number of studies have addressed minimizing waste by dose roundingCitation7,Citation9,Citation11 by identifying and producing an optimal vial size in oncologyCitation12,Citation13, and by implementing waste reduction strategies at the institutional levelCitation14. It is clear there is a need for manufacturers to routinely apply scientific methods to more accurately identify and subsequently produce vial sizes that minimize waste with all new products. The combination of optimal vial sizes and dose rounding together has the potential to further reduce unused drug waste.
While the importance of reducing waste and the importance of manufacturers needing to address this issue is consistent with the current studyCitation6,Citation7,Citation9,Citation11–13, the current analysis adds to the body of knowledge through patient data specific to the breadth of this important problem in NSCLC across multiple agents. An area of future research could evaluate actual drug waste in breast, colorectal and other cancers to better understand how the problem varies by tumor type, or if approaches to dosing and rounding result in consistent waste reduction.
Importantly, this study found multiple sources of potential waste: unused drug and higher doses than recommended. There is a need to ensure that rounding efforts do not exceed the accepted threshold. The EMR dataset in this study did not include reasons for the dose administered. It can only be assumed that doses below the recommended doses were administered for safety reasons, as recommended on the package inserts; however, reduced dosing may also have occurred because of waste minimization efforts or due to other treatment strategies (e.g. such as flat dosing for checkpoint inhibitors or lower weekly dosing schedules for anti-neoplastic agents). While this study was not designed to evaluate patient outcomes, there is a potential risk of reduced efficacy with the use of reduced dosing and a risk of increased toxicity for increased doses that cannot be ignored. If these practices occur only to reduce waste, it is important that patient outcomes be monitored to ensure patients receive the expected benefit without additional risk.
Drug waste has long been a concern in oncologyCitation6,Citation7,Citation9, but only rarely has it been quantified using a dataset from patients in uncontrolled non-research settings. This study provides evidence of amount (average 4.4%) and cost of unused waste (average cost per patient of $1,964 for a 2-year period), as well as evidence of additional waste in the form of unnecessary drug being administered to patients.
Conclusions
Drug wastage is an important concern and has implications on healthcare costs in NSCLC. Evaluation of these real-world data suggest that pharmacists and physicians are able to reduce drug wastage by optimizing vial combinations and sharing vials among patients. Even small amount of reduction in wastage per administration could be useful in further reducing healthcare costs in the US; however, caution is needed with drug rounding efforts to ensure patients receive medication that neither reduces efficacy nor increases toxicity.
Transparency
Declaration of funding
This was an unfunded study.
Declaration of financial/other relationships
All authors are employees of Eli Lilly and Company. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None reported.
Previous presentations
These data were in part presented at the Society of Medical Decision Making 39th Annual North American Meeting in Pittsburgh, PA, October 22, 2017.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30
- Houston KA, Henley SJ, Li J, et al. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung Cancer 2014;86:22-8
- SEER. SEER*Explorer. www.seer.cancer.gov. Accessed January 19, 2018
- NCCN. NCCN clinical practice guidelines in oncology: non-small cell lung cancer ver 8. NCCN; 2017, Available at nccn.org, Accessed January 19, 2018
- Hess LM, Cui ZL, Xiaohong L, et al. Treatment sequencing for the care of patients with advanced or metastatic non-small cell lung cancer in the United States from 2014–2017. Lung Cancer (submitted 2017)
- Bach PB, Conti RM, Muller RJ, et al. Overspending driven by oversized single dose vials of cancer drugs. BMJ 2016;352:i788
- Dooley MJ, Singh S, Michael M. Implications of dose rounding of chemotherapy to the nearest vial size. Support Care Cancer 2004;12:653-6
- Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989;7:1748-56
- Field K, Zelenko A, Kosmider S, et al. Dose rounding of chemotherapy in colorectal cancer: an analysis of clinician attitudes and the potential impact on treatment costs. Asia-Pacific J Clin Oncol 2010;6:203-9
- RED BOOK. Micromedex Clinical Knowledge Suite. Truven Health Analytics, an IBM Company; 2017. Available from: https://truvenhealth.com/products/micromedex/product-suites/clinical-knowledge/red-book
- Patel S, Le A. Rounding rituximab dose to nearest vial size. J Oncol Pharm Pract 2013;19:218-21
- Clark L, Castro AP, Fortes AF, et al. Ideal vial size for bortezomib: real-world data on waste and cost reduction in treatment of multiple myeloma in Brazil. Value Health 2011;14(5 Suppl 1):S82-4
- Sheffield KM, Beyrer J, Watson I, et al. Minimization of olaratumab drug waste using real-world data. Am J Health Syst Pharm 2017;74(11):832-842
- Leung CYW, Cheung MC, Charbonneau LF, et al. Financial impact of cancer drug wastage and potential cost savings from mitigation strategies. J Oncol Pract 2017;13:e646-e652